Crosslinked Structure of Polyacrylic Acid Affects Pulmonary Fibrogenicity in Rats
Abstract
:1. Introduction
2. Results
2.1. Relative Lung Weights
2.2. Cell Analysis and Cell Injury Markers in Bronchoalveolar Lavage Fluid (BALF)
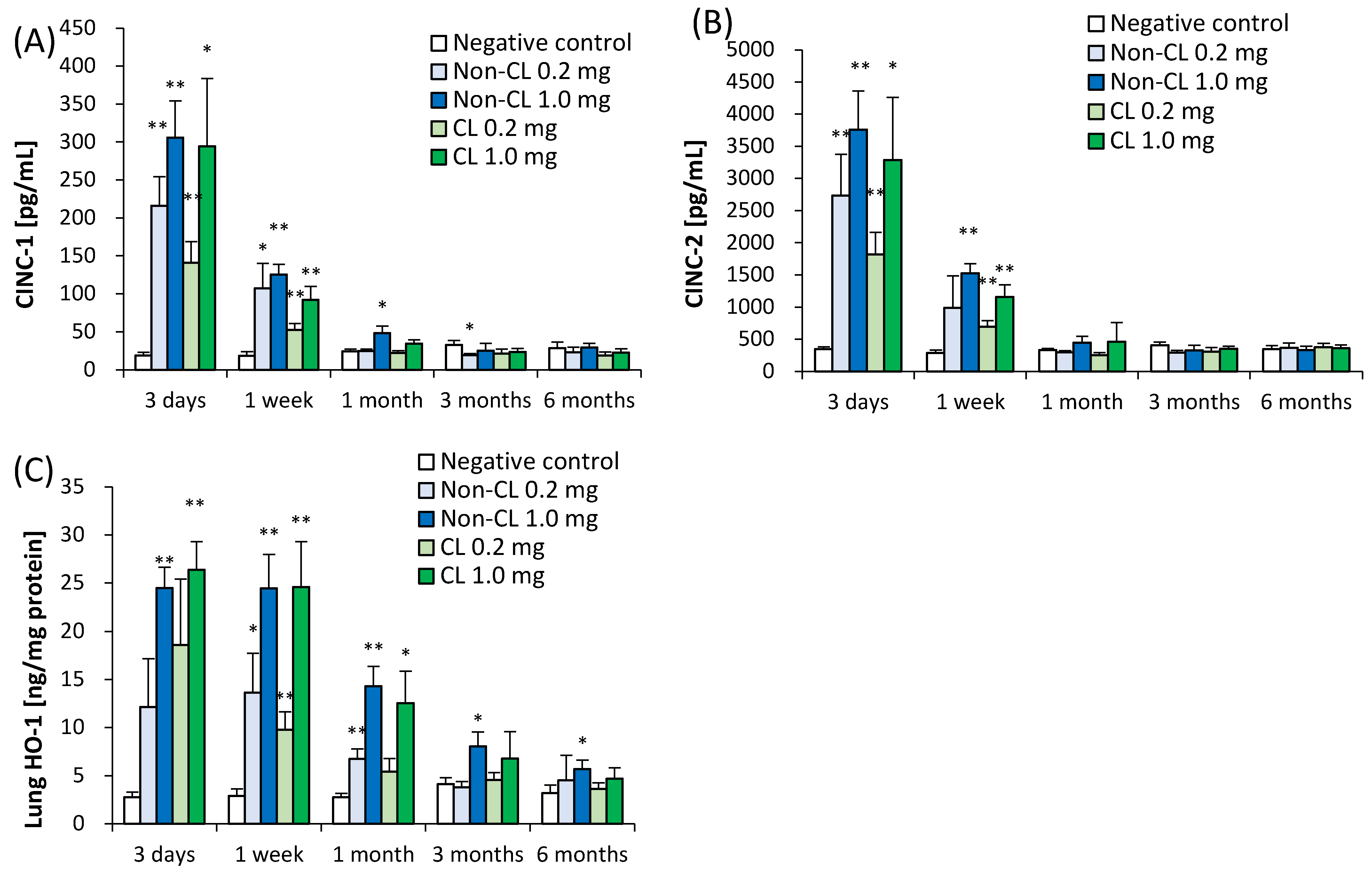
2.3. Concentration of Cytokine-Induced Neutrophil Chemoattractant (CINC) in BALF and Concentration of Heme Oxygenase (HO)-1 in Lung Tissue
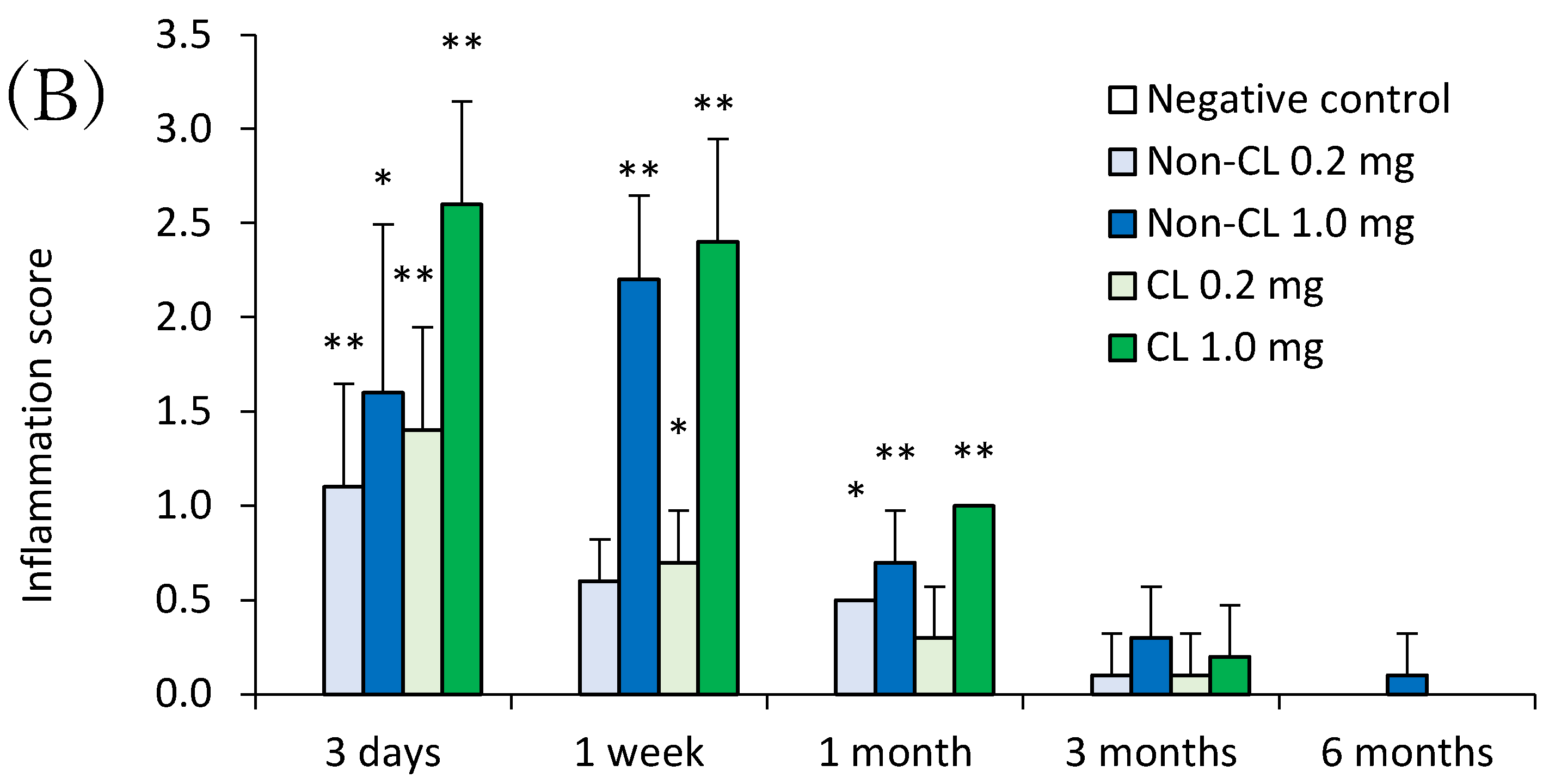
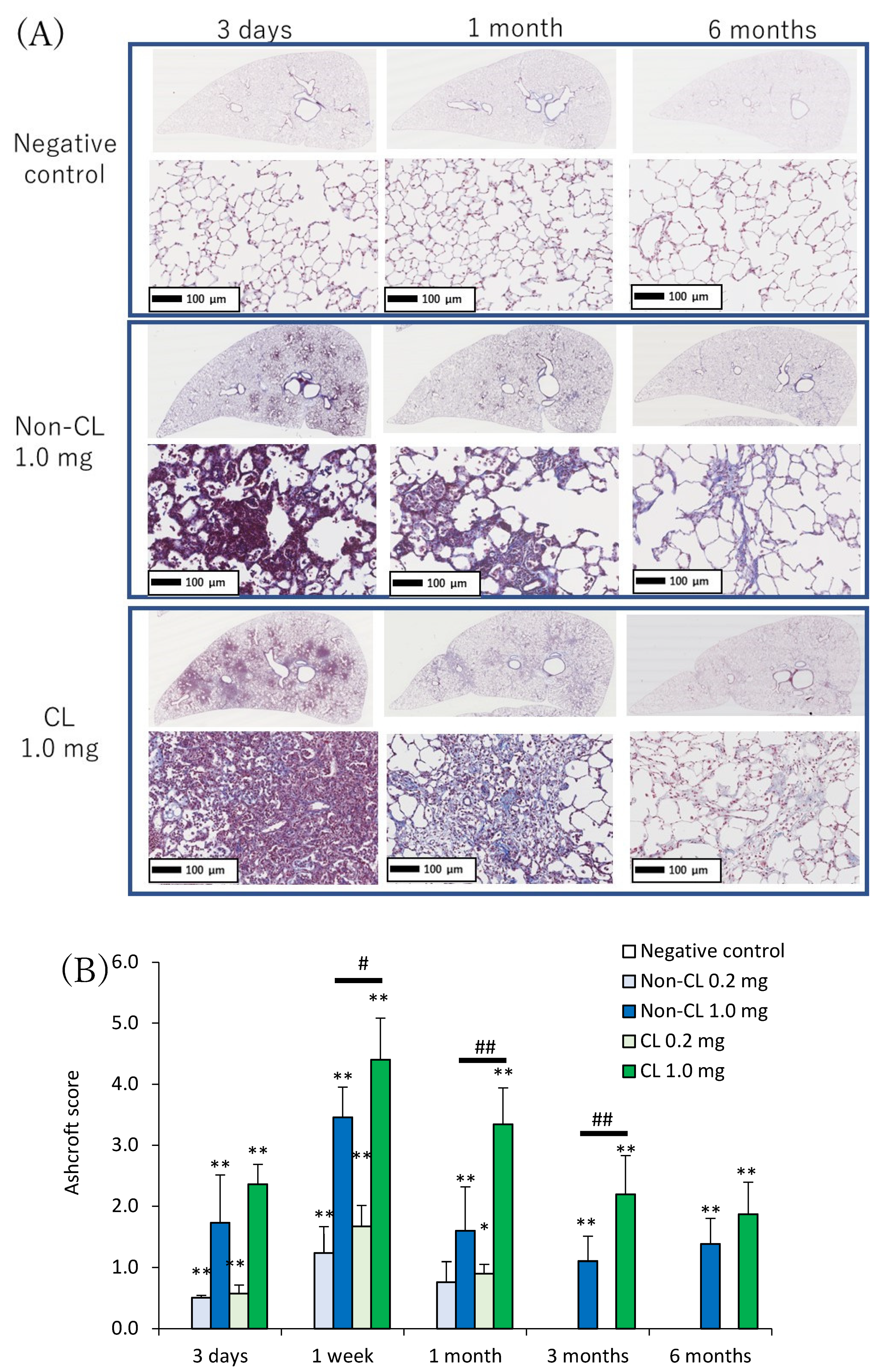
2.4. Histopathological Features in the Lung
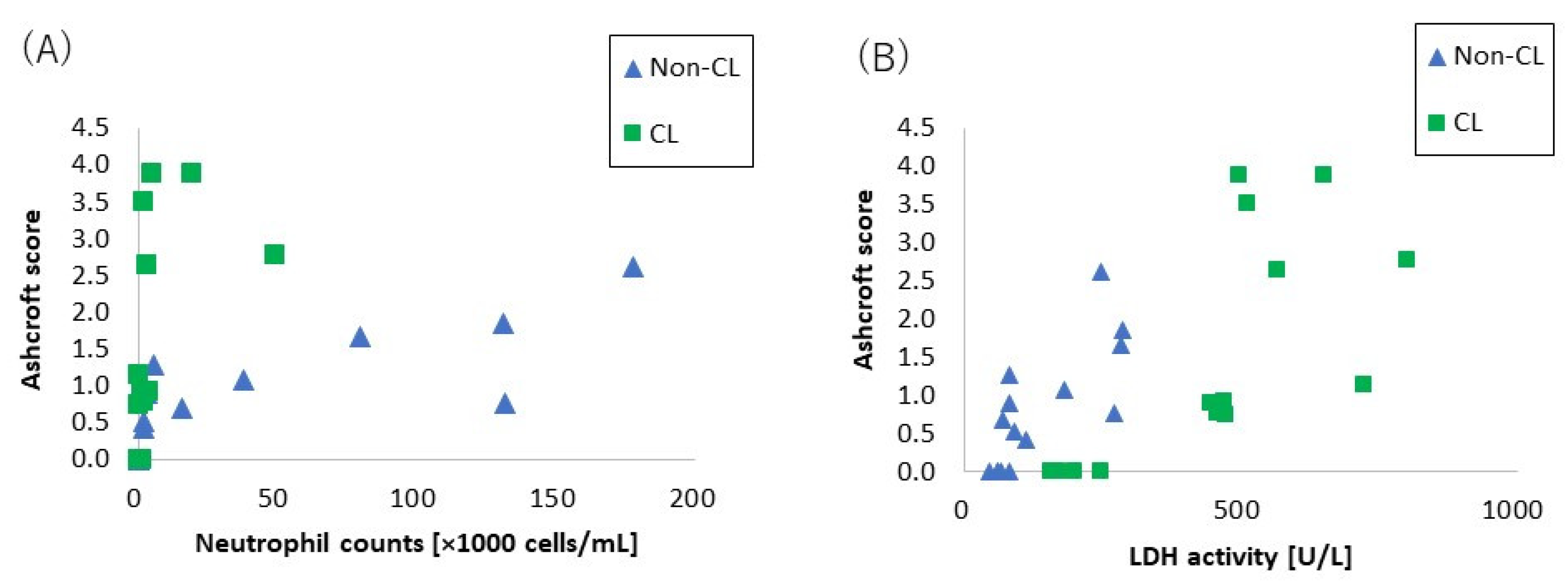
2.5. The Relationship between Lung Fibrosis and Lung Injury
2.6. Gene Expression Analysis
3. Discussion
- (1)
- Inflammogenicity and fibrogenicity induced by both types of PAA. In the present study, both Non-CL-PAA and CL-PAA induced severe lung inflammation in the acute phase but it was transient. In addition, fibrosis occurred to a certain degree in both of the PAAs. In the past, persistent lung inflammation was considered to lead to irreversible fibrosis in lung exposed to inhaled chemicals. In the case of silica and asbestos, which have high pulmonary toxicity, there was persistent inflammation that progressed to fibrosis in rat lung up to 6–12 months after exposure [10,13,14,15]. On the other hand, inhaled chemicals such as titanium dioxide (TiO2) and zinc oxide (ZnO), which have low pulmonary toxicity, show acute-phase lung inflammation and lung injury, but inflammation is recovered from within 1 week to 1 month after exposure and does not progress to fibrosis [16,17,18]. In the present study, lung inflammation was caused for about 1 week, and CINC, a neutrophil chemokine, increased significantly in both PAAs to 1 week in a dose-dependent manner. Total protein and LDH in the BALF (tissue injury indicators) and HO-1 in the lung tissue (oxidative stress indicator) were also significantly increased in the exposed groups until 1 month after exposure.Considering that persistent inflammation is associated with the potential for lung disorder, it is speculated that the lung disorder potential of both PAAs is not high, based on transient inflammation and inflammation-associated cytokine production by both PAAs. However, although the inflammation by PAAs ended at the acute phase, it was accompanied by fibrosis, and we considered that the acute inflammation rapidly transitioned to the next stage, fibrosis—that is, it progressed to an irreversible lesion.Fibrosis after acute inflammation has been observed in animal exposure studies and clinical cases; it is often observed in LPS exposure models that induce acute inflammation and in ARDS that presents with acute respiratory failure. The clinical presentation of ARDS is known to cause lung injury of the alveolar epithelium due to inflammation followed by fibrosis from repair processes [19]. Animal models of acute respiratory failure caused by LPS or bleomycin also show severe lung inflammation that ends in 1–2 weeks, followed by fibrosis [20,21]. Although the persistent inflammation by both PAAs was not observed for long periods, the inflammations did cause fibrosis. Similarly, in other reports of PAA, the inflammation is followed promptly by fibrosis [7,10]. Although asbestosis usually causes fibrosis after 10 years of exposure in clinical cases, PAA causes rapid fibrosis in 1 to 2 years in the clinical cases of workers working with it. The present study is clinically consistent with PAA having fibrotic potential.In the present study, both exposures to PAA caused irreversible fibrosis from 1 month after intratracheal instillation. Proliferation of fibroblasts or deposition of collagen in the lung interstitium has been observed from about 1 month after pharyngeal aspiration exposure of asbestos or MWCNTs in mice [14]. An intratracheal instillation in mice using the organic substance polyhexamethylene guanidine phosphate (PHMG-p) also showed collagen deposition as well as lung inflammation from 2 weeks after intratracheal instillation [22]. Considering these reports, the fibrosis observed in the present study after exposure to Non-CL-PAA and CL-PAA suggests that both polymers have fibrogenic potential.
- (2)
- Pathophysiology accompanied with the crosslinked type. The level of crosslinking of CL-PAA in the present study was below 0.1%, which is commonly used in industrial applications for its thickening and water absorbency properties, and the fibrogenicity of CL-PAA in the lung with the level of crosslinking in industrial applications has been evaluated. In the present study, the CL-PAA caused more fibrosis of the lungs than did the Non-CL-PAA. To investigate the difference in fibrosis progression between the CL-PAA and Non-CL-PAA, we performed a microarray analysis using lung tissue one month after exposure, which is considered to be the fibrosis transition period. Compared with Non-CL-PAA, the expression of elastin, which constitutes elastic fibers, and the collagen 1a1 gene, which is involved in the fibrosis, was higher in CL-PAA. Increased elastin gene and collagen 1a1 gene expression in fibrotic lesions have been reported in a bleomycin-induced pulmonary fibrosis model [23,24], suggesting that increased elastin as well as collagen was involved in the fibrosis of the lung histopathology findings of CL-PAA.In the CL-PAA with strongly induced fibrosis, the LDH in the BALF was found to be higher than in Non-CL-PAA, suggesting that CL-PAA causes greater cellular injury to the constituent cells of the lung. Several reports have recognized that stronger lung injury leads to the development of fibrosis: in inhalation exposure and intratracheal instillation of MWCNTs in mice, it was reported that the increase in the LDH in BALF and the deposition and fibrosis levels of MWCNTs showed similar tendencies to increase [14,25]. In the present study, the relationship between LDH and fibrosis in the BALF with both PAAs showed an almost linear correlation between LDH and fibrosis (Figure 7A). On the other hand, a correlation was observed between the number of neutrophils in the BALF and fibrosis, but not as linear as the correlation between LDH and fibrosis (Figure 7B). It is likely that lung injury has a more direct effect on the fibrosis development of PAA than does inflammatory cell infiltration.As for gene expression involved in lung injury including cell death, KEGG pathway analysis in genes that are more upregulated in CL-PAA showed genes related to apoptosis, including apoptosis and the Phosphoinositide 3-kinase-AKT (PI3K-AKT) signaling pathway, and it is reported that these pathways are involved in fibrosis. In models of idiopathic pulmonary fibrosis and bleomycin-induced pulmonary fibrosis, it has been reported that the expression of genes related to the PI3K-AKT pathway is elevated and involved in the development of fibrosis in the lung [26,27]. Although apoptosis is necessary for normal repair to eliminate inflammatory cells and proliferating fibroblasts that collect in the alveolar walls and alveolar space during lung injury [28], it has been reported that excessive apoptosis is involved in the pathogenesis of ARDS, which is a transition to fibrosis [26]. Considering the increased expression of collagen genes and the enhancement of the apoptotic pathways, including the PI3K-AKT pathway in lung tissue due to lung injury, the progression of fibrosis in CL-PAA may be related to lung injury.
- (3)
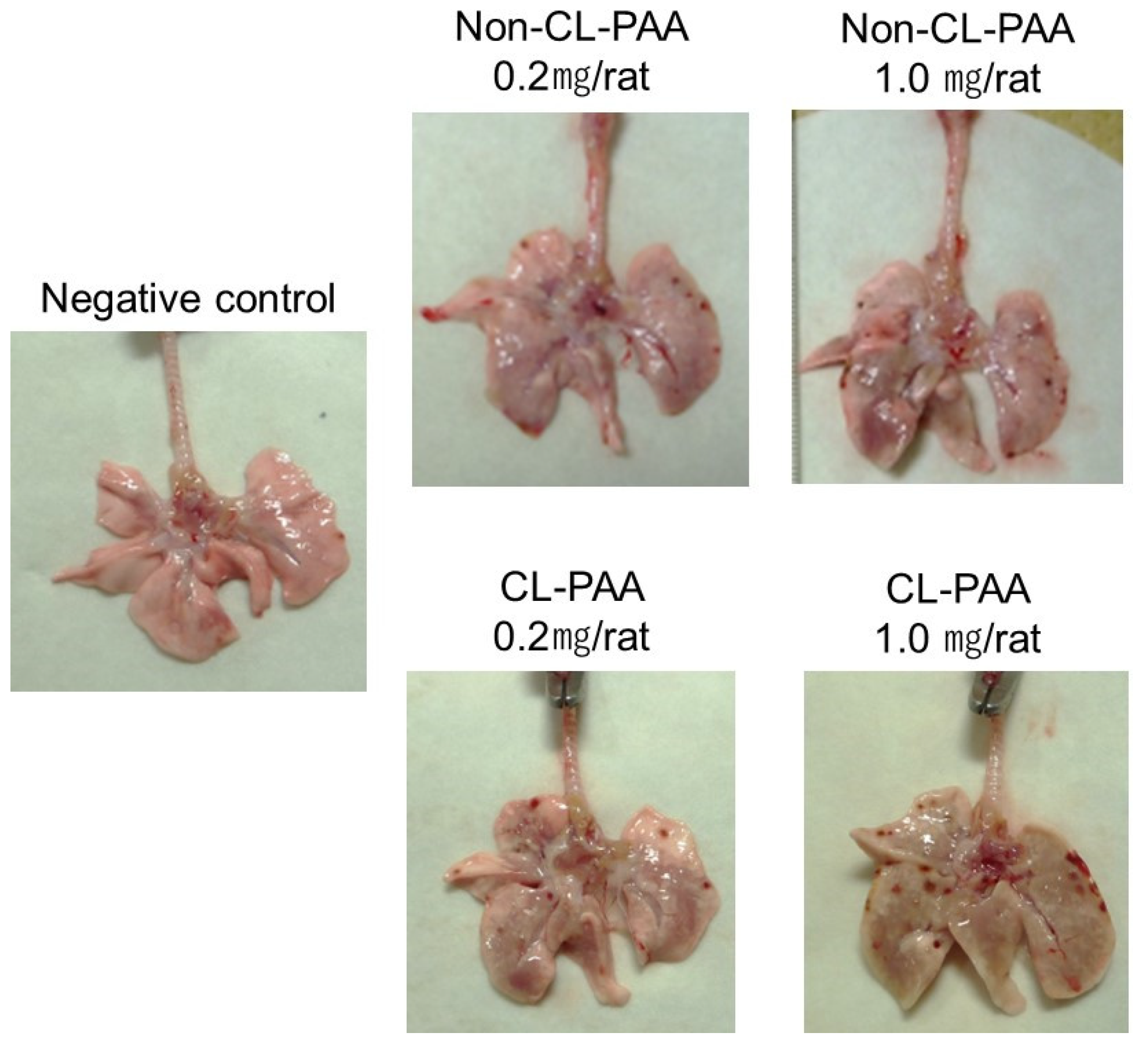
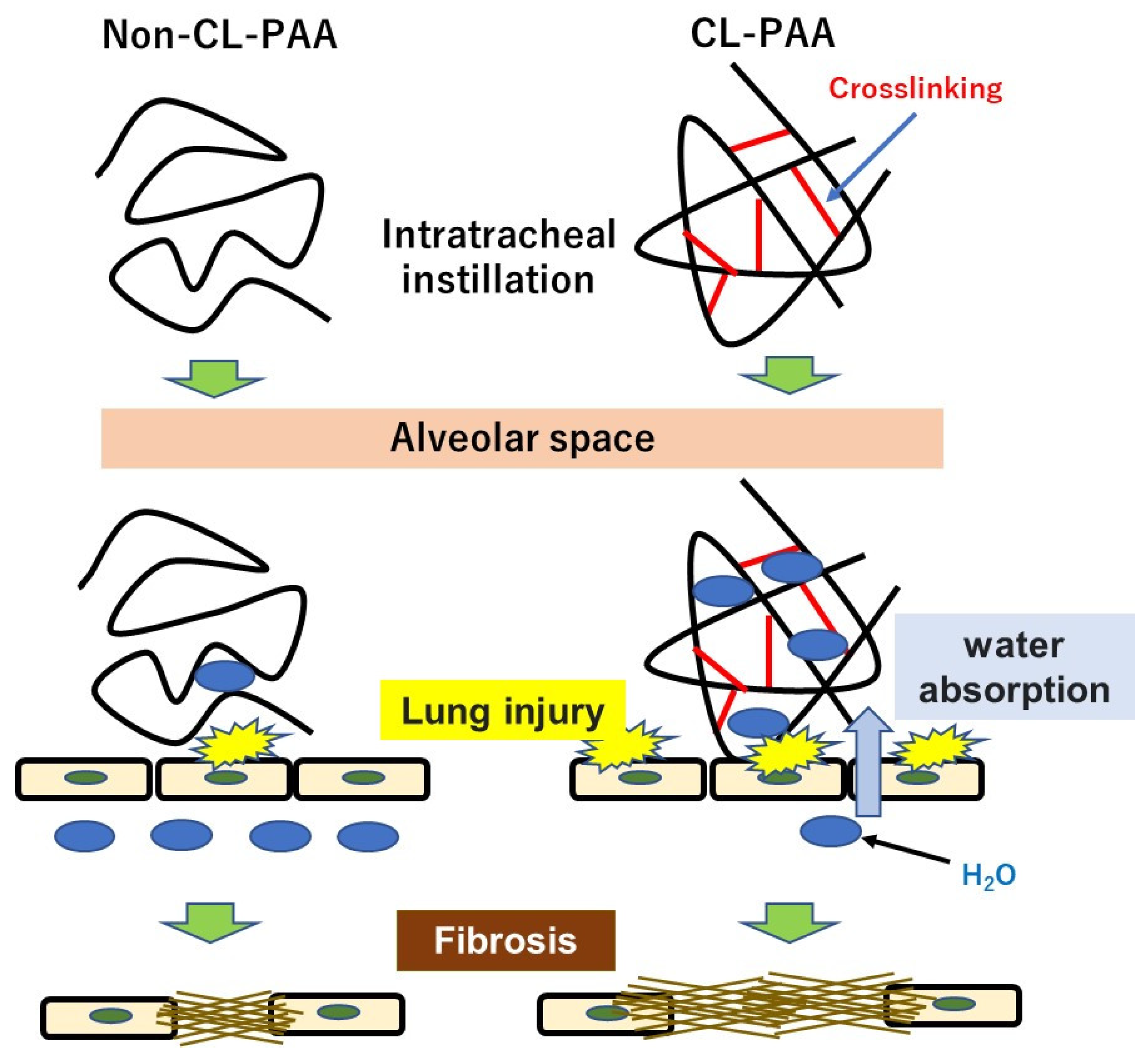
- Factors related to the crosslinking structure of PAA in the progress of fibrosis. The molecular weights of Non-CL-PAA and CL-PAA in this study were 7.53 × 105 g/mol and 7.65 × 105 g/mol, respectively, and the radius of gyration (Rg) indicated that the radii of polymer particles in solution were 74.9 nm and 68.7 nm, respectively. Since both PAAs have similar molecular weights, we considered that the crosslinking structure was responsible for the enhancement of fibrosis by the PAA. In general, the thickening properties of PAA change with increasing molecular weight [8]. It has been reported that there is an optimal viscosity for sputum clearance [29,30]. The thickening that comes with increasing molecular weight may affect the delay in clearance of the PAA from the lungs. In the present study, the rat lungs at dissection 3 days to 1 month after intratracheal instillation of PAA, especially in the 1.0 mg exposure group, were not expanded, and BALF was not recovered sufficiently even when saline was infused into the lung (Figure 1B). Such poor recovery of BALF may reflect the result of a collapsed lung, and a collapsed lung may affect the delay in clearance of PAA. On the other hand, the presence of a crosslinked structure makes a high water-absorbency property and low viscosity when the molecular weight of both PAAs are the same level [31,32]. In other words, CL-PAA incorporates water molecules into the polymer’s strong braided structure by crosslinking in the lung, while Non-CL-PAA has a weak retention of water molecules due to its lack of a strong network structure. It is possible that the water-absorbing property of the CL-PAA changes the osmotic pressure in the lungs by absorbing water molecules in vivo, causing cytotoxicity. In vitro studies using polymeric nanoparticles have shown that cytotoxicity increases with elevation in the osmotic pressure of the culture medium. When the biodegradable polymer Poly(lactideco-glycide) was exposed to macrophages (RAW cells) and alveolar epithelial cells (A549 cells), it caused cell death as the osmotic pressure of the medium increased, and a correlation was observed between the increase in osmotic pressure and cell death [33]. Since PAAs also cause osmotic pressure changes due to water absorption, this difference in water absorption by PAAs may have influenced the difference in fibrosis via cell injury (Figure 8).
4. Materials and Methods
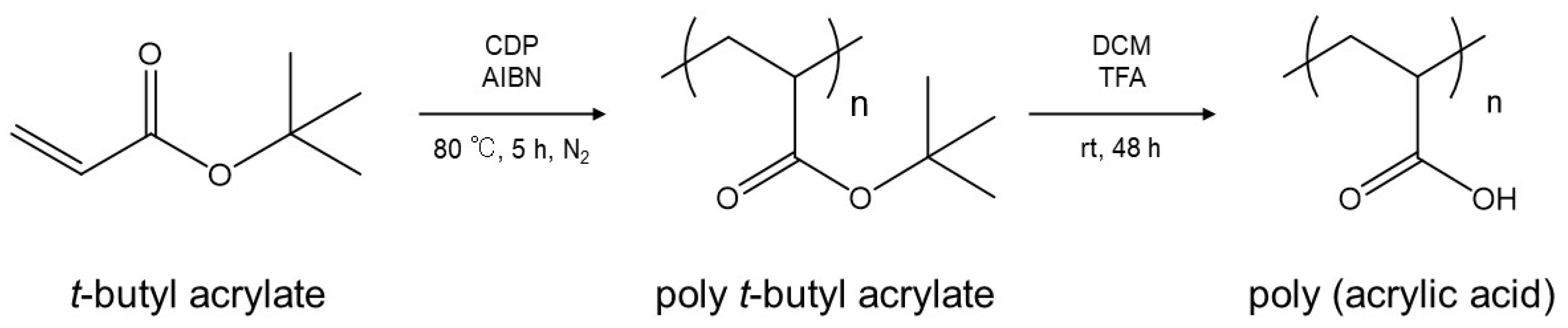
4.1. Sample Polymer
4.2. Animals
4.3. Intratracheal Instillation
4.4. Animals Following Intratracheal Instillation
4.5. Cytospin Analysis of Inflammatory Cells and Measurement of Inflammation Related Markers in BALF
4.6. Measurement of Chemokines in BALF and HO-1 in Lung Tissue
4.7. Total RNA Extraction
4.8. Microarray Analysis
4.9. Histopathology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kim, K.W.; Ahn, K.; Yang, H.J.; Lee, S.Y.; Park, J.D.; Kim, W.K.; Kim, J.-T.; Kim, H.H.; Rha, Y.H.; Park, Y.M.; et al. Humidifier Disinfectant-Associated Children’s Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2013, 26, 131107125401005. [Google Scholar] [CrossRef] [PubMed]
- Paek, D.; Koh, Y.; Park, D.-U.; Cheong, H.-K.; Do, K.-H.; Lim, C.-M.; Hong, S.-J.; Kim, Y.-H.; Leem, J.-H.; Chung, K.H.; et al. Nationwide Study of Humidifier Disinfectant Lung Injury in South Korea, 1994–2011. Incidence and Dose–Response Relationships. Ann. Am. Thorac. Soc. 2015, 12, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.; Markin, C.; Hosmer, D. An Unexpected Consequence of Electronic Cigarette Use. Chest 2012, 141, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Thota, D.; Latham, E. Case Report of Electronic Cigarettes Possibly Associated with Eosinophilic Pneumonitis in a Previously Healthy Active-duty Sailor. J. Emerg. Med. 2014, 47, 15–17. [Google Scholar] [CrossRef]
- Ministry of Health, Labour, and Welfare. Notification about Prevention of Lung Disease Caused by Organic Dust (Published on 28 April 2017). Available online: https://www.mhlw.go.jp/stf/houdou/0000163568.html (accessed on 27 June 2022). (In Japanese)
- Ministry of Health, Labour, and Welfare. Working Group Report to Decide whether Pulmonary Disorder That Occurred at the Workplace of Production of Powder of Cross-Linked Polyacrylic Acid Is Work-Related Disease or Not (Published on 19 April 2019). 2019. Available online: https://www.mhlw.go.jp/content/11402000/000502982.pdf (accessed on 27 June 2022). (In Japanese)
- Morimoto, Y.; Nishida, C.; Tomonaga, T.; Izumi, H.; Yatera, K.; Sakurai, K.; Kim, Y. Lung disorders induced by respirable organic chemicals. J. Occup. Health 2021, 63, e12240. [Google Scholar] [CrossRef]
- Horiuchi, T. Physico-chemical properties of water-soluble polymers in aqueous solution. J. Surf. Finish. Soc. Jpn. 2009, 60, 746–753. [Google Scholar] [CrossRef]
- Fiume, M.Z. Final Report on the Safety Assessment of Acrylates Copolymer and 33 Related Cosmetic Ingredients. Int. J. Toxicol. 2002, 21, 1–50. [Google Scholar] [CrossRef]
- Nishida, C.; Tomonaga, T.; Izumi, H.; Wang, K.-Y.; Higashi, H.; Ishidao, T.; Takeshita, J.; Ono, R.; Sumiya, K.; Fujii, S.; et al. Inflammogenic effect of polyacrylic acid in rat lung following intratracheal instillation. Part. Fibre Toxicol. 2022, 19, 8. [Google Scholar] [CrossRef]
- Takeda, T.; Yamano, S.; Goto, Y.; Hirai, S.; Furukawa, Y.; Kikuchi, Y.; Misumi, K.; Suzuki, M.; Takanobu, K.; Senoh, H.; et al. Dose–response relationship of pulmonary disorders by inhalation exposure to cross-linked water-soluble acrylic acid polymers in F344 rats. Part. Fibre Toxicol. 2022, 19, 27. [Google Scholar] [CrossRef]
- Nishida, C.; Izumi, H.; Tomonaga, T.; Wang, K.-Y.; Higashi, H.; Takeshita, J.-I.; Ono, R.; Sumiya, K.; Fujii, S.; Hata, Y.; et al. Effect of Different Molecular Weights of Polyacrylic Acid on Rat Lung Following Intratracheal Instillation. Int. J. Mol. Sci. 2022, 23, 10345. [Google Scholar] [CrossRef]
- Kajiwara, T.; Ogami, A.; Yamato, H.; Oyabu, T.; Morimoto, Y.; Tanaka, I. Effect of Particle Size of Intratracheally Instilled Crystalline Silica on Pulmonary Inflammation. J. Occup. Health 2007, 49, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder-Talkington, B.N.; Dong, C.; Porter, D.W.; Ducatman, B.; Wolfarth, M.G.; Andrew, M.; Battelli, L.; Raese, R.; Castranova, V.; Guo, N.L.; et al. Multiwalled carbon nanotube-induced pulmonary inflammatory and fibrotic responses and genomic changes following aspiration exposure in mice: A 1-year postexposure study. J. Toxicol. Environ. Health Part A 2016, 79, 352–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyphert, J.M.; Padilla-Carlin, D.J.; Schladweiler, M.C.; Shannahan, J.H.; Nyska, A.; Kodavanti, U.P.; Gavett, S.H. Long-Term Response of Rats to Single Intratracheal Exposure of Libby Amphibole or Amosite. J. Toxicol. Environ. Health Part A 2012, 75, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Hoke, R.A.; Finlay, C.; Donner, E.M.; Reed, K.L.; Sayes, C.M. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol. Lett. 2007, 171, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.-W.; Okada, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Evaluation of pulmonary toxicity of zinc oxide nanoparticles following inhalation and intratracheal instillation. Int. J. Mol. Sci. 2016, 17, 1241. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.; Bellingan, G.; Laurent, G. The acute respiratory distress syndrome: Fibrosis in the fast lane. Thorax 1998, 53, 815–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-N.; Lee, J.-S.; Yang, H.-S.; Cho, J.-W.; Kwon, S.-J.; Kim, Y.-B.; Her, J.-D.; Cho, K.-H.; Song, C.-W.; Lee, K.-H. Dose-response Effects of Bleomycin on Inflammation and Pulmonary Fibrosis in Mice. Toxicol. Res. 2010, 26, 217–222. [Google Scholar] [CrossRef] [Green Version]
- de Souza Xavier Costa, N.; Ribeiro Júnior, G.; dos Santos Alemany, A.A.; Belotti, L.; Zati, D.H.; Frota Cavalcante, M.; Matera Veras, M.; Ribeiro, S.; Kallás, E.G.; Nascimento Saldiva, P.H.; et al. Early and late pulmonary effects of nebulized LPS in mice: An acute lung injury model. PLoS ONE 2017, 12, e0185474. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Park, J.-H.; Lee, J.-Y.; Jeong, Y.-J.; Song, J.A.; Lee, K.; Kim, D.-J. Establishment of a mouse model for pulmonary inflammation and fibrosis by intratracheal instillation of polyhexamethyleneguanidine phosphate. J. Toxicol. Pathol. 2016, 29, 95–102. [Google Scholar] [CrossRef]
- Blaauboer, M.E.; Boeijen, F.R.; Emson, C.L.; Turner, S.M.; Zandieh-Doulabi, B.; Hanemaaijer, R.; Smit, T.H.; Stoop, R.; Everts, V. Extracellular matrix proteins: A positive feedback loop in lung fibrosis? Matrix Biol. 2014, 34, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Barbayianni, I.; Ninou, I.; Tzouvelekis, A.; Aidinis, V. Bleomycin Revisited: A Direct Comparison of the Intratracheal Micro-Spraying and the Oropharyngeal Aspiration Routes of Bleomycin Administration in Mice. Front. Med. 2018, 5, 269. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.W.; Hubbs, A.F.; Chen, B.T.; McKinney, W.; Mercer, R.R.; Wolfarth, M.G.; Battelli, L.; Wu, N.; Sriram, K.; Leonard, S.; et al. Acute pulmonary dose–responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 2012, 7, 1179–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Hu, K.; Cai, X.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin. B 2022, 12, 18–32. [Google Scholar] [CrossRef]
- Hsu, H.-S.; Liu, C.-C.; Lin, J.-H.; Hsu, T.-W.; Hsu, J.-W.; Su, K.; Hung, S.-C. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci. Rep. 2017, 7, 14272. [Google Scholar] [CrossRef] [Green Version]
- Polunovsky, V.A.; Chen, B.; Henke, C.; Snover, D.; Wendt, C.; Ingbar, D.H.; Bitterman, P.B. Role of mesenchymal cell death in lung remodeling after injury. J. Clin. Investig. 1993, 92, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.M.; Dulfano, M.J. Mucus viscoelasticity and mucociliary transport rate. J. Lab. Clin. Med. 1978, 91, 423–431. [Google Scholar]
- Zhong, L.; Xiong, Y.; Zheng, Z.; Liu, N.; Hu, J.; Yang, F.; Chen, R. Effect of short-term inhalation of warm saline atomised gas on patients with non-cystic fibrosis bronchiectasis. ERJ Open Res. 2020, 6, 130–2019. [Google Scholar] [CrossRef] [Green Version]
- Urahama, Y.; Hasegawa, Y.; Murata, J.; Kishi, H. Investigation of the Mechanical Properties of aPressure-sensitive Adhesive Part 3Effect of Polydispersity Index (PDI) on Crosslinking Property of Acrylic Polymers. J. Adhes. Soc. Jpn. 2020, 56, 4–11. [Google Scholar] [CrossRef]
- Xu, J.; Kim, K.O.; Yoon, K.J. Effect of Cross-Linker Length on the Absorption Characteristics of the Sodium Salt of Cross-Linked Polyaspartic Acid. Polymers 2022, 14, 2244. [Google Scholar] [CrossRef]
- Singh, R.P.; Ramarao, P. Accumulated Polymer Degradation Products as Effector Molecules in Cytotoxicity of Polymeric Nanoparticles. Toxicol. Sci. 2013, 136, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Morrow, P.E.; Muhle, H.; Mermelstein, R. Chronic Inhalation Study Findings as a Basis for Proposing a New Occupational Dust Exposure Limit. Int. J. Toxicol. 1991, 10, 279–290. [Google Scholar] [CrossRef]
- Bellmann, B.; Muhle, H.; Creutzenberg, O.; Mermelstein, R. Irreversible pulmonary changes induced in rat lung by dust overload. Environ. Health Perspect. 1992, 97, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Oyabu, T.; Morimoto, Y.; Hirohashi, M.; Horie, M.; Kambara, T.; Lee, B.W.; Hashiba, M.; Mizuguchi, Y.; Myojo, T.; Kuroda, E. Dose-dependent pulmonary response of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J. Nanoparticle Res. 2013, 15, 1600. [Google Scholar] [CrossRef]
- Sumiya, K.; Matsunaga, T.; Tanaka, M.; Mochizuki, S.; Sakurai, K. Oligo-DNA Stoichiometrically Binds β-1,3-Glucan with the Best Fit Length. Biomacromolecules 2020, 21, 4823–4834. [Google Scholar] [CrossRef]
- Doan, V.T.H.; Lee, J.H.; Takahashi, R.; Nguyen, P.T.M.; Nguyen, V.A.T.; Pham, H.T.T.; Fujii, S.; Sakurai, K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: Potential carriers of α-mangostin for cancer therapy. Polym. J. 2020, 52, 457–466. [Google Scholar] [CrossRef]
- Nishida, C.; Izumi, H.; Tomonaga, T.; Takeshita, J.; Wang, K.-Y.; Yamasaki, K.; Yatera, K.; Morimoto, Y. Predictive Biomarkers for the Ranking of Pulmonary Toxicity of Nanomaterials. Nanomaterials 2020, 10, 2032. [Google Scholar] [CrossRef]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Hübner, R.-H.; Gitter, W.; Eddine El Mokhtari, N.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–517. [Google Scholar] [CrossRef]



| Gene Symbol | Gene Description | Fold Change (ACTB) | Fold Change (GAPDH) |
|---|---|---|---|
| Mt3 | metallothionein 3 | 5.6 | 8.0 |
| AABR07056026.1 | - | 3.1 | 4.4 |
| Olr1523 | olfactory receptor 1523 | 3.0 | 4.3 |
| Hbq1b | hemoglobin, theta 1B | 2.8 | 4.1 |
| Mt2A | metallothionein 2A | 2.8 | 4.1 |
| Rab24 | RAB24, member RAS oncogene family | 2.8 | 4.0 |
| Tuba3b | tubulin, alpha 3B | 2.8 | 4.0 |
| Nr1d1 | nuclear receptor subfamily 1, group D, member 1 | 2.6 | 3.8 |
| Igh-6 | immunoglobulin heavy chain 6 | 2.6 | 3.7 |
| RGD1565617 | similar to Ig variable region, light chain | 2.6 | 3.7 |
| Ttll11 | tubulin tyrosine ligase like11 | 2.5 | 3.6 |
| Atp13a2 | ATPase 13A2 | 2.4 | 3.4 |
| Sec14l1 | SEC14-like lipid binding 1 | 2.3 | 3.3 |
| Igsf8 | immunoglobulin superfamily, member 8 | 2.2 | 3.1 |
| Egfl7 | EGF-like-domain, multiple 7 | 2.2 | 3.1 |
| Fau | Finkel–Biskis–Reilly murine sarcoma virus (FBR-MuSV) ubiquitously expressed | 2.1 | 3.0 |
| Eln | elastin | 2.1 | 3.0 |
| Ep300 | E1A binding protein p300 | 2.0 | 2.9 |
| Arl2 | ADP-ribosylation factor like GTPase 2 | 2.0 | 2.9 |
| Otop2 | otopetrin 2 | 1.5 | 2.1 |
| Gene Symbol | Gene Description | Fold Change (ACTB) | Fold Change (GAPDH) |
|---|---|---|---|
| Eln | elastin | 2.1 | 3.0 |
| Akt1 | v-akt murine thymoma viral oncogene homolog 1 | 1.8 | 2.6 |
| Ccm2l | CCM2-like scaffolding protein | 1.7 | 2.4 |
| Mif | macrophage migration inhibitory factor (glycosylation-inhibiting factor) | 1.6 | 2.3 |
| Parp10 | poly (ADP-ribose) polymerase family, member 10 | 1.6 | 2.3 |
| Pdgfrb | platelet derived growth factor receptor beta | 1.5 | 2.1 |
| Col1a1 | collagen, type I, alpha 1 | 1.4 | 2.1 |
| Fgfr4 | fibroblast growth factor receptor 4 | 1.4 | 2.0 |
| Ctgf | connective tissue growth factor | 1.4 | 2.0 |
| Hyal2 | hyaluronoglucosaminidase 2 | 1.4 | 2.0 |
| Terms of KEGG Pathway | Gene Counts | % | p-Value |
|---|---|---|---|
| Salmonella infection | 10 | 5.08 | 0.0012 |
| Alzheimer disease | 12 | 6.09 | 0.0021 |
| Tuberculosis | 8 | 4.06 | 0.0022 |
| Apoptosis | 7 | 3.55 | 0.0027 |
| Fluid shear stress and atherosclerosis | 7 | 3.55 | 0.0045 |
| PI3K-Akt signaling pathway | 10 | 5.08 | 0.0088 |
| Sphingolipid signaling pathway | 6 | 3.05 | 0.0089 |
| Physiochemical Characterization | Non-Crosslinked Polyacrylic Acid | Crosslinked Polyacrylic Acid |
|---|---|---|
| Structural formula |  | |
| Weight average molecular weight (MW) | 7.53 × 105 g/mol | 7.65 × 105 g/mol |
| Number average molecular weight (Mn) | 6.10 × 105 g/mol | 4.14 × 105 g/mol |
| Poly dispersity index (PDI) | 1.24 | 1.85 |
| Degree of crosslinking | None | ~0.1% |
| Viscosity (mPa·s) at 25 °C | 3.039 mPa·s | 2.383 mPa·s |
| radius of gyration (Rg) | 74.9 nm | 68.7 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomonaga, T.; Nishida, C.; Izumi, H.; Kawai, N.; Wang, K.-Y.; Higashi, H.; Takeshita, J.-I.; Ono, R.; Sumiya, K.; Fujii, S.; et al. Crosslinked Structure of Polyacrylic Acid Affects Pulmonary Fibrogenicity in Rats. Int. J. Mol. Sci. 2022, 23, 13870. https://doi.org/10.3390/ijms232213870
Tomonaga T, Nishida C, Izumi H, Kawai N, Wang K-Y, Higashi H, Takeshita J-I, Ono R, Sumiya K, Fujii S, et al. Crosslinked Structure of Polyacrylic Acid Affects Pulmonary Fibrogenicity in Rats. International Journal of Molecular Sciences. 2022; 23(22):13870. https://doi.org/10.3390/ijms232213870
Chicago/Turabian StyleTomonaga, Taisuke, Chinatsu Nishida, Hiroto Izumi, Naoki Kawai, Ke-Yong Wang, Hidenori Higashi, Jun-Ichi Takeshita, Ryohei Ono, Kazuki Sumiya, Shota Fujii, and et al. 2022. "Crosslinked Structure of Polyacrylic Acid Affects Pulmonary Fibrogenicity in Rats" International Journal of Molecular Sciences 23, no. 22: 13870. https://doi.org/10.3390/ijms232213870






