GmWRI1c Increases Palmitic Acid Content to Regulate Seed Oil Content and Nodulation in Soybean (Glycine max)
Abstract
:1. Introduction
2. Results
2.1. Identification of WRI Gene Family Members in Soybean
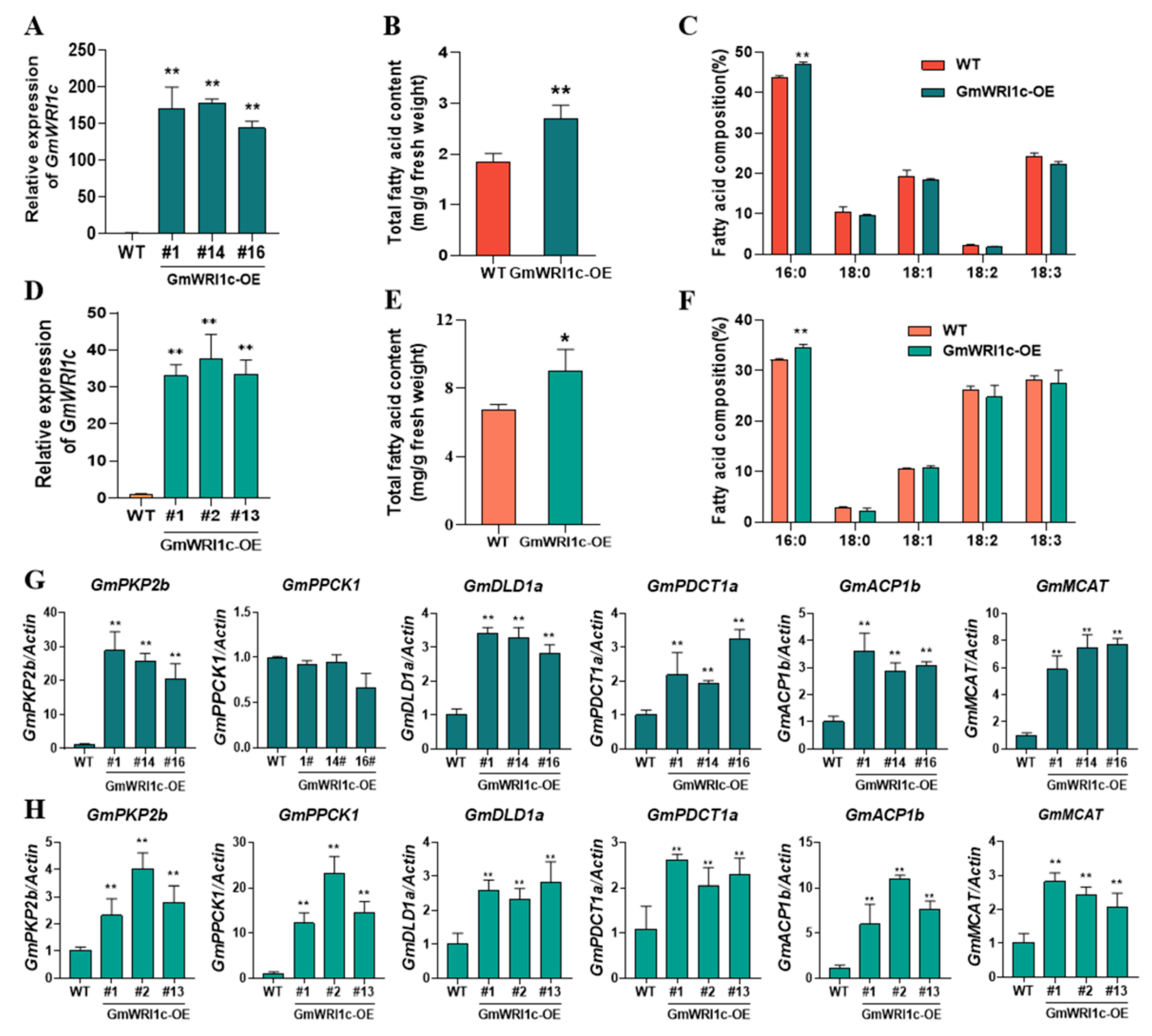
2.2. Conserved Function of GmWRI1c in Fatty Acid Synthesis
2.3. GmWRI1c Specifically Increases Palmitic Acid Content by Regulating Genes Related to Glycolysis and De Novo Lipogenesis
2.4. Overexpression of GmWRI1c Promotes Nodulation Genes’ Expression and Increases Nodule Numbers in Soybean
2.5. Superior Allele of GmWRI1cHap1 Increases Seed Oil Content and Nodule Numbers in Soybean
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Identification and Bioinformatics Analysis of GmWRI1c
4.3. Subcellular Localisation Analysis
4.4. Generation of Transgenic Arabidopsis
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative Real-Time PCR Analysis
4.7. Soybean Hairy Root Transformation and Nodulation Assay
4.8. Determination of Soluble Sugars Content, Total Fatty Acid Content, and Fatty Acid Composition
4.9. Determination of GUS Activity
4.10. Soybean Oil Content Determination
4.11. Tassel Analysis and Genotyping
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cleaved amplified polymorphic sequence | CAPS |
| Coding sequence | CDS |
| Haplotype | Hap |
| Hidden Markov model | HMM |
| Quantitative real-time PCR | qRT-PCR |
| Recombinant inbred line | RIL |
References
- Tyug, T.S.; Prasad, K.N.; Ismail, A. Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chem. 2010, 123, 583–589. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (alpha-amylase and alpha-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.D.; Perrone, D. Characterization and stability of bioactive compounds from soybean meal. LWT-Food Sci. Technol. 2015, 63, 992–1000. [Google Scholar] [CrossRef]
- Li, H.; Peng, Z.Y.; Yang, X.H.; Wang, W.D.; Fu, J.J.; Wang, J.H.; Han, Y.J.; Chai, Y.C.; Guo, T.T.; Yang, N.; et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 2013, 45, 43–50. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Faergeman, O.; Bourne, P.G. Action plan for a healthy agriculture, healthy nutrition, healthy people. World Rev. Nutr. Diet. 2011, 102, 1–5. [Google Scholar]
- de Lorgeril, M.; Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012, 10, 50. [Google Scholar] [CrossRef]
- Cernac, A.; Benning, C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004, 40, 575–585. [Google Scholar] [CrossRef]
- Baud, S.; Mendoza, M.S.; To, A.; Harscoet, E.; Lepiniec, L.; Dubreucq, B. WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J. 2007, 50, 825–838. [Google Scholar] [CrossRef]
- Focks, N.; Benning, C. wrinkled1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 1998, 118, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hua, W.; Zhan, G.M.; Wei, F.; Wang, X.F.; Liu, G.H.; Wang, H.Z. Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol. Biochem. 2010, 48, 9–15. [Google Scholar] [CrossRef]
- Wu, X.L.; Liu, Z.H.; Hu, Z.H.; Huang, R.Z. BnWRI1 coordinates fatty acid biosynthesis and photosynthesis pathways during oil accumulation in rapeseed. J. Integr. Plant Biol. 2014, 56, 582–593. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, Y.H.; Dong, Z.M.; Meng, F.F.; Sun, X.M.; Fan, X.H.; Zhang, Y.F.; Wang, M.L.; Wang, S.M. Soybean (Glycine max) WRINKLED1 transcription factor, GmWRI1a, positively regulates seed oil accumulation. Mol. Genet. Genom. 2018, 293, 401–415. [Google Scholar] [CrossRef]
- Zhou, X.F.; Dai, Y.Q.; Wu, H.J.; Zhong, P.Q.; Luo, L.J.; Shang, Y.J.; Tan, P.P.; Peng, F.R.; Tian, Z.X. Characterization of CiWRI1 from Carya illinoinensis in Seed Oil Biosynthesis. Forests 2020, 10, 818. [Google Scholar] [CrossRef]
- Lim, A.R.Q.; Kong, Q.; Singh, S.K.; Guo, L.; Yuan, L.; Ma, W. Sunflower WRINKLED1 Plays a Key Role in Transcriptional Regulation of Oil Biosynthesis. Int. J. Mol. Sci. 2022, 23, 3054. [Google Scholar] [CrossRef]
- Wang, Z.K.; Wang, Y.Z.; Shang, P.; Yang, C.; Yang, M.M.; Huang, J.X.; Ren, B.Z.; Zuo, Z.H.; Zhang, Q.Y.; Li, W.B.; et al. Overexpression of Soybean GmWRI1a Stably Increases the Seed Oil Content in Soybean. Int. J. Mol. Sci. 2022, 23, 5084. [Google Scholar] [CrossRef]
- Guo, W.; Chen, L.M.; Chen, H.F.; Yang, H.L.; You, Q.B.; Bao, A.L.; Chen, S.L.; Hao, Q.N.; Huang, Y.; Qiu, D.Z.; et al. Overexpression of GmWRI1b in soybean stably improves plant architecture and associated yield parameters, and increases total seed oil production under field conditions. Plant Biotechnol. J. 2020, 18, 1639–1641. [Google Scholar] [CrossRef]
- Chen, B.B.; Zhang, G.Y.; Li, P.H.; Yang, J.H.; Guo, L.; Benning, C.; Wang, X.M.; Zhao, J. Multiple GmWRI1s are redundantly involved in seed filling and nodulation by regulating plastidic glycolysis, lipid biosynthesis and hormone signalling in soybean (Glycine max). Plant Biotechnol. J. 2020, 18, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Udvardi, M.; Poole, P.S. Transport and Metabolism in Legume-Rhizobia Symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.C.; Katinakis, P.; Hendriks, P.; Smolders, A.; de Vries, F.; Spee, J.; van Kammen, A.; Bisseling, T.; Franssen, H. Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. Cell Mol. Biol. 1993, 3, 573–585. [Google Scholar] [CrossRef]
- Murakami, Y.; Miwa, H.; Imaizumi-Anraku, H.; Kouchi, H.; Downie, J.A.; Kawaguchi, M.; Kawasaki, S. Positional cloning identifies Lotus japonicus NSP2, a putative transcription factor of the GRAS family, required for NIN and ENOD40 gene expression in nodule initiation. DNA Res. 2006, 13, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E.T. Hormone modulation of legume-rhizobial symbiosis. J. Integr. Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.L.P.; Hassan, S.; Truong, T.T.; Hocart, C.H.; Laffont, C.; Frugier, F.; Mathesius, U. Flavonoids and Auxin Transport Inhibitors Rescue Symbiotic Nodulation in the Medicago truncatula Cytokinin Perception Mutant cre1. Plant Cell 2015, 27, 2210–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.M.; Wang, Y.N.; Zhu, L.; Tian, Y.P.; Chen, L.; Sun, Z.X.; Ullah, I.; Li, X. GmTIR1/GmAFB3-based auxin perception regulated by miR393 modulates soybean nodulation. New Phytol. 2017, 215, 672–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Sanchez, M.; Laffont, C.; Boivin, S.; Le Signor, C.; Thompson, R.; Frugier, F.; Brault, M. A Cytokinin Signaling Type-B Response Regulator Transcription Factor Acting in Early Nodulation. Plant Physiol. 2020, 183, 1319–1330. [Google Scholar] [CrossRef]
- Suzuki, A.; Akune, M.; Kogiso, M.; Imagama, Y.; Osuki, K.; Uchiumi, T.; Higashi, S.; Han, S.Y.; Yoshida, S.; Asami, T.; et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004, 45, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.L.; Kalo, P.; Yendrek, C.; Sun, J.H.; Liang, Y.; Marsh, J.F.; Harris, J.M.; Oldroyd, G.E.D. Abscisic Acid Coordinates Nod Factor and Cytokinin Signaling during the Regulation of Nodulation in Medicago truncatula. Plant Cell 2008, 20, 2681–2695. [Google Scholar] [CrossRef] [Green Version]
- Taylor, B.N.; Menge, D.N.L. Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants 2018, 4, 655–661. [Google Scholar] [CrossRef]
- Wang, X.D.; Chen, K.; Zhou, M.M.; Gao, Y.K.; Huang, H.M.; Liu, C.; Fan, Y.Y.; Fan, Z.H.; Wang, Y.N.; Li, X. GmNAC181 promotes symbiotic nodulation and salt tolerance of nodulation by directly regulating GmNINa expression in soybean. New Phytol. 2022, 236, 656–670. [Google Scholar] [CrossRef]
- Guan, R.X.; Qu, Y.; Guo, Y.; Yu, L.L.; Liu, Y.; Jiang, J.H.; Chen, J.G.; Ren, Y.L.; Liu, G.Y.; Tian, L.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Toth, K.; Tanaka, K.; Nguyen, C.T.; Yan, Z.; Brechenmacher, L.; Dahmen, J.; Chen, M.J.; Thelen, J.J.; Qiu, L.J.; et al. A Soybean Acyl Carrier Protein, GmACP, Is Important for Root Nodule Symbiosis. Mol. Plant-Microbe Interact. 2014, 27, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Y.; Ahmad, M.Z.; Chen, B.B.; Manan, S.; Zhang, Y.L.; Jin, H.N.; Wang, X.M.; Zhao, J. Lipidomic and transcriptomic profiling of developing nodules reveals the essential roles of active glycolysis and fatty acid and membrane lipid biosynthesis in soybean nodulation. Plant J. 2020, 103, 1351–1371. [Google Scholar] [CrossRef]
- Lee, S.H.; Bailey, M.A.; Mian, M.A.; Carter, T.E., Jr.; Shipe, E.R.; Ashley, D.A.; Parrott, W.A.; Hussey, R.S.; Boerma, H.R. RFLP loci associated with soybean seed protein and oil content across populations and locations. TAG. Theor. Appl. Genet. Theor. Und Angew. Genet. 1996, 93, 649–657. [Google Scholar] [CrossRef]
- Brummer, E.C.; Graef, G.L.; Orf, J.H.; Wilcox, J.R.; Shoemaker, R.C. Mapping QTL for seed protein and oil content in eight soybean populations. Crop Sci. 1997, 37, 370–378. [Google Scholar] [CrossRef]
- Reinprecht, Y.; Poysa, V.W.; Yu, K.F.; Rajcan, I.; Ablett, G.R.; Pauls, K.P. Seed and agronomic QTL in low linolenic acid, lipoxygenase-free soybean (Glycine max (L.) Merrill) germplasm. Genome 2006, 49, 1510–1527. [Google Scholar] [CrossRef]
- Qi, Z.M.; Wu, Q.; Han, X.; Sun, Y.N.; Du, X.Y.; Liu, C.Y.; Jiang, H.W.; Hu, G.H.; Chen, Q.S. Soybean oil content QTL mapping and integrating with meta-analysis method for mining genes. Euphytica 2011, 179, 499–514. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Downie, J.M. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Minami, E.; Kouchi, H.; Cohn, J.R.; Ogawa, T.; Stacey, G. Expression of the early nodulin, ENOD40, in soybean roots in response to various lipo-chitin signal molecules. Plant J. Cell Mol. Biol. 1996, 10, 23–32. [Google Scholar] [CrossRef]
- Liu, M.; Soyano, T.; Yano, K.; Hayashi, M.; Kawaguchi, M. ERN1 and CYCLOPS coordinately activate NIN signaling to promote infection thread formation in Lotus japonicus. J. Plant Res. 2019, 132, 641–653. [Google Scholar] [CrossRef]
- Charon; Sousa; Crespi; Kondorosi, Alteration of enod40 expression modifies medicago truncatula root nodule development induced by sinorhizobium meliloti. Plant Cell 1999, 11, 1953–1966.
- Xu, H.Y.; Li, Y.J.; Zhang, K.F.; Li, M.J.; Fu, S.Y.; Tian, Y.Z.; Qin, T.F.; Li, X.X.; Zhong, Y.J.; Liao, H. miR169c-NFYA-C-ENOD40 modulates nitrogen inhibitory effects in soybean nodulation. New Phytol. 2021, 229, 3377–3392. [Google Scholar] [CrossRef]
- Kouchi, H.; Kinoshita, E.; Ridge, R.W.; Kumagai, H. RNAi knockdown of ENOD40s leads to significant suppression of nodule formation in Lotus japonicus. Plant Cell Physiol. 2005, 46, S91. [Google Scholar]
- Wang, L.X.; Sun, Z.X.; Su, C.; Wang, Y.L.; Yan, Q.Q.; Chen, J.H.; Ott, T.; Li, X. A GmNINa-miR172c-NNC1 Regulatory Network Coordinates the Nodulation and Autoregulation of Nodulation Pathways in Soybean. Mol. Plant 2019, 12, 1211–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Du, C.; Song, H.; Aziz, U.; Wang, L.L.; Zhao, C.Z.; Zhang, M. Genome-wide analysis reveals the evolution and structural features of WRINKLED1 in plants. Mol. Genet. Genom. 2019, 294, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.R.; Rahman, M.M.; Bhatia, S.; Shockey, J.; Kilaru, A. Functional and Predictive Structural Characterization of WRINKLED2, A Unique Oil Biosynthesis Regulator in Avocado. Front. Plant Sci. 2021, 12, 648494. [Google Scholar] [CrossRef] [PubMed]
- Zakim, D.; Herman, R.H. Regulation of fatty acid synthesis. Am. J. Clin. Nutr. 1969, 22, 200–213. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, M.; Zhang, B.L.; Shrestha, P.; Petrie, J.; Green, A.G.; Singh, S.P. Genetic enhancement of palmitic acid accumulation in cotton seed oil through RNAi down-regulation of ghKAS2 encoding beta-ketoacyl-ACP synthase II (KASII). Plant Biotechnol. J. 2017, 15, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Sedivy, E.J.; Wu, F.Q.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.D.; Liu, S.L.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.Q.; et al. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef]
- Duan, Z.B.A.; Zhang, M.; Zhang, Z.F.; Liang, S.; Fan, L.; Yang, X.; Yuan, Y.Q.; Pan, Y.; Zhou, G.A.; Liu, S.L.; et al. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant Biotechnol. J. 2022, 20, 1807–1818. [Google Scholar] [CrossRef]
- Goettel, W.; Zhang, H.Y.; Li, Y.; Qiao, Z.Z.; Jiang, H.; Hou, D.Y.; Song, Q.J.; Pantalone, V.R.; Song, B.H.; Yu, D.Y.; et al. POWR1 is a domestication gene pleiotropically regulating seed quality and yield in soybean. Nat. Commun. 2022, 13, 3051. [Google Scholar] [CrossRef]
- Zhu, W.W.; Yang, C.; Yong, B.; Wang, Y.; Li, B.B.; Gu, Y.Z.; Wei, S.M.; An, Z.H.; Sun, W.K.; Qiu, L.J.; et al. An enhancing effect attributed to a nonsynonymous mutation in SOYBEAN SEED SIZE 1, a SPINDLY-like gene, is exploited in soybean domestication and improvement. New Phytol. 2022, 236, 1375–1392. [Google Scholar] [CrossRef]
- Li, Q.T.; Lu, X.; Song, Q.X.; Chen, H.W.; Wei, W.; Tao, J.J.; Bian, X.H.; Shen, M.; Ma, B.A.; Zhang, W.K.; et al. Selection for a Zinc-Finger Protein Contributes to Seed Oil Increase during Soybean Domestication. Plant Physiol. 2017, 173, 2208–2224. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Wei, W.; Li, Q.T.; Bian, X.H.; Lu, X.; Hu, Y.; Cheng, T.; Wang, Z.Y.; Jin, M.; Tao, J.J.; et al. A transcriptional regulatory module controls lipid accumulation in soybean. New Phytol. 2021, 231, 661–678. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.H.; Ma, R.R.; Huang, W.X.; Hou, J.J.; Fang, C.; Wang, L.S.; Yuan, Z.H.; Sun, Q.; Dong, X.H.; et al. Identification of ST1 reveals a selection involving hitchhiking of seed morphology and oil content during soybean domestication. Plant Biotechnol. J. 2022, 20, 1110–1121. [Google Scholar] [CrossRef]
- Lu, X.; Li, Q.T.; Xiong, Q.; Li, W.; Bi, Y.D.; Lai, Y.C.; Liu, X.L.; Man, W.Q.; Zhang, W.K.; Ma, B.; et al. The transcriptomic signature of developing soybean seeds reveals the genetic basis of seed trait adaptation during domestication. Plant J. 2016, 86, 530–544. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Li, M.M.; Xu, C.J.; Yang, X.; Li, D.M.; Zhao, X.; Wang, K.; Li, Y.H.; Zhang, X.M.; Liu, L.X.; et al. Natural variation in GmGBP1 promoter affects photoperiod control of flowering time and maturity in soybean. Plant J. 2018, 96, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Sun, Y.Y.; Shan, Z.; He, J.B.; Wang, N.; Gai, J.Y.; Li, Y. Natural variation in the promoter of GsERD15B affects salt tolerance in soybean. Plant Biotechnol. J. 2021, 19, 1155–1169. [Google Scholar] [CrossRef]
- Li, D.; Jin, C.Y.; Duan, S.W.; Zhu, Y.N.; Qi, S.H.; Liu, K.G.; Gao, C.H.; Ma, H.L.; Zhang, M.; Liao, Y.C.; et al. MYB89 Transcription Factor Represses Seed Oil Accumulation. Plant Physiol. 2017, 173, 1211–1225. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, 12. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Li, P.H.; Dong, Q.; Ge, S.J.; He, X.Z.; Verdier, J.; Li, D.Q.; Zhao, J. Metabolic engineering of proanthocyanidin production by repressing the isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotechnol. J. 2016, 14, 1604–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Rehman, N.U.; Yu, S.W.; Zhou, Y.Z.; ul Haq, B.; Wang, J.J.; Li, P.H.; Zeng, Z.X.; Zhao, J. GmMAX2-D14 and -KAI interaction-mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J. 2020, 101, 334–351. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, H.F.; Wang, X.C.; Qiu, Y.J.; Tian, L.H.; Qi, X.Q.; Qu, L. Cytochrome P450 family member CYP96B5 hydroxylates alkanes to primary alcohols and is involved in rice leaf cuticular wax synthesis. New Phytol. 2020, 225, 2094–2107. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.X.; Indrasumunar, A.; Nguyen, C.D.T.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Zhang, Y.; Zeng, X.; Li, P.; Wang, X.; Benedito, V.A.; Zhao, J. Isoflavone malonyl-CoA acyltransferase GmMaT2 is involved in nodulation of soybean by modifying synthesis and secretion of isoflavones. J. Exp. Bot. 2021, 72, 1349–1369. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, Q.T.; Lu, X.; Song, Q.X.; Lam, S.M.; Zhang, W.K.; Ma, B.; Lin, Q.; Man, W.Q.; Du, W.G.; et al. Soybean GmMYB73 promotes lipid accumulation in transgenic plants. BMC Plant Biol. 2014, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Zhao, D.; Shao, W.; Lu, Y.; Wang, W.; Hu, Y.; Li, J.; Zhu, S.; Wang, X. GmWRI1c Increases Palmitic Acid Content to Regulate Seed Oil Content and Nodulation in Soybean (Glycine max). Int. J. Mol. Sci. 2022, 23, 13793. https://doi.org/10.3390/ijms232213793
Zheng H, Zhao D, Shao W, Lu Y, Wang W, Hu Y, Li J, Zhu S, Wang X. GmWRI1c Increases Palmitic Acid Content to Regulate Seed Oil Content and Nodulation in Soybean (Glycine max). International Journal of Molecular Sciences. 2022; 23(22):13793. https://doi.org/10.3390/ijms232213793
Chicago/Turabian StyleZheng, Haowei, Duo Zhao, Wentao Shao, Yun Lu, Wenhui Wang, Yanjiao Hu, Jiajia Li, Shangshang Zhu, and Xiaobo Wang. 2022. "GmWRI1c Increases Palmitic Acid Content to Regulate Seed Oil Content and Nodulation in Soybean (Glycine max)" International Journal of Molecular Sciences 23, no. 22: 13793. https://doi.org/10.3390/ijms232213793



