Telomere Length Is Correlated with Resting Metabolic Rate and Aerobic Capacity in Women: A Cross-Sectional Study
Abstract
:1. Introduction
2. Results
2.1. Demographic and Physiological Characteristics
2.2. RMR, RFO, Aerobic Capacity, Oxidative Stress, Inflammation, and RTL
2.3. Heatmaps of Raw Data and Correlations between RTL and All the Variables
2.4. Correlation between RTL and All Adjusted Variables
2.4.1. RMR, RFO, and Aerobic Capacity
2.4.2. Association between RTL and Oxidative Stress and Inflammation
2.4.3. Associations between RTL and Age, Anthropometry, and Body Composition
2.5. Associations between RTL and RMR, RFO, Aerobic Capacity, Oxidative Stress, and Inflammation in Age Subgroups
3. Discussion
4. Methods and Materials
4.1. Subjects
4.2. Research Design, Setting, and Protocol
4.3. Measurements of Physiological Characteristics
4.4. Measurements of Anthropometry and Body Composition
4.5. Measurement of RMR and RFO
4.6. Measurement of Aerobic Capacity
4.7. Measurements of Plasma Malondialdehyde, Vitamin C, and Inflammation
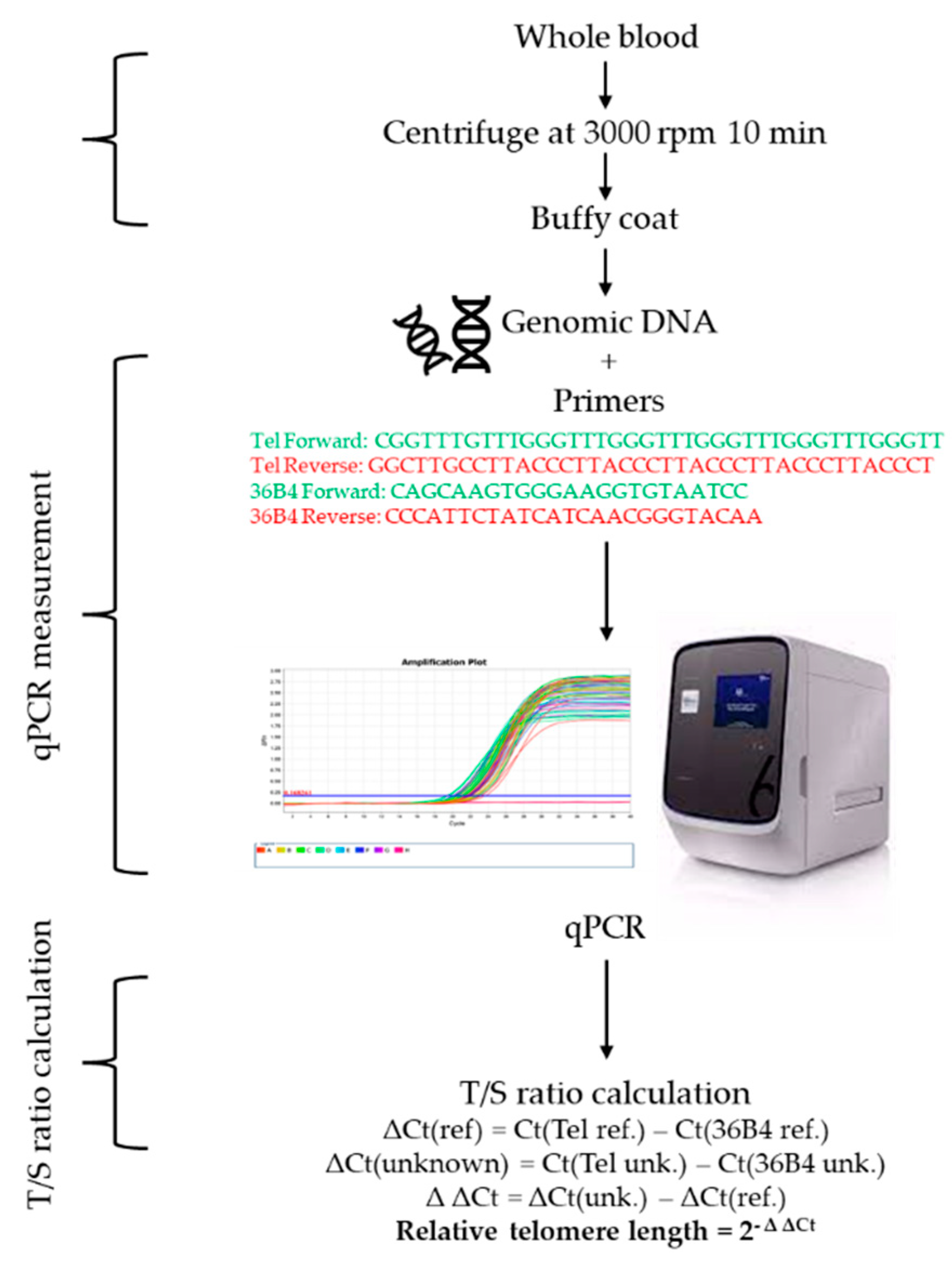
4.8. Telomere Length Measurement
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, E.H.; Gall, J.G. A Tandemly Repeated Sequence at the Termini of the Extrachromosomal Ribosomal RNA Genes in Tetrahymena. J. Mol. Biol. 1978, 120, 33–53. [Google Scholar] [CrossRef]
- Blackburn, E.H. Telomere States and Cell Fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef]
- Srettabunjong, S.; Satitsri, S.; Thongnoppakhun, W.; Tirawanchai, N. The Study on Telomere Length for Age Estimation in a Thai Population. Am. J. Forensic Med. Pathol. 2014, 35, 148–153. [Google Scholar] [CrossRef]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. Body Mass Index and Leukocyte Telomere Length in Adults: A Systematic Review and Meta-Analysis. Obes. Rev. 2014, 15, 192–201. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Vasquez, E.; Germain, C.M.; Emeny, R.T.; Rippberger, P.; Lopez-Jimenez, F.; Bartels, S.J. Association of Adiposity, Telomere Length and Mortality: Data from the NHANES 1999–2002. Int. J. Obes. (Lond) 2018, 42, 198–204. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Lo, K.; Feng, Y.-Q.; Zhang, B. The Association of Mean Telomere Length with All-Cause, Cerebrovascular and Cardiovascular Mortality. Biosci. Rep. 2019, 39, BSR20192306. [Google Scholar] [CrossRef] [Green Version]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S.; American Society for Nutrition; NAASO, The Obesity Society. Obesity in Older Adults: Technical Review and Position Statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes. Res. 2005, 13, 1849–1863. [Google Scholar] [CrossRef]
- Verga, S.; Buscemi, S.; Caimi, G. Resting Energy Expenditure and Body Composition in Morbidly Obese, Obese and Control Subjects. Acta Diabetol. 1994, 31, 47–51. [Google Scholar] [CrossRef]
- Lazzer, S.; Bedogni, G.; Lafortuna, C.L.; Marazzi, N.; Busti, C.; Galli, R.; De Col, A.; Agosti, F.; Sartorio, A. Relationship between Basal Metabolic Rate, Gender, Age, and Body Composition in 8,780 White Obese Subjects. Obesity (Silver Spring) 2010, 18, 71–78. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ravussin, E. Indirect Calorimetry: An Indispensable Tool to Understand and Predict Obesity. Eur. J. Clin. Nutr. 2017, 71, 318–322. [Google Scholar] [CrossRef]
- Bonen, A.; Tandon, N.N.; Glatz, J.F.C.; Luiken, J.J.F.P.; Heigenhauser, G.J.F. The Fatty Acid Transporter FAT/CD36 Is Upregulated in Subcutaneous and Visceral Adipose Tissues in Human Obesity and Type 2 Diabetes. Int. J. Obes. (Lond) 2006, 30, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucci, M.; Borra, R.; Någren, K.; Maggio, R.; Tuunanen, H.; Oikonen, V.; Del Ry, S.; Viljanen, T.; Taittonen, M.; Rigazio, S.; et al. Human Obesity Is Characterized by Defective Fat Storage and Enhanced Muscle Fatty Acid Oxidation, and Trimetazidine Gradually Counteracts These Abnormalities. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E105–E112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Ruiz, J.R.; Castillo, M.J. Association of Basal Metabolic Rate and Fuel Oxidation in Basal Conditions and during Exercise, with Plasma S-Klotho: The FIT-AGEING Study. Aging (Albany NY) 2019, 11, 5319–5333. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; LaMonte, M.J.; Laditka, J.N.; Hardin, J.W.; Chase, N.; Hooker, S.P.; Blair, S.N. Cardiorespiratory Fitness and Adiposity as Mortality Predictors in Older Adults. JAMA 2007, 298, 2507–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaRocca, T.J.; Seals, D.R.; Pierce, G.L. Leukocyte Telomere Length Is Preserved with Aging in Endurance Exercise-Trained Adults and Related to Maximal Aerobic Capacity. Mech. Ageing Dev. 2010, 131, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D. Cardiorespiratory Capacity and Leukocyte Telomere Length Among Adults in the United States. Am. J. Epidemiol. 2015, 182, 198–201. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Masi, S.; Salpea, K.D.; Li, K.; Parkar, M.; Nibali, L.; Donos, N.; Patel, K.; Taddei, S.; Deanfield, J.E.; D’Aiuto, F.; et al. Oxidative Stress, Chronic Inflammation, and Telomere Length in Patients with Periodontitis. Free Radic. Biol. Med. 2011, 50, 730–735. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Mahdi, F. Telomere Length Variations in Aging and Age-Related Diseases. Curr. Aging Sci. 2014, 7, 161–167. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Hewitt, G.; Passos, J.F. Telomeres, Oxidative Stress and Inflammatory Factors: Partners in Cellular Senescence? Longev. Healthspan 2014, 3, 1. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-Specific DNA Damage at GGG Sequence by Oxidative Stress May Accelerate Telomere Shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Rhee, D.B.; Ghosh, A.; Lu, J.; Bohr, V.A.; Liu, Y. Factors That Influence Telomeric Oxidative Base Damage and Repair by DNA Glycosylase OGG1. DNA Repair (Amst.) 2011, 10, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Zhu, L.; Cui, X.; Feng, L.; Zhao, X.; He, S.; Ping, F.; Li, W.; Li, Y. Influence of Diet on Leukocyte Telomere Length, Markers of Inflammation and Oxidative Stress in Individuals with Varied Glucose Tolerance: A Chinese Population Study. Nutr. J. 2016, 15, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoy, P.; Yuktanandana, P.; Tanavalee, A.; Tanpowpong, T.; Ittipanichpong, T.; Honsawek, S. Telomere Shortening Is Associated with Poor Physical Performance in Knee Osteoarthritis. Biomed. Rep. 2020, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Al-Attas, O.S.; Al-Daghri, N.M.; Alokail, M.S.; Alfadda, A.; Bamakhramah, A.; Sabico, S.; Pritlove, D.; Harte, A.; Tripathi, G.; McTernan, P.G.; et al. Adiposity and Insulin Resistance Correlate with Telomere Length in Middle-Aged Arabs: The Influence of Circulating Adiponectin. Eur. J. Endocrinol. 2010, 163, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Sen, A.; Marsche, G.; Freudenberger, P.; Schallert, M.; Toeglhofer, A.M.; Nagl, C.; Schmidt, R.; Launer, L.J.; Schmidt, H. Association between Higher Plasma Lutein, Zeaxanthin, and Vitamin C Concentrations and Longer Telomere Length: Results of the Austrian Stroke Prevention Study. J. Am. Geriatr. Soc. 2014, 62, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Krasnienkov, D.S.; Khalangot, M.D.; Kravchenko, V.I.; Kovtun, V.A.; Guryanov, V.G.; Chizhova, V.P.; Korkushko, O.V.; Shatilo, V.B.; Kukharsky, V.M.; Vaiserman, A.M. Hyperglycemia Attenuates the Association between Telomere Length and Age in Ukrainian Population. Exp. Gerontol. 2018, 110, 247–252. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Sleiman, F.; Nasreddine, L.; Nasrallah, M.; Nakhoul, N.; Isma’eel, H.; Tamim, H. Short Telomere Length Is Associated with Aging, Central Obesity, Poor Sleep and Hypertension in Lebanese Individuals. Aging Dis. 2018, 9, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, Cigarette Smoking, and Telomere Length in Women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A.G. Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Péronnet, F.; Massicotte, D. Table of Nonprotein Respiratory Quotient: An Update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Boonthongkaew, C.; Tong-Un, T.; Kanpetta, Y.; Chaungchot, N.; Leelayuwat, C.; Leelayuwat, N. Vitamin C Supplementation Improves Blood Pressure and Oxidative Stress after Acute Exercise in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled, Cross-over Study. Chin. J. Physiol. 2021, 64, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-Predicted Maximal Heart Rate Revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A Comparative Evaluation of Thiobarbituric Acid Methods for the Determination of Malondialdehyde in Biological Materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Wang, K.; Du, X.; Li, Q. A Simple and Sensitive Assay for Ascorbate Using Potassium Ferricyanide as Spectroscopic Probe Reagent. Anal. Biochem. 2009, 388, 40–46. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Measurement by Quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [Green Version]
- Udomsinprasert, W.; Poovorawan, Y.; Chongsrisawat, V.; Vejchapipat, P.; Zhan, D.; Honsawek, S. Telomere Length in Peripheral Blood Leukocytes Is Associated with Severity of Biliary Atresia. PLoS ONE 2015, 10, e0134689. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 27 September 2022).



| All (n = 134) | NW (n = 68) | OB (n = 66) | p-Value | |
|---|---|---|---|---|
| RMRAB (kcal/day) | 1373.1 ± 271.8 | 1250.4 ± 194.1 | 1499.6 ± 283.5 | <0.001 |
| RMRBM (kcal/kg BM/day) | 21.9 ± 4.5 | 24.4 ± 3.6 | 19.4 ± 3.9 | <0.001 |
| RMRFFM (kcal/kg FFM/day) | 32.9 ± 5.2 | 34.2 ± 4.8 | 31.5 ± 5.3 | 0.002 |
| RFOAB (g/min) | 0.056 ± 0.03 | 0.049 ± 0.02 | 0.063 ± 0.03 | 0.007 |
| RFOBM (g/kg BM/min) | 0.0009 ± 0.0004 | 0.0010 ± 0.0005 | 0.0008 ± 0.0004 | 0.048 |
| RFOFFM (g/kg FFM/min) | 0.0013 ± 0.0007 | 0.0013 ± 0.0006 | 0.0013 ± 0.0007 | 0.880 |
| O2max (mL/kg BM/min) | 23.6 ± 5.9 | 26.3 ± 5.8 | 20.9 ± 4.6 | <0.001 |
| O2max (mL/kg FFM/min) | 35.5 ± 8.1 | 37.1 ± 8.4 | 33.8 ± 7.5 | 0.040 |
| Plasma vitamin C (µmol/L) | 51.4 ± 21.8 | 40.8 ± 16.7 | 62.4 ± 21.4 | <0.001 |
| Plasma MDA (µmol/mL) | 6.8 (4.5–9.3) | 5.6 (3.4–8.6) | 8.3 (6.1–9.8) | 0.001 |
| Serum hsCRP (mg/L) | 1.2 (0.4–3.1) | 0.5 (0.2–1.2) | 2.8 (1.4–4.1) | <0.001 |
| Model | All (n = 134; 68 NW, 66OB) | p Value | NW (n = 68) | p Value | OB (n = 66) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (yr) | Model1# | −0.004 (−0.009; 0.002) | 0.164 | −0.010 (−0.018; −0.001) | 0.026 | 0.000 (−0.005; 0.005) | 0.916 |
| Model3 | −0.005 (−0.010; −0.008) | 0.020 | −0.012 (−0.018; −0.005) | 0.001 | −0.001 (−0.005; 0.004) | 0.797 | |
| BMI (kg/m2) | Model1 | −0.023 (−0.031; −0.014) | <0.001 | 0.021 (−0.033; 0.075) | 0.443 | 0.003 (−0.019; 0.025) | 0.778 |
| Model3 | −0.021 (−0.296; −0.013) | <0.001 | 0.021 (−0.036; 0.078) | 0.465 | 0.004 (−0.018; 0.025) | 0.752 | |
| W (cm) | Model1 | −0.008 (−0.012; −0.004) | <0.001 | 0.010 (−0.002; 0.022) | 0.109 | −0.002 (−0.009; 0.005) | 0.599 |
| Model3 | −0.008 (−0.012; −0.005) | <0.001 | 0.004 (−0.009; 0.017) | 0.514 | −0.002 (−0.009; 0.004) | 0.513 | |
| BF (%)e | Model1 | −0.021 (−0.030; −0.013) | <0.001 | 0.002 (−0.019; 0.024) | 0.830 | −0.003 (−0.029; 0.023) | 0.818 |
| Model3 | −0.023 (−0.031; −0.015) | <0.001 | −0.013 (−0.034; 0.007) | 0.210 | −0.004 (−0.027; 0.020) | 0.760 | |
| FFM (kg) | Model1 | −0.016 (−0.024; −0.008) | <0.001 | 0.016 (−0.005; 0.037) | 0.141 | −0.003 (−0.016; 0.010) | 0.667 |
| Model3 | −0.015 (−0.019; −0.004) | 0.002 | 0.028 (0.009; 0.047) | 0.004 | −0.001 (−0.013; 0.011) | 0.916 | |
| RMRAB (kcal/day) | Model1 | −0.000 (−0.000; −0.000) | 0.006 | −0.000 (−0.001; 0.000) | 0.239 | −0.000 (−0.000; 0.000) | 0.756 |
| Model2 | −0.000 (−0.000; 0.000) | 0.296 | −0.000 (−0.001; −0.000) | 0.049 | −0.000 (−0.000; 0.000) | 0.900 | |
| RMRBM (kcal/kg BM/day) | Model1 | 0.009 (−0.002; 0.021) | 0.115 | −0.024 (−0.045; −0.004) | 0.021 | −0.001 (−0.014; 0.013) | 0.903 |
| Model2 | −0.008 (−0.020; 0.005) | 0.230 | −0.023 (−0.046; −0.000) | 0.049 | −0.001 (−0.015; 0.013) | 0.877 | |
| RMRFFM (kcal/kg FFM/day) | Model1 | −0.001 (−0.011; 0.009) | 0.841 | −0.019 (−0.034; −0.003) | 0.017 | −0.001 (−0.011; 0.009) | 0.901 |
| Model2 | −0.007 (−0.016; 0.003) | 0.169 | −0.016 (−0.0327; −0.000) | 0.045 | −0.001 (−0.011; 0.010) | 0.905 | |
| Model3 | −0.002 (−0.013; 0.008) | 0.647 | −0.018 (−0.034; −0.002) | 0.027 | 0.000 (−0.010; 0.010) | 0.998 | |
| RFOAB (g/min) | Model1 | −1.386 (−3.196; 0.424) | 0.133 | −1.038 (−4.616; 2.540) | 0.570 | −0.038 (−1.638; 1.562) | 0.963 |
| Model2 | −0.192 (−1.935; 1.551) | 0.829 | −1.196 (−4.806; 2.414) | 0.516 | −0.179 (−1.836; 1.478) | 0.833 | |
| RFOBM (g/kg BM/min) | Model1 | 14.6 (−106.5; 135.7) | 0.813 | −96.5 (−274.3; 81.3) | 0.288 | 8.7 (−114.6; 132.0) | 0.890 |
| Model2 | −25.1 (−139.4; 89.3) | 0.667 | −67.8 (−250.4; 114.8) | 0.467 | −6.2 (−135.5; 123.2) | 0.926 | |
| RFOFFM (g/kg FFM/min) | Model1 | −25.4 (−105.9; 55.1) | 0.536 | −65.2 (−194.0; 63.6) | 0.321 | 2.8 (−72.2; 77.9) | 0.941 |
| Model2 | −14.0(−88.9; 61.0) | 0.715 | −46.6 (−176.9; 83.7) | 0.484 | −6.438 (−85.2; 72.3) | 0.873 | |
| O2max (mL/kg BM/min) | Model1 | 0.010 (0.001; 0.019) | 0.026 | −0.001 (−0.014; 0.013) | 0.899 | −0.003 (−0.015; 0.009) | 0.640 |
| Model2 | −0.000 (−0.010; 0.010) | 0.982 | 0.001 (−0.013; 0.016) | 0.858 | −0.006 (−0.019; 0.008) | 0.407 | |
| Model3 | 0.013 (0.004; 0.022) | 0.005 | 0.007 (−0.007; 0.021) | 0.316 | −0.002 (−0.013; 0.010) | 0.787 | |
| O2max (mL/kg FFM/min) | Model1 | 0.006 (−0.001; 0.012) | 0.086 | 0.004 (−0.006; 0.013) | 0.444 | 0.001 (−0.006; 0.008) | 0.801 |
| Model2 | 0.003 (−0.003; 0.010) | 0.332 | 0.004 (−0.005; 0.013) | 0.394 | −0.000 (−0.008; 0.008) | 0.939 | |
| Model3 | 0.007 (0.000; 0.013) | 0.046 | 0.006 (−0.003; 0.016) | 0.202 | 0.002 (−0.005; 0.009) | 0.615 | |
| Plasma vitamin C (µmol/L) | Model1 | −0.001 (−0.003; 0.002) | 0.484 | 0.003 (−0.002; 0.008) | 0.184 | 0.002 (−0.000; 0.005) | 0.053 |
| Model2 | 0.003 (0.000; 0.005) | 0.049 | 0.003 (−0.003; 0.008) | 0.324 | 0.002 (−0.000; 0.005) | 0.079 | |
| Model3 | −0.000 (−0.003; 0.002) | 0.742 | 0.005 (0.000; 0.009) | 0.048 | 0.002 (−0.000; 0.005) | 0.060 | |
| Model4 | −0.000 (−0.003; 0.002) | 0.892 | 0.004 (−0.000; 0.009) | 0.066 | 0.003 (0.000; 0.005) | 0.032 | |
| Plasma MDA (µmol/mL) | Model1 | −0.005 (−0.017; 0.007) | 0.418 | 0.010 (−0.009; 0.029) | 0.301 | −0.005 (−0.016; 0.007) | 0.398 |
| Model2 | 0.001 (−0.011; 0.012) | 0.913 | 0.006 (−0.013; 0.026) | 0.528 | −0.005 (−0.017; 0.007) | 0.434 | |
| Serum hsCRP (mg/L) | Model1 | −0.035 (−0.054; −0.015) | < 0.001 | −0.025 (−0.063; 0.012) | 0.190 | −0.001 (−0.022; 0.020) | 0.925 |
| Model2 | −0.017 (−0.038; 0.004) | 0.109 | −0.031 (−0.071; 0.009) | 0.132 | −0.003 (−0.025; 0.019) | 0.785 | |
| Model3 | −0.032 (−0.052; −0.012) | 0.002 | −0.032 (−0.071; 0.006) | 0.096 | 0.000 (−0.019; 0.0196) | 0.982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonsa-ard, R.; Aneknan, P.; Tong-un, T.; Honsawek, S.; Leelayuwat, C.; Leelayuwat, N. Telomere Length Is Correlated with Resting Metabolic Rate and Aerobic Capacity in Women: A Cross-Sectional Study. Int. J. Mol. Sci. 2022, 23, 13336. https://doi.org/10.3390/ijms232113336
Nonsa-ard R, Aneknan P, Tong-un T, Honsawek S, Leelayuwat C, Leelayuwat N. Telomere Length Is Correlated with Resting Metabolic Rate and Aerobic Capacity in Women: A Cross-Sectional Study. International Journal of Molecular Sciences. 2022; 23(21):13336. https://doi.org/10.3390/ijms232113336
Chicago/Turabian StyleNonsa-ard, Rujira, Ploypailin Aneknan, Terdthai Tong-un, Sittisak Honsawek, Chanvit Leelayuwat, and Naruemon Leelayuwat. 2022. "Telomere Length Is Correlated with Resting Metabolic Rate and Aerobic Capacity in Women: A Cross-Sectional Study" International Journal of Molecular Sciences 23, no. 21: 13336. https://doi.org/10.3390/ijms232113336






