Morphological and Mechanical Characterization of Extracellular Vesicles and Parent Human Synoviocytes under Physiological and Inflammatory Conditions
Abstract
:1. Introduction
2. Results
2.1. Choice of the Optimal Analysis Conditions for FLS and Secreted EV
2.1.1. Selection of the Appropriate Time-Point to Perform Morphological and Mechanical Characterization
2.1.2. Choice of the Appropriate Number of Cell Passages
2.2. Impact of Disease on FLS and on Synovial Vesicles
2.2.1. Ultrastructural Characterization at Cell Level by Confocal Microscopy
2.2.2. Ultrastructural Characterization of Synovial Vesicles under Healthy and Pathological Conditions
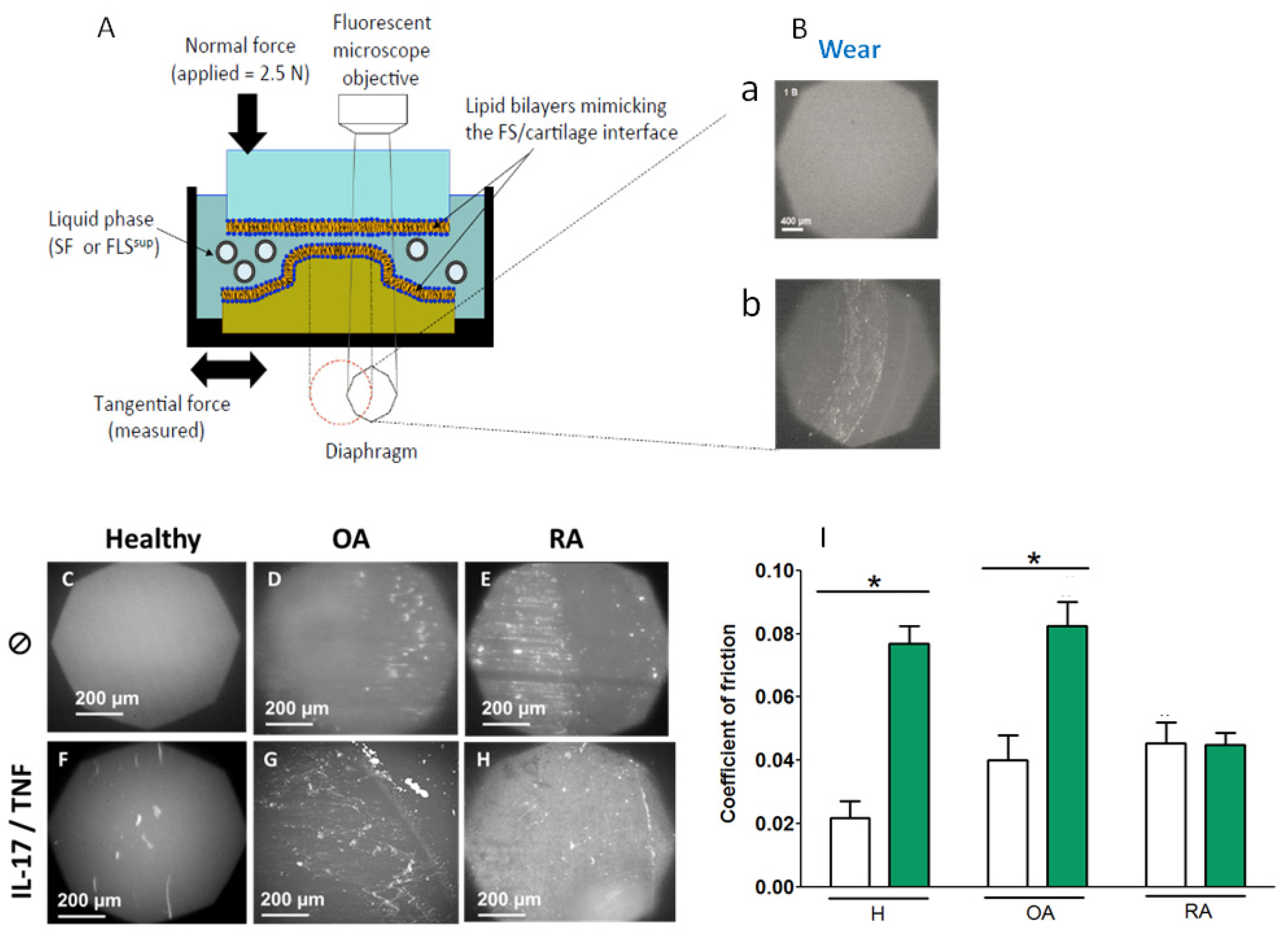
2.3. Mechanical and Tribological Characterization
2.3.1. Tribological Properties of Supernatant in the Case of OA and RA
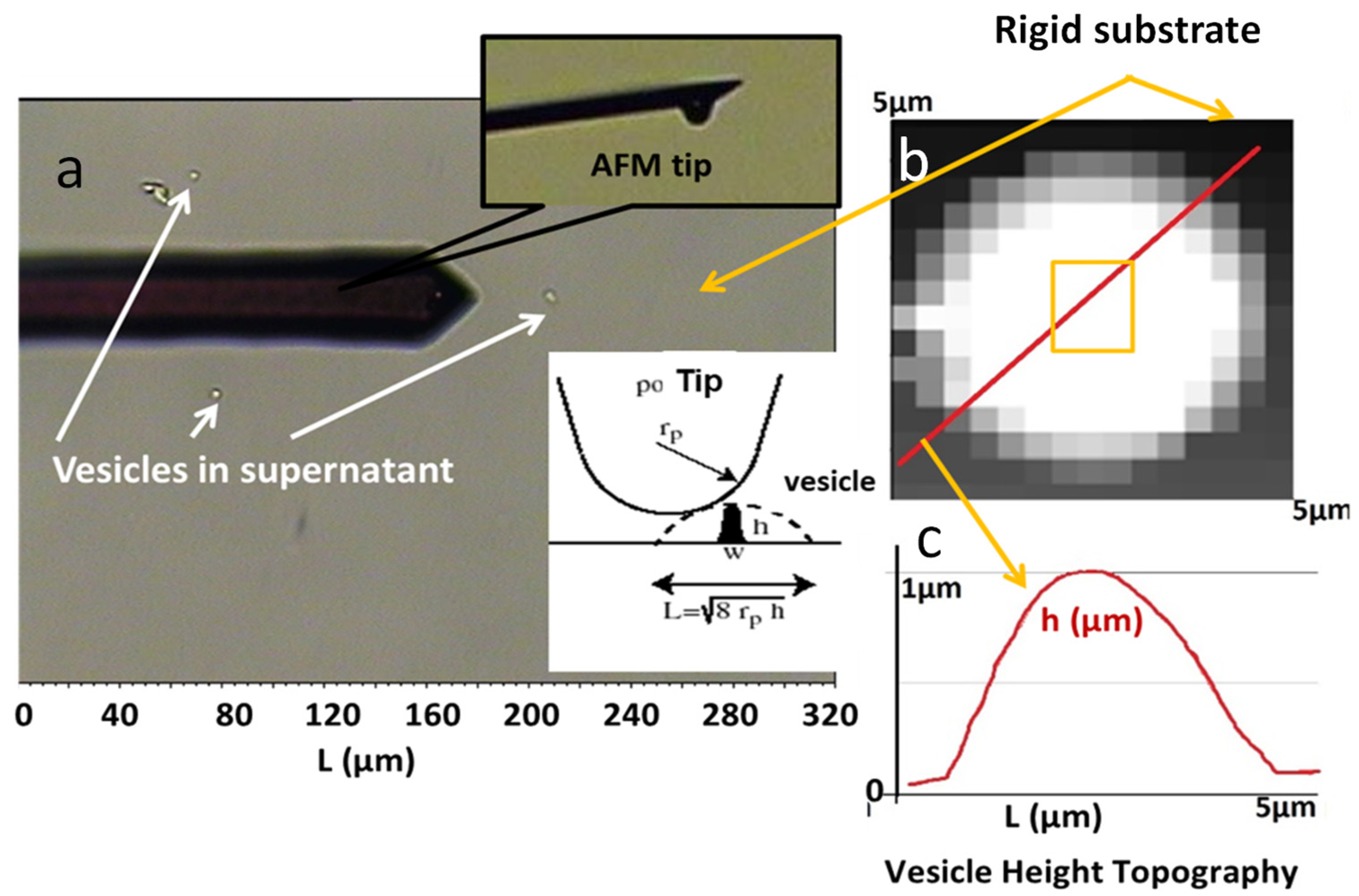
2.3.2. Mechanical Properties of Synovial Vesicles Evaluated by Atomic Force Microscopy
2.3.3. Morphology of Synovial Vesicles by Atomic Force Microscopy
2.4. Response to Mechanical Stress at the Scale of a Single Vesicle
3. Discussion
4. Materials and Method
4.1. Biological Samples and Ethic Statements
4.2. Cell Culture Conditions
4.3. Visualization of Cells and Synovial Vesicles by Confocal Microscopy
4.4. Vesicle Visualization by Transmission Electron Microscopy
4.5. Determination of Phospholipid Concentration Using Stewart Assay
4.6. Tribological Tests
4.7. Analysis of the Intrinsic Rigidity of Vesicles by Atomic Force Microscopy (AFM)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirea, D.A.; Trunfio-Sfarghiu, A.-M.; Matei, C.I.; Munteanu, B.; Piednoir, A.; Rieu, J.P.; Blanchin, M.-G.; Berthier, Y. Role of the biomolecular interactions in the structure and tribological properties of synovial fluid. Tribol. Int. 2013, 59, 302–311. [Google Scholar] [CrossRef]
- Murakami, T.; Yarimitsu, S.; Sakai, N.; Nakashima, K.; Yamaguchi, T.; Sawae, Y.; Suzuki, A. Superior lubrication mechanism in poly(vinyl alcohol) hybrid gel as artificial cartilage. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2017, 231, 1160–1170. [Google Scholar] [CrossRef]
- Noble, P.; Collin, B.; Letcombe-Beckers, J.; Magnée, A.; Denoix, J.-M.; Serteyn, D.A. L’articulation synoviale: Un système tribologique parfait [The synovial joint: A perfect tribological system]. Ann. Méd. Vét. 2010, 154, 83–93. [Google Scholar]
- Nuki, G.; Ferguson, J. Studies on the nature and significance of macromolecular complexes in the rheology of synovial fluid from normal and diseased human joints. Rheol. Acta 1971, 10, 8–14. [Google Scholar] [CrossRef]
- Prekasan, D.; Saju, K.K. Review of the Tribological Characteristics of Synovial Fluid. Procedia Technol. 2016, 25, 1170–1174. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.E.; Deodhar, S.; Nuki, G.; Whaley, K.; Buchanan, W.W.; Dick, W.C. Radioisotope study of small joint inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 1971, 30, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Emmi, A.; Stocco, E.; Boscolo-Berto, R.; Contran, M.; Belluzzi, E.; Favero, M.; Ramonda, R.; Porzionato, A.; Ruggieri, P.; De Caro, R.; et al. Infrapatellar Fat Pad-Synovial Membrane Anatomo-Fuctional Unit: Microscopic Basis for Piezo1/2 Mechanosensors Involvement in Osteoarthritis Pain. Front. Cell Dev. Biol. 2022, 10, 886604. [Google Scholar] [CrossRef]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. Biomed. Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef]
- Syx, D.; Tran, P.B.; Miller, R.E.; Malfait, A.-M. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr. Rheumatol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Sánchez-Flórez, J.C.; Seija-Butnaru, D.; Valero, E.G.; Acosta, C.D.P.A.; Amaya, S. Pain Management Strategies in Rheumatoid Arthritis: A Narrative Review. J. Pain Palliat. Care Pharmacother. 2021, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Khir, N.A.M.; Noh, A.S.M.; Long, I.; Ismail, N.I.; Siran, R.; Ismail, C.A.N. Inflammatory-associated apoptotic markers: Are they the culprit to rheumatoid arthritis pain? Mol. Biol. Rep. 2022, 49, 10077–10090. [Google Scholar] [CrossRef] [PubMed]
- Shikichi, M.; Kitamura, H.P.; Yanase, H.; Konno, A.; Takahashi-Iwanaga, H.; Iwanaga, T. Three-dimensional Ultrastructure of Synoviocytes in the Horse Joint as Revealed by the Scanning Electron Microscope. Arch. Histol. Cytol. 1999, 62, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.C. The nature and origins of synovium: Experimental approaches to the study of synoviocyte differentiation. J. Anat. 1994, 184 Pt 3, 493–501. [Google Scholar] [PubMed]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and Functional Roles of Synoviocytes in the Joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, D.E.; Kaabachi, W.; Sassi, N.; Tarhouni, L.; Rekik, S.; Jemmali, S.; Sehli, H.; Kallel-Sellami, M.; Cheour, E.; Laadhar, L. The synovial fluid fibroblast-like synoviocyte: A long-neglected piece in the puzzle of rheumatoid arthritis pathogenesis. Front. Immunol. 2022, 13, 942417. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. Two Main Cellular Components in Rheumatoid Arthritis: Communication Between T Cells and Fibroblast-Like Synoviocytes in the Joint Synovium. Front. Immunol. 2022, 13, 922111. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, D.; Yang, H.; Gao, J.; Zhang, G.; Xu, K.; Zhang, L. Fibroblast-like synoviocytes in rheumatoid arthritis: Surface markers and phenotypes. Int. Immunopharmacol. 2021, 93, 107392. [Google Scholar] [CrossRef]
- Han, D.; Fang, Y.; Tan, X.; Jiang, H.; Gong, X.; Wang, X.; Hong, W.; Tu, J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell Mol. Med. 2020, 24, 9518–9532. [Google Scholar] [CrossRef]
- Hunter, D.J.; McDougall, J.J.; Keefe, F.J. The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. North Am. 2008, 34, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Sofat, N.; Ejindu, V.; Kiely, P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology 2011, 50, 2157–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, L.-D.; Thiele, G.M.; Tian, J.; Wang, D. The Development of Novel Therapies for Rheumatoid Arthritis. Expert Opin. Ther. Pat. 2008, 18, 723–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreev, D.; Kachler, K.; Schett, G.; Bozec, A. Rheumatoid arthritis and osteoimmunology: The adverse impact of a deregulated immune system on bone metabolism. Bone 2022, 162, 116468. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990{\textendash}2017: A systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef]
- Ballara, S.; Taylor, P.C.; Reusch, P.; Marmé, D.; Feldmann, M.; Maini, R.N.; Paleolog, E.M. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001, 44, 2055–2064. [Google Scholar] [CrossRef]
- Feldmann, M.; Brennan, F.M.; Paleolog, E.; Cope, A.; Taylor, P.; Williams, R.; Woody, J.; Maini, R.N. Anti-TNFalpha therapy of rheumatoid arthritis: What can we learn about chronic disease? Novartis Found. Symp. 2004, 256, 53–69. [Google Scholar]
- Shukla, P.; Mansoori, M.N.; Kakaji, M.; Shukla, M.; Gupta, S.K.; Singh, D. Interleukin 27 (IL-27) Alleviates Bone Loss in Estrogen-deficient Conditions by Induction of Early Growth Response-2 Gene. J. Biol. Chem. 2017, 292, 4686–4699. [Google Scholar] [CrossRef] [Green Version]
- Noack, M.; Miossec, P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P. Local and systemic effects of IL-17 in joint inflammation: A historical perspective from discovery to targeting. Cell. Mol. Immunol. 2021, 18, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.F.; Isaacs, J.D. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef]
- Granet, C.; Maslinski, W.; Miossec, P. Increased AP-1 and NF-kappaB activation and recruitment with the combination of the proinflammatory cytokines IL-1beta, tumor necrosis factor alpha and IL-17 in rheumatoid synoviocytes. Arthritis Res. Ther. 2004, 6, R190. [Google Scholar] [CrossRef] [Green Version]
- Hot, A.; Lenief, V.; Miossec, P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann. Rheum. Dis. 2012, 71, 768–776. [Google Scholar] [CrossRef]
- Miossec, P. Cytokines in rheumatoid arthritis: Is it all TNF-alpha? Cell. Mol. Biol. (Noisy-Le-Grand) 2001, 47, 675–678. [Google Scholar]
- Hueber, A.J.; Asquith, D.L.; Miller, A.M.; Reilly, J.; Kerr, S.; Leipe, J.; Melendez, A.J.; McInnes, I.B. Mast cells express IL-17A in rheumatoid arthritis synovium. J. Immunol. 2010, 184, 3336–3340. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.A.A.; Hueber, A.J.; Wilson, S.; Galm, M.; Baum, W.; Kitson, C.; Auer, J.; Lorenz, S.H.; Moelleken, J.; Bader, M.; et al. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: Development and characterization of a novel bispecific antibody. Arthritis Rheumatol. (Hoboken N.J.) 2015, 67, 51–62. [Google Scholar] [CrossRef]
- Noack, M.; Beringer, A.; Miossec, P. Additive or Synergistic Interactions Between IL-17A or IL-17F and TNF or IL-1β Depend on the Cell Type. Front. Immunol. 2019, 10, 1726. [Google Scholar] [CrossRef] [Green Version]
- Mimpen, J.Y.; Baldwin, M.J.; Cribbs, A.P.; Philpott, M.; Carr, A.J.; Dakin, S.G.; Snelling, S.J.B. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front. Immunol. 2021, 12, 676173. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, H.M.; El-Tawab, S.S.; Rizk, M.M.; Aboeladl, N.A. Is interleukin-17 implicated in early knee osteoarthritis pathogenesis as in rheumatoid arthritis? Egypt. Rheumatol. Rehabil. 2022, 49, 29. [Google Scholar] [CrossRef]
- Mimpen, J.Y.; Carr, A.J.; Dakin, S.G.; Snelling, S.J. Inhibition of interleukin-17-induced effects in osteoarthritis—An in vitro study. Osteoarthr. Cartil. 2018, 26, S118. [Google Scholar] [CrossRef]
- Mazzucco, D.; Scott, R.; Spector, M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: Correlation with flow properties. Biomaterials 2004, 25, 4433–4445. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Mashiah, R.; Seror, J.; Kadar, A.; Dolkart, O.; Pritsch, T.; Goldberg, R.; Klein, J. Lipid-hyaluronan synergy strongly reduces intrasynovial tissue boundary friction. Acta Biomater. 2019, 83, 314–321. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Klein, J. Designer Nanoparticles as Robust Superlubrication Vectors. ACS Nano 2020, 14, 7008–7017. [Google Scholar] [CrossRef]
- Veselack, T.; Aldebert, G.; Trunfio-Sfarghiu, A.M.; Schmid, T.M.; Laurent, M.P.; Wimmer, M.A. Phospholipid vesicles in media for tribological studies against live cartilage. Lubricants 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Ben-Trad, L.; Matei, C.I.; Sava, M.M.; Duclos, M.-E.; Filali, S.; Berthier, Y.; Guichardant, M.; Bernoud Hubac, N.; Maniti, O.; Landoulsi, A.; et al. Synovial Extracellular Vesicles: Structure and Role in Synovial Fluid Tribological Performances. Int. J. Mol. Sci. 2022, 23, 11998. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhu, W.; Li, H.; Ma, D.; Liu, W.; Yu, W.; Wang, L.; Cao, Y.; Jiang, Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2020, 30, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Esa, A.; Connolly, K.D.; Williams, R.; Archer, C.W. Extracellular Vesicles in the Synovial Joint: Is there a Role in the Pathophysiology of Osteoarthritis? Malays. Orthop. J. 2019, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Zhonghui, C.; Zhang, Y.; Li, J.; Liu, S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Huang, Q.; Sun, S.; Ji, Z.; Deng, W.; LI, T.W. AB0050 A NOVEL METHOD FOR ISOLATION OF EXOSOMES FROM SYNOVIAL FLUID. Ann. Rheum. Dis. 2021, 80, 1057. [Google Scholar] [CrossRef]
- Goldberg, R.; Klein, J. Liposomes as lubricants: Beyond drug delivery. Chem. Phys. Lipids 2012, 165, 374–381. [Google Scholar] [CrossRef]
- Goldberg, R.; Schroeder, A.; Barenholz, Y.; Klein, J. Interactions between adsorbed hydrogenated soy phosphatidylcholine (HSPC) vesicles at physiologically high pressures and salt concentrations. Biophys. J. 2011, 100, 2403–2411. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.; Schroeder, A.; Silbert, G.; Turjeman, K.; Barenholz, Y.; Klein, J. Boundary lubricants with exceptionally low friction coefficients based on 2D close-packed phosphatidylcholine liposomes. Adv. Mater. 2011, 23, 3517–3521. [Google Scholar] [CrossRef]
- Klein, J. Molecular mechanisms of synovial joint lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 691–710. [Google Scholar] [CrossRef]
- Matei, C.I.; Boulocher, C.; Boulé, C.; Schramme, M.; Viguier, E.; Roger, T.; Berthier, Y.; Trunfio-Sfarghiu, A.-M.; Blanchin, M.-G. Ultrastructural analysis of healthy synovial fluids in three mammalian species. Microsc. Microanal. Off. J. Microsc. Soc. Am. Microbeam Anal. Soc. Microsc. Soc. Can. 2014, 20, 903–911. [Google Scholar] [CrossRef]
- Sava, M.-M.; Boulocher, C.; Matei, C.I.; Munteanu, B.; Schramme, M.; Viguier, E.; Roger, T.; Berthier, Y.; Blanchin, M.-G.; Trunfio-Sfarghiu, A.-M. Structural and tribological study of healthy and biomimetic SF. Comput. Methods Biomech. Biomed. Engin. 2013, 16, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Trunfio-Sfarghiu, A.-M.; Berthier, Y.; Meurisse, M.-H.; Rieu, J.-P. Role of nanomechanical properties in the tribological performance of phospholipid biomimetic surfaces. Langmuir 2008, 24, 8765–8771. [Google Scholar] [CrossRef] [PubMed]
- Bostan, L.; Trunfio-Sfarghiu, A.M.; Verestiuc, L.; Popa, M.I.; Munteanu, F.; Rieu, J.P.; Berthier, Y. Mechanical and tribological properties of poly(hydroxyethyl methacrylate) hydrogels as articular cartilage substitutes. Tribol. Int. 2012, 46, 215–224. [Google Scholar] [CrossRef]
- Zouaoui, J.; Trunfio-Sfarghiu, A.M.; Brizuela, L.; Piednoir, A.; Maniti, O.; Munteanu, B.; Mebarek, S.; Girard-Egrot, A.; Landoulsi, A.; Granjon, T. Multi-scale mechanical characterization of prostate cancer cell lines: Relevant biological markers to evaluate the cell metastatic potential. Biochim. Biophys. Acta–Gen. Subj. 2017, 1861, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef]
- Forsey, R.W.; Fisher, J.; Thompson, J.; Stone, M.H.; Bell, C.; Ingham, E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials 2006, 27, 4581–4590. [Google Scholar] [CrossRef]
- Filali, S.; Geloën, A.; Lysenko, V.; Pirot, F.; Miossec, P. Live-stream characterization of cadmium-induced cell death using visible CdTe-QDs. Sci. Rep. 2018, 8, 12614. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Sranley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Corneci, M.C.; Dekkiche, F.; Trunfio-Sfarghiu, A.M.; Meurisse, M.H.; Berthier, Y.; Rieu, J.P. Tribological properties of fluid phase phospholipid bilayers. Tribol. Int. 2011, 44, 1959–1968. [Google Scholar] [CrossRef]
- Trunfio-Sfarghiu, A.-M.; Berthier, Y.; Meurisse, M.-H.; Rieu, J.-P.; Dowson, D. Multiscale analysis of the tribological role of the molecular assemblies of synovial fluid: Case of a healthy joint and implants. Tribol. Int. 2007, 40, 1728. [Google Scholar] [CrossRef]
- Berquand, A.; Mazeran, P.E.; Pantigny, J.; Proux-Delrouyre, V.; Laval, J.M.; Bourdillon, C. Two-step formation of streptavidin-supported lipid bilayers by PEG-triggered vesicle fusion. Fluorescence and atomic force microscopy characterization. Langmuir 2003, 19, 1700–1707. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filali, S.; Darragi-Raies, N.; Ben-Trad, L.; Piednoir, A.; Hong, S.-S.; Pirot, F.; Landoulsi, A.; Girard-Egrot, A.; Granjon, T.; Maniti, O.; et al. Morphological and Mechanical Characterization of Extracellular Vesicles and Parent Human Synoviocytes under Physiological and Inflammatory Conditions. Int. J. Mol. Sci. 2022, 23, 13201. https://doi.org/10.3390/ijms232113201
Filali S, Darragi-Raies N, Ben-Trad L, Piednoir A, Hong S-S, Pirot F, Landoulsi A, Girard-Egrot A, Granjon T, Maniti O, et al. Morphological and Mechanical Characterization of Extracellular Vesicles and Parent Human Synoviocytes under Physiological and Inflammatory Conditions. International Journal of Molecular Sciences. 2022; 23(21):13201. https://doi.org/10.3390/ijms232113201
Chicago/Turabian StyleFilali, Samira, Nesrine Darragi-Raies, Layth Ben-Trad, Agnès Piednoir, Saw-See Hong, Fabrice Pirot, Ahmed Landoulsi, Agnès Girard-Egrot, Thierry Granjon, Ofelia Maniti, and et al. 2022. "Morphological and Mechanical Characterization of Extracellular Vesicles and Parent Human Synoviocytes under Physiological and Inflammatory Conditions" International Journal of Molecular Sciences 23, no. 21: 13201. https://doi.org/10.3390/ijms232113201





