Systematic Identification and Analysis of OSC Gene Family of Rosa rugosa Thunb
Abstract
:1. Introduction
2. Results
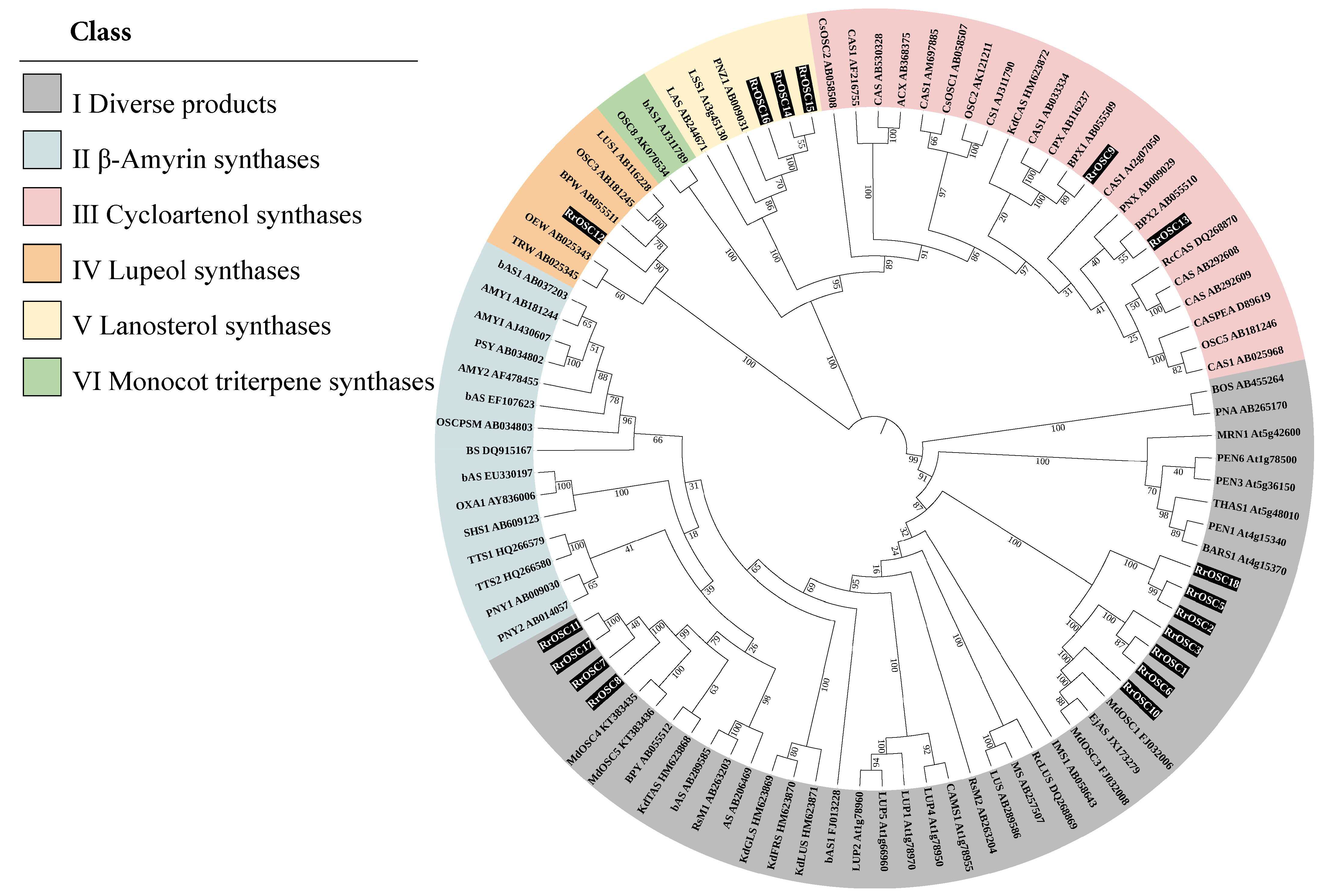
2.1. Identification and Phylogenetic Analysis of RrOSCs
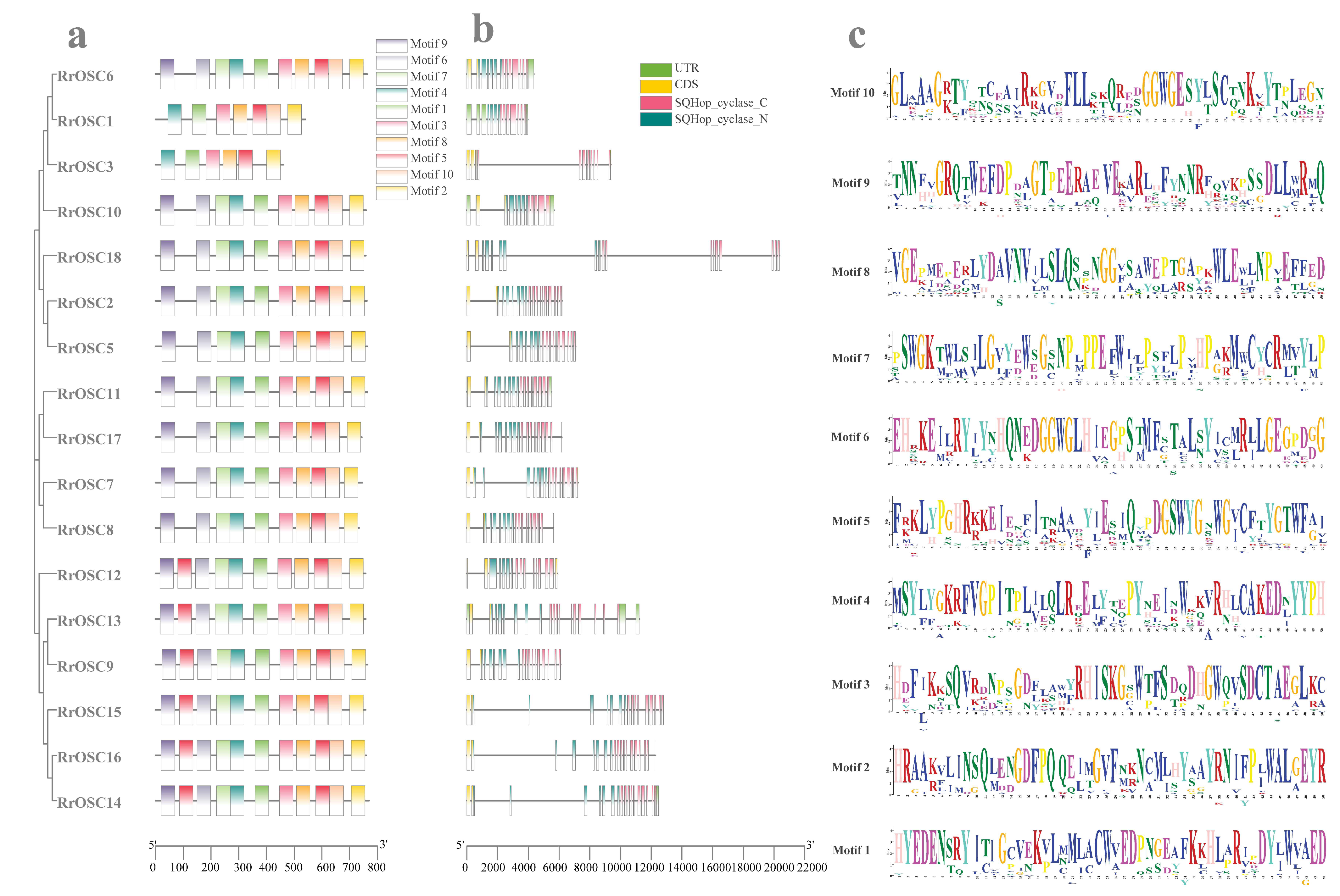
2.2. The Conserved Motifs and Gene Structures of RrOSCs
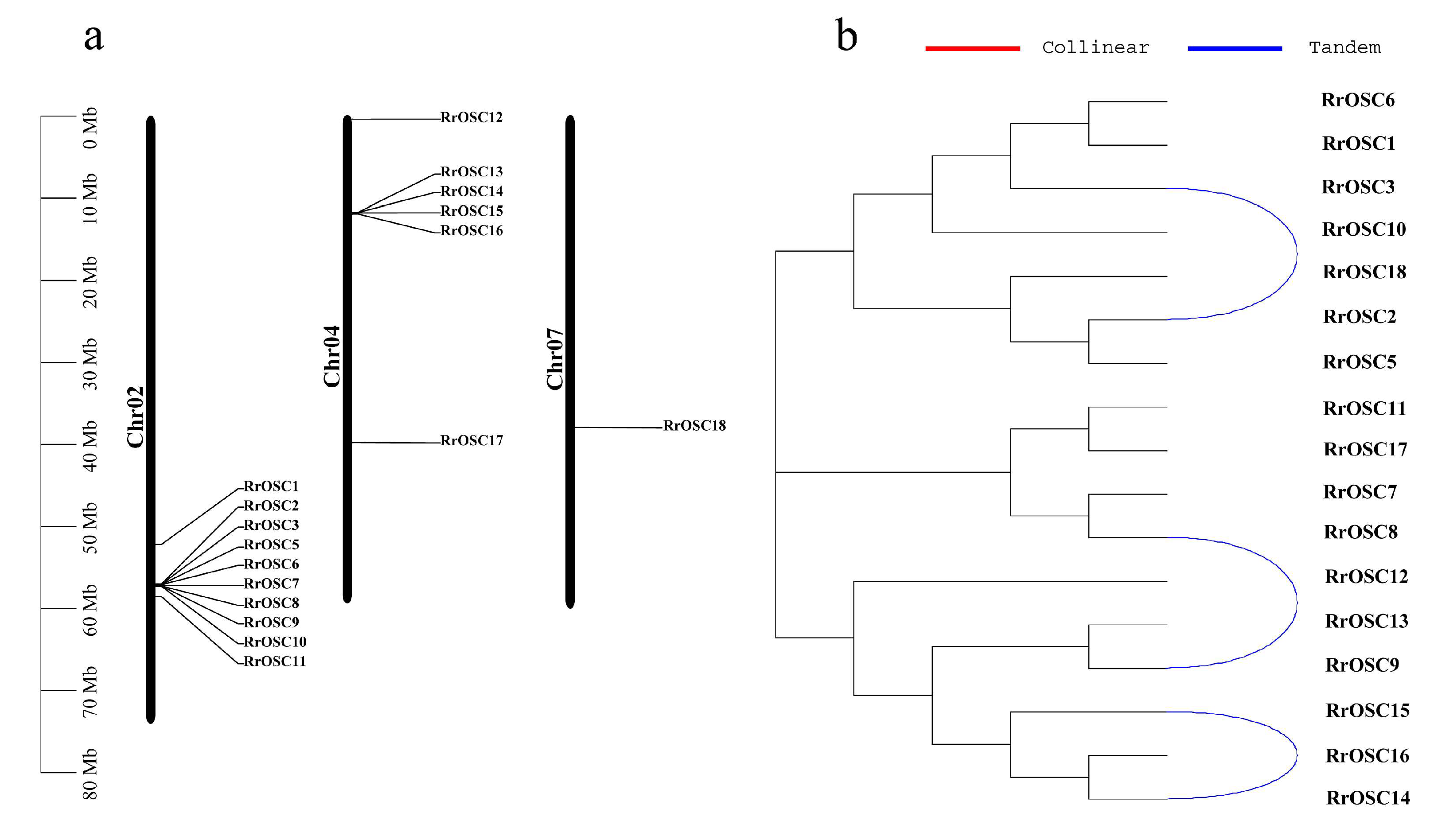
2.3. Clusters and Gene Duplications of RrOSCs
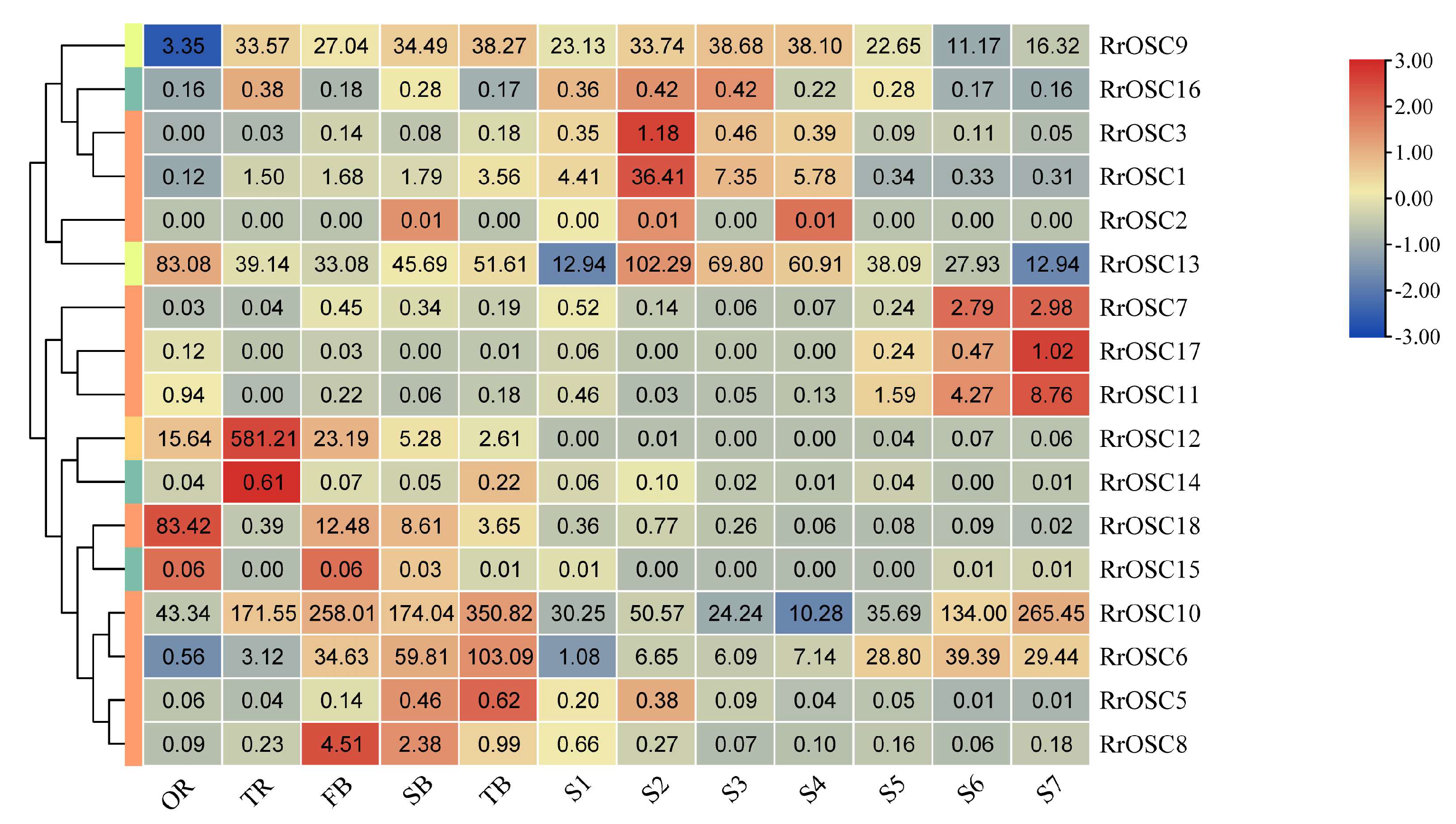
2.4. Expression Analysis of RrOSCs
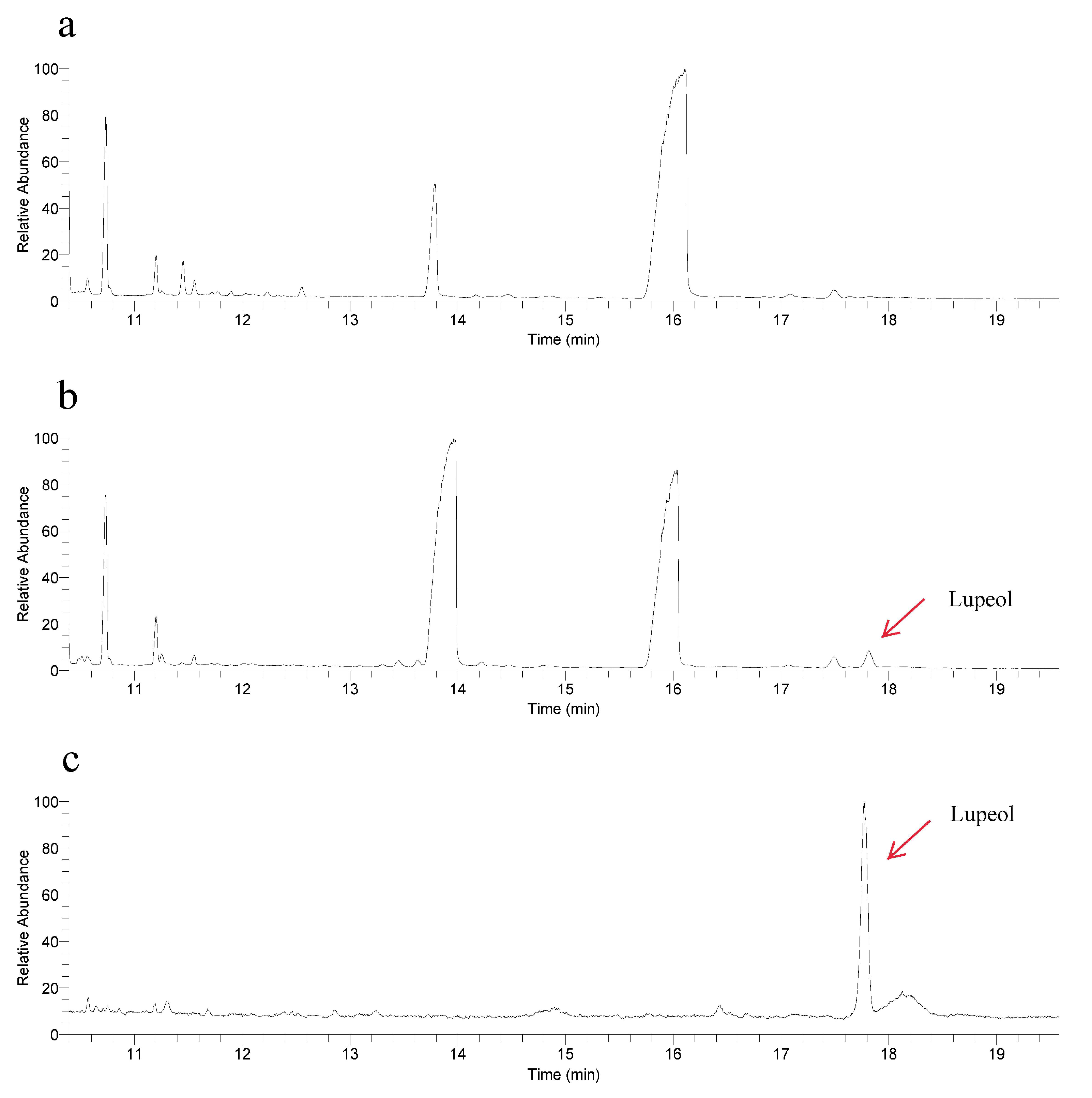
2.5. Functional Characterization of RrOSC12
3. Discussion
4. Materials and Methods
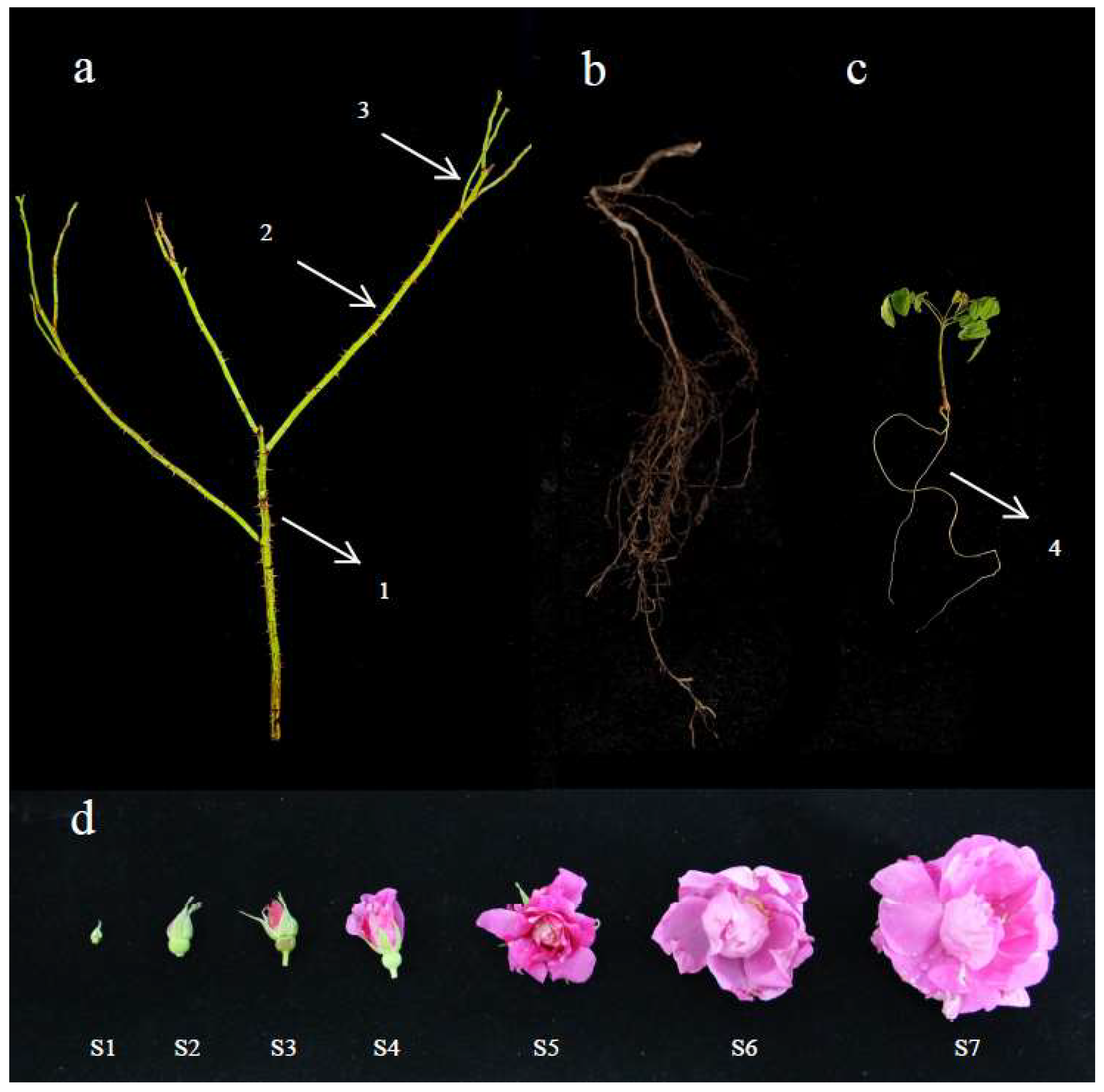
4.1. Plant Materials
4.2. Identification of RrOSCs
4.3. Phylogenetic Analyses
4.4. Gene Structures and Conserved Motifs Analysis
4.5. Chromosome Location and Synteny Analysis
4.6. Expression Analysis and Functional Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002, 75, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Zhang, H.; Endo, A.; Shishikura, K.; Kushiro, T.; Ebizuka, Y. Two branches of the lupeol synthase gene in the molecular evolution of plant oxidosqualene cyclases. Eur. J. Biochem. 1999, 266, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Basyuni, M.; Oku, H.; Tsujimoto, E.; Kinjo, K.; Baba, S.; Takara, K. Triterpene synthases from the Okinawan mangrove tribe, Rhizophoraceae. FEBS J. 2007, 274, 5028–5042. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P.T. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Duan, L.; Liu, D.; Guo, J.; Ge, S.; Dicks, J.; ÓMáille, P.; Osbourn, A.; Qi, X. Divergent evolution of oxidosqualene cyclases in plants. New Phytol. 2012, 193, 1022–1038. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Shibuya, M.; Katsube, Y.; Tsutsumi, T.; Otsuka, M.; Zhang, H.; Masuda, K.; Ebizuka, Y. A new triterpene synthase from Arabidopsis thaliana produces a tricyclic triterpene with two hydroxyl groups. Org. Lett. 2006, 8, 2835–2838. [Google Scholar] [CrossRef]
- Kushiro, T.; Shibuya, M.; Masuda, K.; Ebizuka, Y. A novel multifunctional triterpene synthase from Arabidopsis thaliana. Tetrahedron Lett. 2000, 41, 7705–7710. [Google Scholar] [CrossRef]
- Lodeiro, S.; Xiong, Q.; Wilson, W.K.; Kolesnikova, M.D.; Onak, C.S.; Matsuda, S.P.T. An oxidosqualene cyclase makes numerous products by diverse mechanisms: A challenge to prevailing concepts of triterpene biosynthesis. J. Am. Chem. Soc. 2007, 129, 11213–11222. [Google Scholar]
- Xiong, Q.; Wilson, W.K.; Matsuda, S.P. An Arabidopsis oxidosqualene cyclase catalyzes iridal skeleton formation by Grob fragmentation. Angew. Chem. Int. Ed. Engl. 2006, 45, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.J.R.; Meyer, M.M.; Matsuda, S.P.T. Arabidopsis thaliana LUP1 converts oxidosqualene to multiple triterpene alcohols and a triterpene diol. Org. Lett. 2000, 2, 2257–2259. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.F.; Larondelle, Y.; et al. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Chang, P.; Yu, H.; Ren, H.; Hong, D.; Li, Z.; Wang, Y.; Song, H.; Huo, Y.; Li, C. Productive Amyrin Synthases for Efficient α-Amyrin Synthesis in Engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Hashidoko, Y. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.D.; Lee, Y.H.; Rhee, H.; Choi, Y.S. Influence of extract of Rosa rugosa roots on lipid levels in serum and liver of rats. Life Sci. 1991, 49, 947–951. [Google Scholar] [CrossRef]
- Na, J.R.; Oh, D.R.; Han, S.; Kim, Y.J.; Choi, E.; Bae, D.; Oh, D.H.; Lee, Y.H.; Kim, S.; Jun, W. Antistress Effects of Rosa rugosa Thunb. On Total Sleep Deprivation-Induced Anxiety-Like Behavior and Cognitive Dysfunction in Rat: Possible Mechanism of Action of 5-HT6 Receptor Antagonist. J. Med. Food 2016, 19, 870–881. [Google Scholar] [CrossRef]
- Jung, H.-J.; Nam, J.-H.; Choi, J.; Lee, K.-T.; Park, H.-J. 19Alpha-hydroxyursane-type triterpenoids: Antinociceptive anti-inflammatory principles of the roots of Rosa rugosa. Biol. Pharm. Bull. 2005, 28, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Park, J.C.; Kim, S.C.; Choi, M.R.; Song, S.H.; Yoo, E.J.; Kim, S.H.; Miyashiro, H.; Hattori, M. Anti-HIV protease activity from rosa family plant extracts and rosamultin from Rosa rugosa. J. Med. Food. 2005, 8, 107–109. [Google Scholar] [CrossRef]
- Liu, X.; Su, M.; Wu, S.; Li, H. Clone of Amyrin Synthase Gene Conservative District from Eriobotrya japonica ‘Jie Fang Zhong’. Subtrop. Plant Sci. 2013, 42, 1–4. (In Chinese) [Google Scholar]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharm. Res. 2021, 164, 105373. [Google Scholar] [CrossRef] [PubMed]
- Guhling, O.; Hobl, B.; Yeats, T.; Jetter, R. Cloning and characterization of a lupeol synthase involved in the synthesis of epicuticular wax crystals on stem and hypocotyl surfaces of Ricinus communis. Arch. Biochem. Biophys. 2006, 448, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rasool, A.; Liu, H.; Lv, B.; Chang, P.; Song, H.; Wang, Y.; Li, C. Engineering Saccharomyces Cerevisiae for High Yield Production of A-Amyrin Via Synergistic Remodeling of A-Amyrin Synthase and Expanding the Storage Pool. Metab. Eng. 2020, 62, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, S.; Zhang, X. Antisense Suppression of Cycloartenol Synthase Results in Elevated Ginsenoside Levels in Panax Ginseng Hairy Roots. Plant Mol. Biol. Rep. 2009, 27, 298–304. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing Aglycons of Ginsenosides in Bakers’ Yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; C. Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. Expasy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. Mafft Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, P.; Xu, M.; Chen, Y.; Feng, L. Systematic Identification and Analysis of OSC Gene Family of Rosa rugosa Thunb. Int. J. Mol. Sci. 2022, 23, 13884. https://doi.org/10.3390/ijms232213884
Wang J, Wang P, Xu M, Chen Y, Feng L. Systematic Identification and Analysis of OSC Gene Family of Rosa rugosa Thunb. International Journal of Molecular Sciences. 2022; 23(22):13884. https://doi.org/10.3390/ijms232213884
Chicago/Turabian StyleWang, Jianwen, Pengqing Wang, Mengmeng Xu, Yudie Chen, and Liguo Feng. 2022. "Systematic Identification and Analysis of OSC Gene Family of Rosa rugosa Thunb" International Journal of Molecular Sciences 23, no. 22: 13884. https://doi.org/10.3390/ijms232213884




