Amyloidogenesis: What Do We Know So Far?
Abstract
:1. Introduction
2. Single Amino Acid Self-Assemblies
3. The History of Amyloid Formation
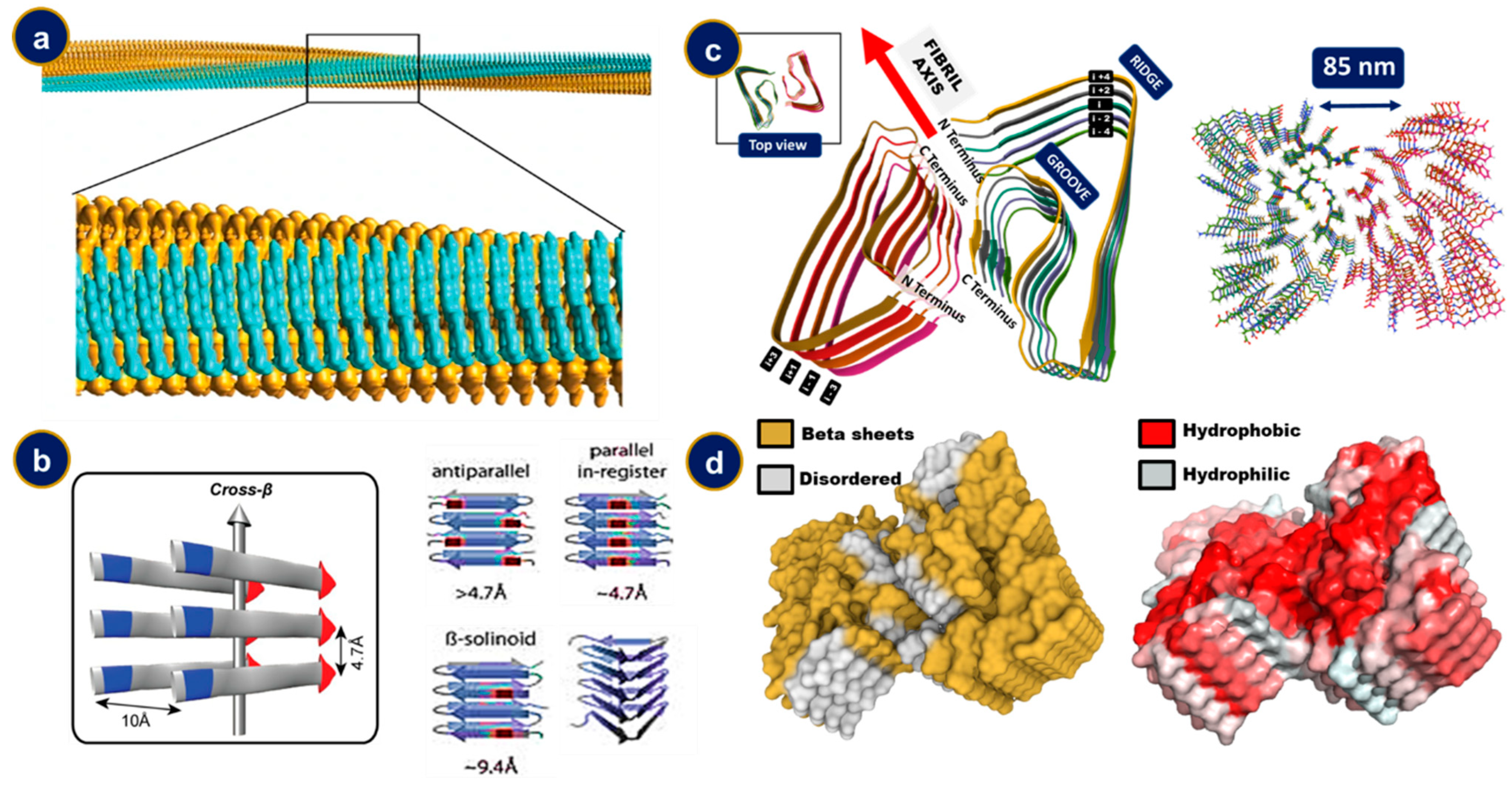
4. The Structural Features of Amyloid Fibrils
5. Characterization of Amyloid Fibril Formation

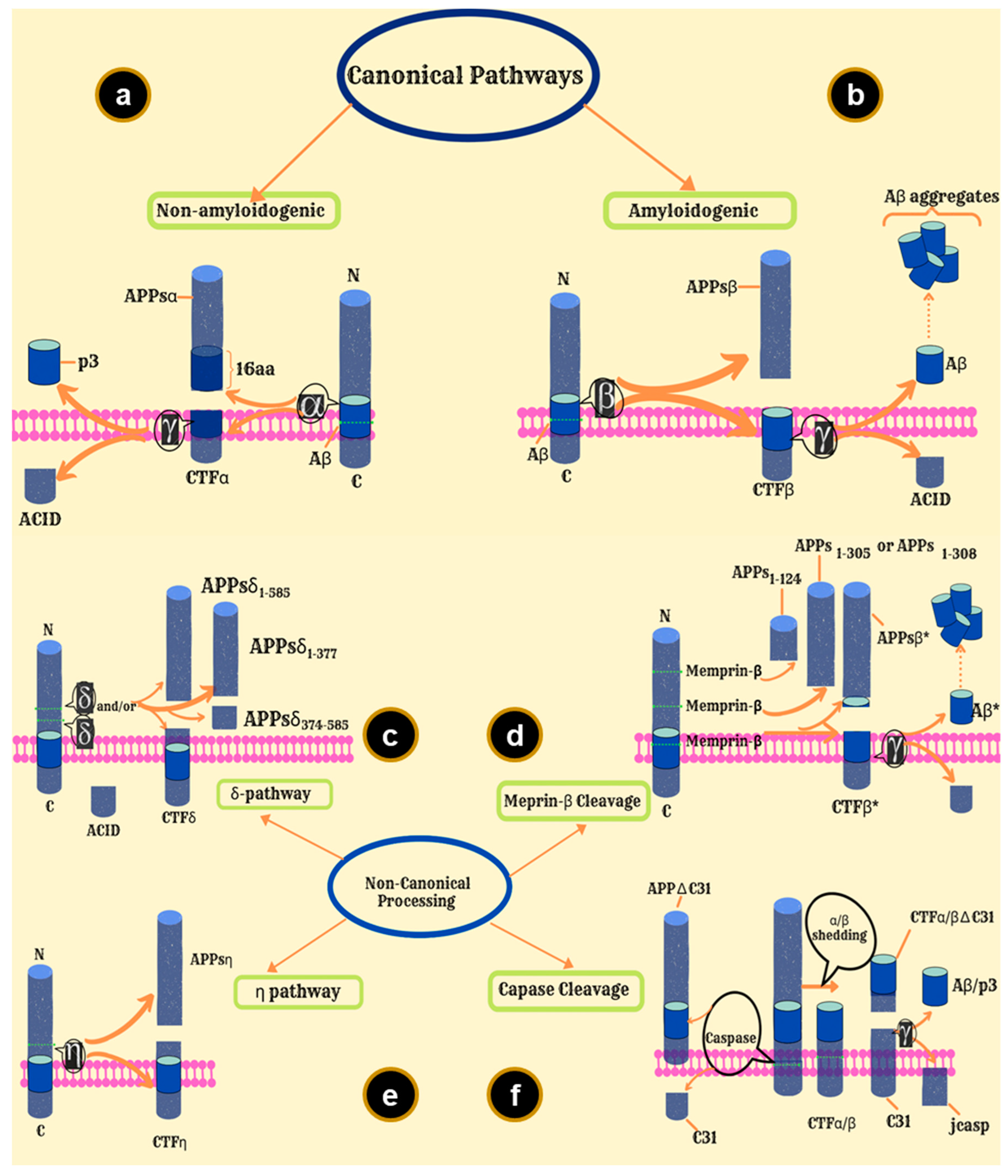
6. Amyloid Precursor Protein and Its Involvement in Amyloid Formation
7. APP Processing Pathways
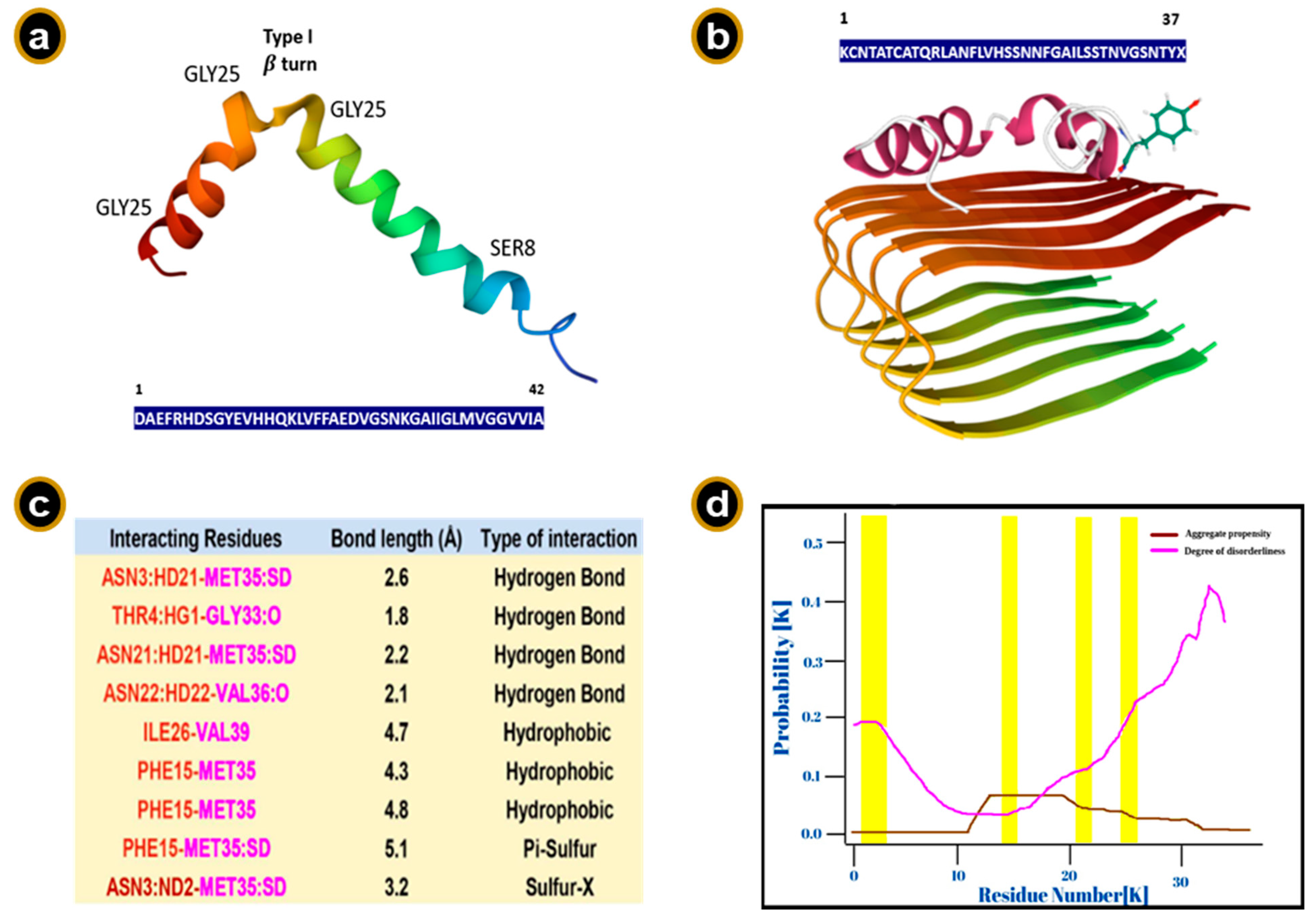
8. The Aβ Peptide
9. Sequence Requirements
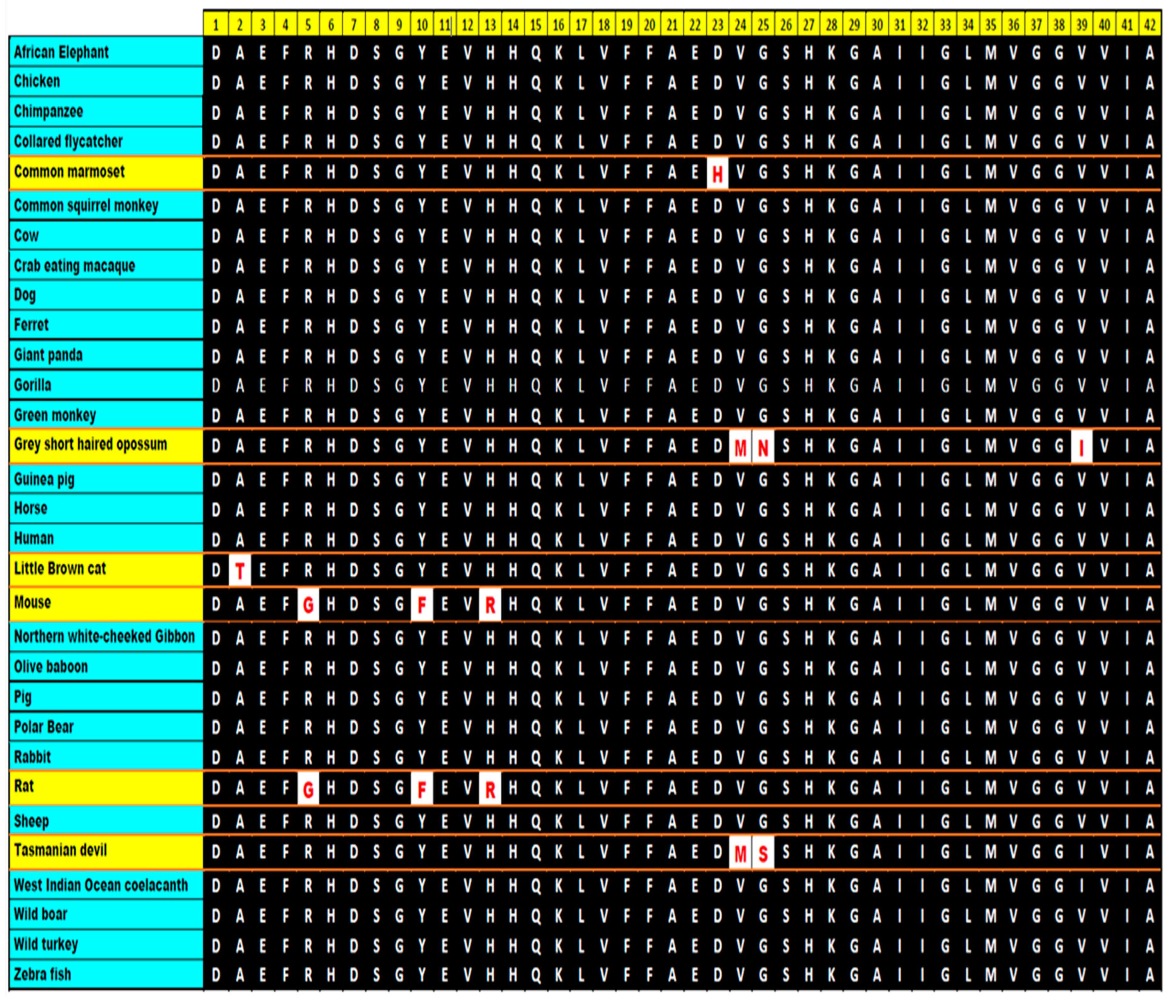
10. Conservation of the Sequence
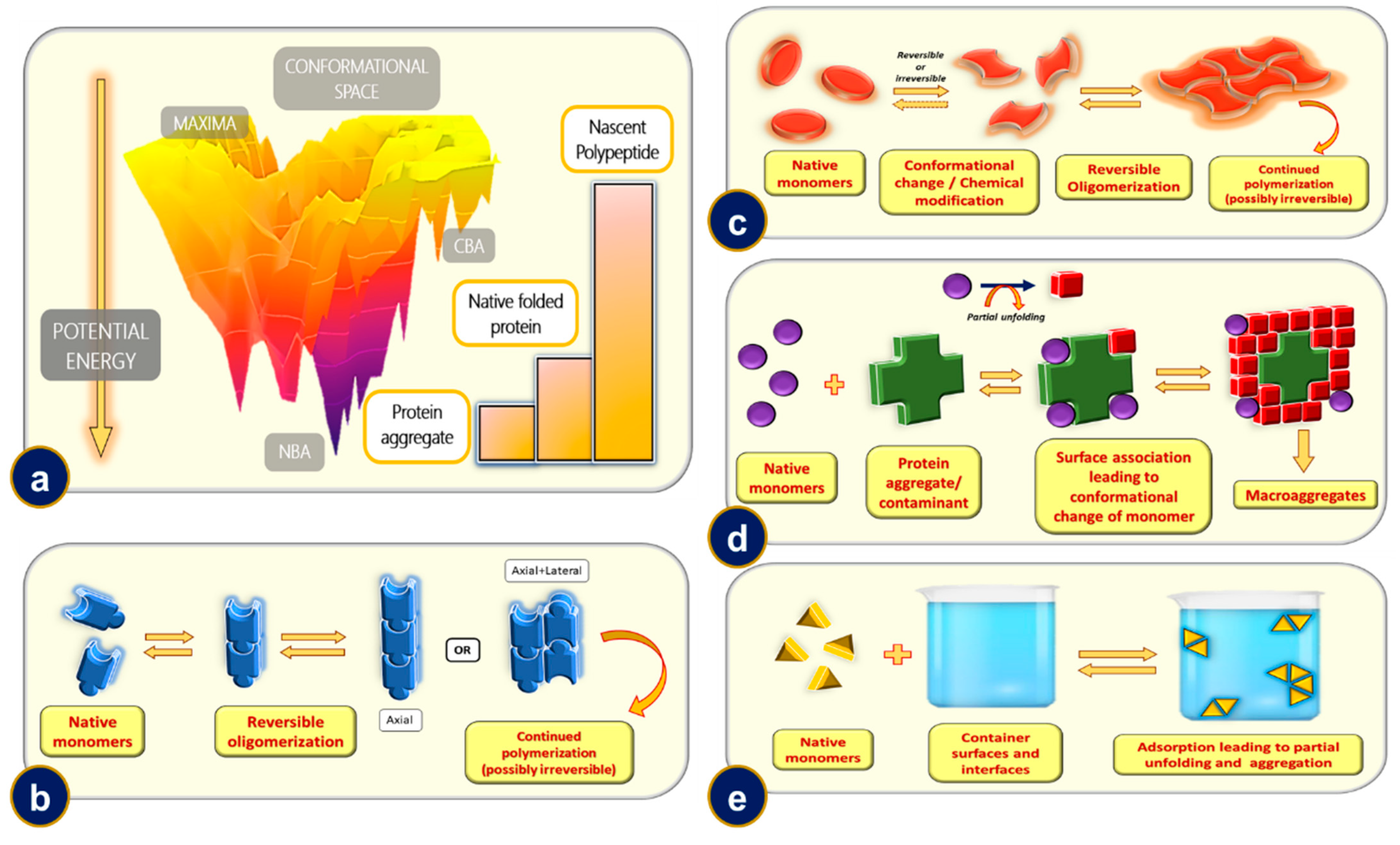
11. Protein Aggregation and Its Mechanisms
11.1. Mechanism-1: Reversible Oligomerization of Native Monomers
11.2. Mechanism-2: Aggregation of Conformationally/Chemically Modified Monomers
11.3. Mechanism-3: Microaggregate and/or Contaminant Induced Aggregation
11.4. Mechanism-4: Phase Interface or Rough Surface Induced Aggregation
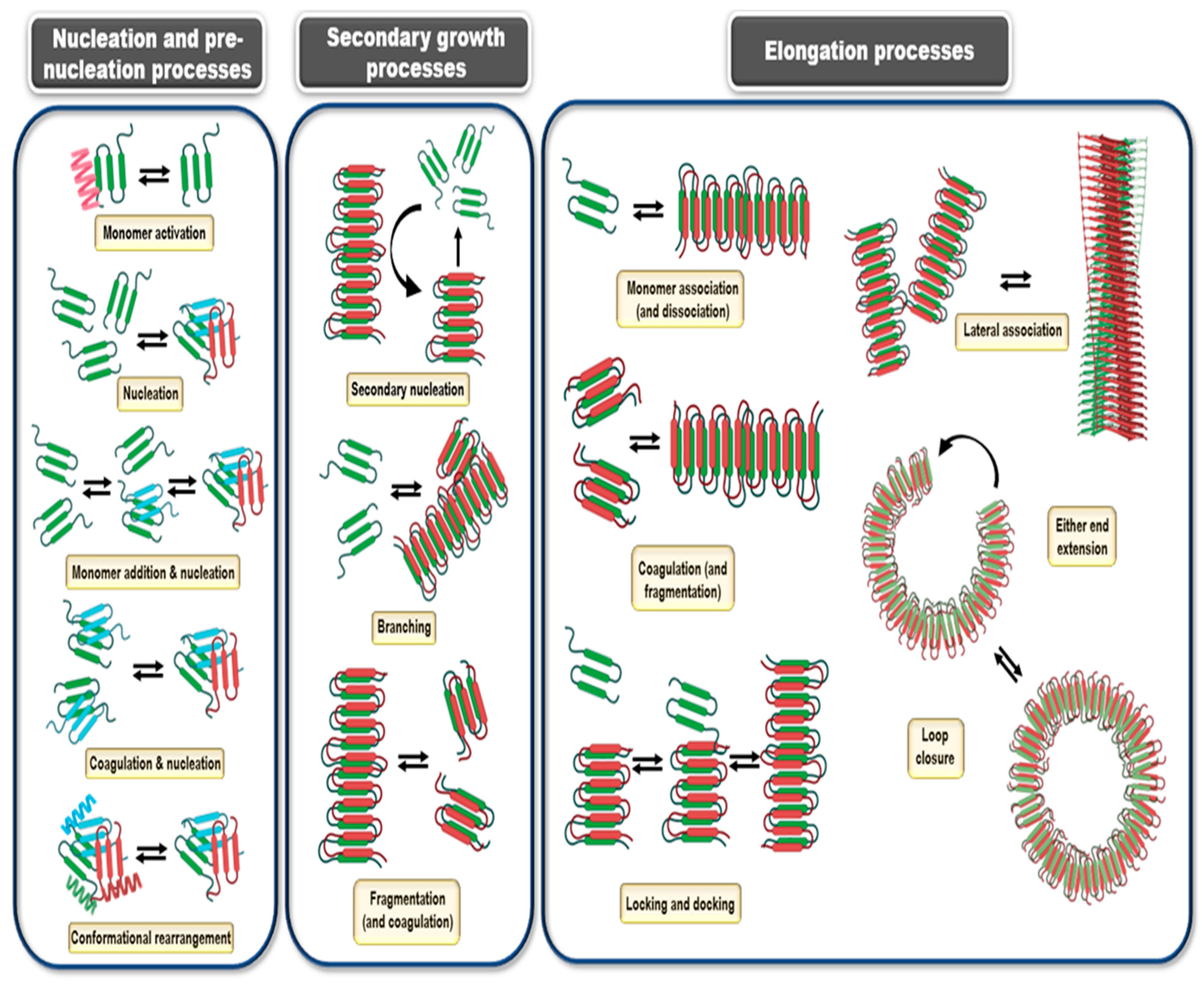
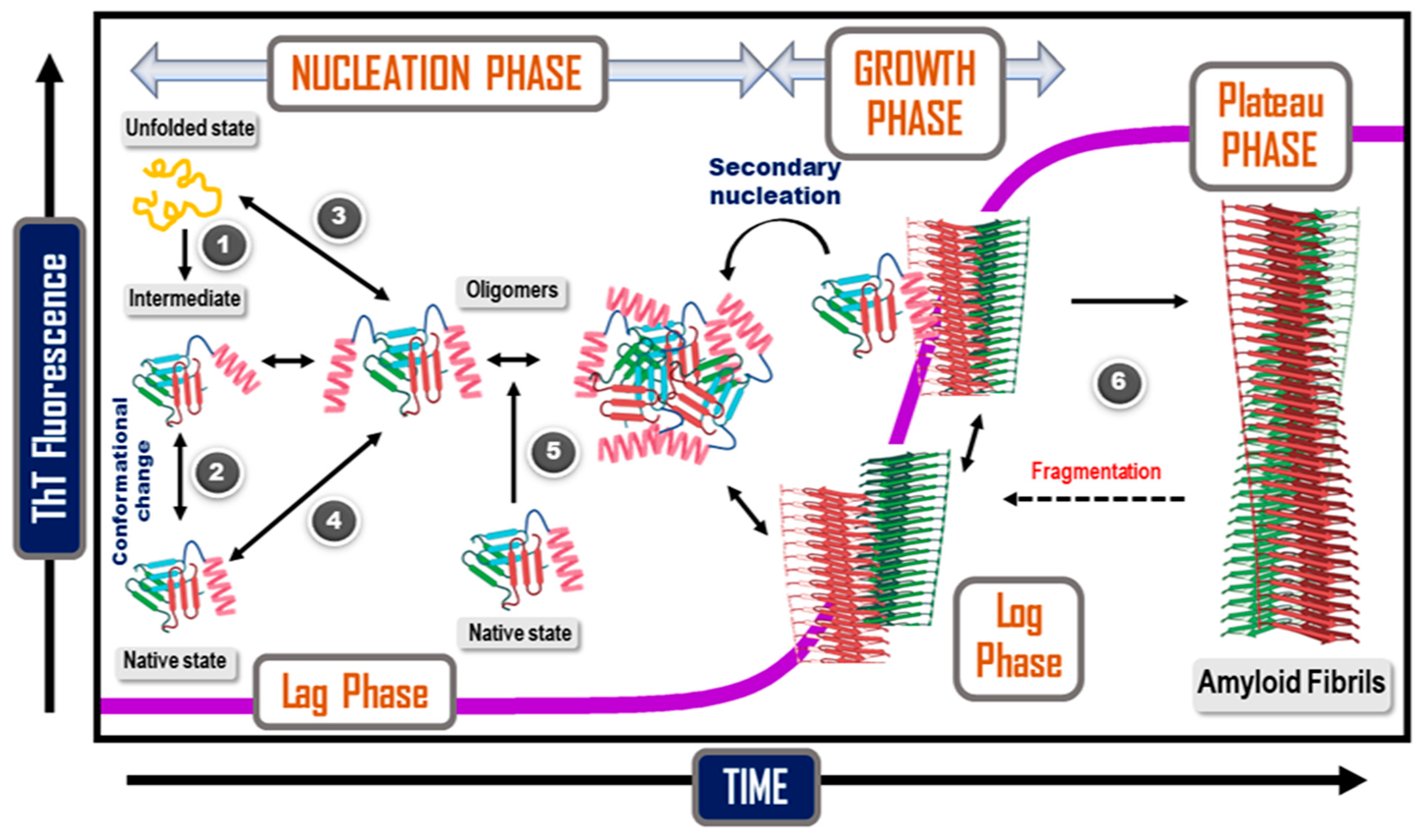
12. Kinetics and Mechanisms of Amyloid Formation
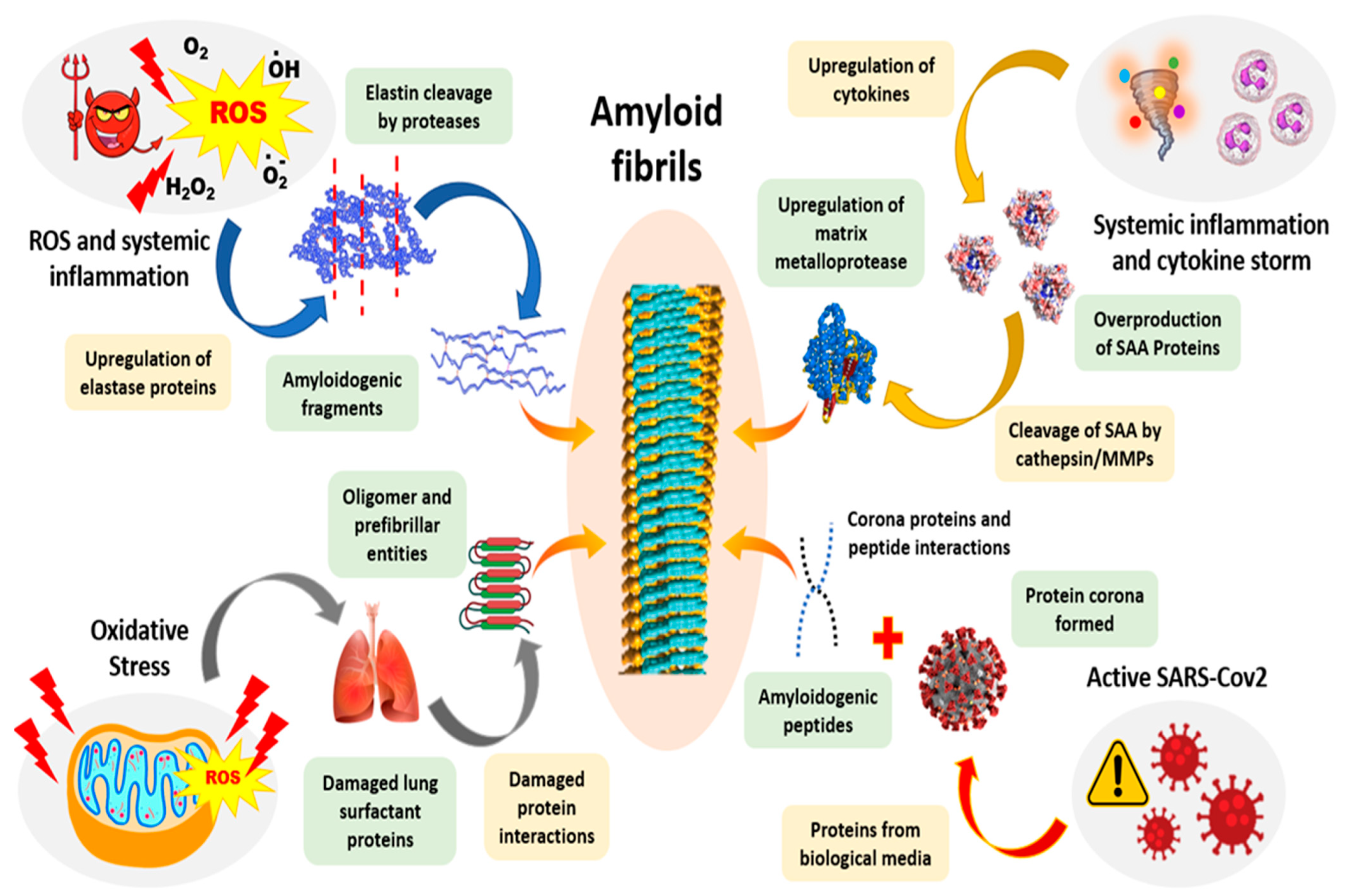
13. The Clinical Effects of Amyloid Formation
14. Prion
15. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Gallego, J.M.; de Parga, A.L.V.; Martin, N.; Miranda, R. Molecular Self-Assembly at Solid Surfaces. Adv. Mater. 2011, 23, 5148–5176. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-assembly of hyperbranched polymers and its biomedical applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Bromley, K.M.; Moradian-Oldak, J.; DeYoreo, J.J. In situ AFM study of amelogenin assembly and disassembly dynamics on charged surfaces provides insights on matrix protein self-assembly. J. Am. Chem. Soc. 2011, 133, 17406–17413. [Google Scholar] [CrossRef] [Green Version]
- Agheli, H.; Malmström, J.; Larsson, E.M.; Textor, M.; Sutherland, D.S. Large area protein nanopatterning for biological applications. Nano Lett. 2006, 6, 1165–1171. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Molecular self-assembly of peptide nanostructures: Mechanism of association and potential uses. Curr. Nanosci. 2006, 2, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Kumaraswamy, P.; Sethuraman, S.; Krishnan, U.M. Hierarchical self-assembly of Tjernberg peptide at nanoscale. Soft Matter 2013, 9, 2684–2694. [Google Scholar] [CrossRef]
- Lesk, A.M.; Rose, G.D. Folding units in globular proteins. Proc. Natl. Acad. Sci. USA 1981, 78, 4304–4308. [Google Scholar] [CrossRef]
- Weber, J.K.; Pande, V.S. Protein folding is mechanistically robust. Biophys. J. 2012, 102, 859–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale self-assembly for therapeutic delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haburcak, R.; Shi, J.; Du, X.; Yuan, D.; Xu, B. Ligand–receptor interaction modulates the energy landscape of enzyme-instructed self-assembly of small molecules. J. Am. Chem. Soc. 2016, 138, 15397–15404. [Google Scholar] [CrossRef] [PubMed]
- Kar, K.; Ibrar, S.; Nanda, V.; Getz, T.M.; Kunapuli, S.P.; Brodsky, B. Aromatic interactions promote self-association of collagen triple-helical peptides to higher-order structures. Biochemistry 2009, 48, 7959–7968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaham-Niv, S.; Rehak, P.; Vuković, L.; Adler-Abramovich, L.; Král, P.; Gazit, E. Formation of apoptosis-inducing amyloid fibrils by tryptophan. Isr. J. Chem. 2017, 57, 729–737. [Google Scholar] [CrossRef]
- Koshti, B.; Ramesh, S.; Kshtriya, V.; Walia, S.; Bhatia, D.; Gour, N. Amyloid like aggregates formed by the self-assembly of proline and Hydroxyproline. Biol. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Koshti, B.; Kshtriya, V.; Singh, R.; Walia, S.; Bhatia, D.; Joshi, K.B.; Gour, N. Unusual Aggregates Formed by the Self-Assembly of Proline, Hydroxyproline, and Lysine. ACS Chem. Neurosci. 2021, 12, 3237–3249. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef]
- Makwana, K.M.; Mahalakshmi, R. Implications of aromatic–aromatic interactions: From protein structures to peptide models. Protein Sci. 2015, 24, 1920–1933. [Google Scholar] [CrossRef] [Green Version]
- Uversky, V.N. The alphabet of intrinsic disorder: II. Various roles of glutamic acid in ordered and intrinsically disordered proteins. Intrinsically Disord. Proteins 2013, 1, e24684. [Google Scholar] [CrossRef]
- Wei, G.; Su, Z.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-assembling peptide and protein amyloids: From structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.H.; Chen, J.; McKoy, A.F.; Hecht, M.H. Mutations that replace aromatic side chains promote aggregation of the Alzheimer’s Aβ peptide. Biochemistry 2011, 50, 4058–4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancalana, M.; Makabe, K.; Koide, A.; Koide, S. Aromatic cross-strand ladders control the structure and stability of β-rich peptide self-assembly mimics. J. Mol. Biol. 2008, 383, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaham-Niv, S.; Adler-Abramovich, L.; Schnaider, L.; Gazit, E. Extension of the generic amyloid hypothesis to nonproteinaceous metabolite assemblies. Sci. Adv. 2015, 1, e1500137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagorski, M.G.; Barrow, C.J. NMR studies of amyloid. beta.-peptides: Proton assignments, secondary structure, and mechanism of an. alpha.-helix. fwdarw.. beta.-sheet conversion for a homologous, 28-residue, N-terminal fragment. Biochemistry 1992, 31, 5621–5631. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.-H.; Raleigh, D.P. Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry 2013, 52, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Koshti, B.; Shah, D.; Kshatriya, V.S.; Agrawal-Rajput, R.; Pandey, M. Single amino acid based self-assemblies of Cysteine and Methionine. ACS Chem. Neurosci. 2018, 10. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Vaks, L.; Carny, O.; Trudler, D.; Magno, A.; Caflisch, A.; Frenkel, D.; Gazit, E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 2012, 8, 701–706. [Google Scholar] [CrossRef]
- Liu, G.; Gaines, J.C.; Robbins, K.J.; Lazo, N.D. Kinetic profile of amyloid formation in the presence of an aromatic inhibitor by nuclear magnetic resonance. ACS Med. Chem. Lett. 2012, 3, 856–859. [Google Scholar] [CrossRef] [Green Version]
- Tanskanen, M. “Amyloid”—Historical Aspects. In Amyloidosis; IntechOpen: London, UK, 2013; p. 1. [Google Scholar]
- Kyle, R.A. Amyloidosis: A convoluted story. Br. J. Haematol. 2001, 114, 529–538. [Google Scholar] [CrossRef]
- Nishitsuji, K.; Saito, H.; Uchimura, K. Enzymatic remodeling of heparan sulfate: A therapeutic strategy for systemic and localized amyloidoses? Neural Regen. Res. 2016, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Prion hypothesis: The end of the controversy? Trends Biochem. Sci. 2011, 36, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabel, M.D.; Reid, C. A brief history of prions. Pathog. Dis. 2015, 73, ftv087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, C.G. The history of Parkinson’s disease: Early clinical descriptions and neurological therapies. Cold Spring Harb. Perspect. Med. 2011, 1, a008862. [Google Scholar] [CrossRef] [Green Version]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [Green Version]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39, BSR20181415. [Google Scholar] [CrossRef] [Green Version]
- Howie, A.J.; Owen-Casey, M.P. Discrepancies between descriptions and illustrations of colours in Congo red-stained amyloid, and explanation of discrepant colours. Amyloid 2010, 17, 109–117. [Google Scholar] [CrossRef]
- Linke, R.P. Congo red staining of amyloid: Improvements and practical guide for a more precise diagnosis of amyloid and the different amyloidoses. In Protein Misfolding, Aggregation, and Conformational Diseases; Springer: Berlin/Heidelberg, Germany, 2006; pp. 239–276. [Google Scholar]
- Toyama, B.H.; Weissman, J.S. Amyloid Structure: Conformational Diversity and Consequences. Annu. Rev. Biochem. 2011, 80, 557–585. [Google Scholar] [CrossRef] [Green Version]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C.F. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Kollmer, M.; Meinhardt, K.; Haupt, C.; Liberta, F.; Wulff, M.; Linder, J.; Handl, L.; Heinrich, L.; Loos, C.; Schmidt, M.; et al. Electron tomography reveals the fibril structure and lipid interactions in amyloid deposits. Proc. Natl. Acad. Sci. USA 2016, 113, 5604–5609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorkovskiy, A.; Thurber, K.R.; Tycko, R.; Wickner, R.B. Locating folds of the in-register parallel β-sheet of the Sup35p prion domain infectious amyloid. Proc. Natl. Acad. Sci. USA 2014, 111, E4615–E4622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.R.; Makin, O.S.; Morris, K.L.; Marshall, K.E.; Tian, P.; Sikorski, P.; Serpell, L.C. The common architecture of cross-β amyloid. J. Mol. Biol. 2010, 395, 717–727. [Google Scholar] [CrossRef] [PubMed]
- DeForte, S.; Uversky, V.N. Order, disorder, and everything in between. Molecules 2016, 21, 1090. [Google Scholar] [CrossRef] [Green Version]
- Van Der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Xu, S.H. Cross-beta-Sheet Structure in Amyloid Fiber Formation. J. Phys. Chem. B 2009, 113, 12447–12455. [Google Scholar] [CrossRef]
- Schmidt, M.; Wiese, S.; Adak, V.; Engler, J.; Agarwal, S.; Fritz, G.; Westermark, P.; Zacharias, M.; Fändrich, M. Cryo-EM structure of a transthyretin-derived amyloid fibril from a patient with hereditary ATTR amyloidosis. Nat. Commun. 2019, 10, 5008. [Google Scholar] [CrossRef]
- Smith, J.E.; Mowles, A.K.; Mehta, A.K.; Lynn, D.G. Looked at life from both sides now. Life 2014, 4, 887–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, G.; Lee, M.; Kim, J.I.; Na, S.; Eom, K. Role of sequence and structural polymorphism on the mechanical properties of amyloid fibrils. PLoS ONE 2014, 9, e88502. [Google Scholar] [CrossRef] [PubMed]
- Makin, O.S.; Atkins, E.; Sikorski, P.; Johansson, J.; Serpell, L.C. Molecular basis for amyloid fibril formation and stability. Proc. Natl. Acad. Sci. USA 2005, 102, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancalana, M.; Makabe, K.; Koide, S. Minimalist design of water-soluble cross-β architecture. Proc. Natl. Acad. Sci. USA 2010, 107, 3469–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pozo-Yauner, L.; Becerril, B.; Ochoa-Leyva, A.; Rodríguez-Ambriz, S.L.; Carrión, J.I.P.; Zavala-Padilla, G.; Sánchez-López, R.; Velasco, D.A.F. The structural determinants of the immunoglobulin light chain amyloid aggregation. In Physical Biology of Proteins and Peptides; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–28. [Google Scholar]
- Maity, B.K.; Das, A.K.; Dey, S.; Moorthi, U.K.; Kaur, A.; Dey, A.; Surendran, D.; Pandit, R.; Kallianpur, M.; Chandra, B.; et al. Ordered and Disordered Segments of Amyloid-β Drive Sequential Steps of the Toxic Pathway. ACS Chem. Neurosci. 2019, 10, 2498–2509. [Google Scholar] [CrossRef]
- Schmidt, A.; Annamalai, K.; Schmidt, M.; Grigorieff, N.; Fändrich, M. Cryo-EM reveals the steric zipper structure of a light chain-derived amyloid fibril. Proc. Natl. Acad. Sci. USA 2016, 113, 6200–6205. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Lee, W.; Lee, H.; Woo Lee, S.; Sung Yoon, D.; Eom, K.; Kwon, T. Mapping the surface charge distribution of amyloid fibril. Appl. Phys. Lett. 2012, 101, 043703. [Google Scholar] [CrossRef]
- Bhak, G.; Lee, J.-H.; Hahn, J.-S.; Paik, S.R. Granular assembly of α-synuclein leading to the accelerated amyloid fibril formation with shear stress. PLoS ONE 2009, 4, e4177. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Bhak, G.; Lee, S.-G.; Paik, S.R. Instantaneous amyloid fibril formation of α-synuclein from the oligomeric granular structures in the presence of hexane. Biophys. J. 2008, 95, L16–L18. [Google Scholar] [CrossRef] [Green Version]
- Ha, C.; Park, C.B. Template-directed self-assembly and growth of insulin amyloid fibrils. Biotechnol. Bioeng. 2005, 90, 848–855. [Google Scholar] [CrossRef]
- Wu, J.W.; Breydo, L.; Isas, J.M.; Lee, J.; Kuznetsov, Y.G.; Langen, R.; Glabe, C. Fibrillar oligomers nucleate the oligomerization of monomeric amyloid β but do not seed fibril formation. J. Biol. Chem. 2010, 285, 6071–6079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayed, R.; Pensalfini, A.; Margol, L.; Sokolov, Y.; Sarsoza, F.; Head, E.; Hall, J.; Glabe, C. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J. Biol. Chem. 2009, 284, 4230–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado, I.; Wieligmann, K.; Bereza, M.; Rönicke, R.; Meinhardt, K.; Annamalai, K.; Baumann, M.; Wacker, J.; Hortschansky, P.; Malešević, M. Molecular basis of β-amyloid oligomer recognition with a conformational antibody fragment. Proc. Natl. Acad. Sci. USA 2012, 109, 12503–12508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Anderson, D.H.; Liang, W.Y.; Chou, J.; Saelices, L. The inhibition of cellular toxicity of amyloid-β by dissociated transthyretin. J. Biol. Chem. 2020, 295, 14015–14024. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Et Biophys. Acta-Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Levine Iii, H. Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, A.I.; Kumar, T.K.S.; Kathir, K.M.; Srisailam, S.; Wang, H.M.; Leena, P.S.T.; Chi, Y.H.; Chen, H.C.; Wu, C.H.; Wu, R.T. Oligomerization of acidic fibroblast growth factor is not a prerequisite for its cell proliferation activity. Protein Sci. 2002, 11, 1050–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srisailam, S.; Kumar, T.K.S.; Rajalingam, D.; Kathir, K.M.; Sheu, H.-S.; Jan, F.-J.; Chao, P.-C.; Yu, C. Amyloid-like Fibril Formation in an All β-Barrel Protein PARTIALLY STRUCTURED INTERMEDIATE STATE (S) IS A PRECURSOR FOR FIBRIL FORMATION. J. Biol. Chem. 2003, 278, 17701–17709. [Google Scholar] [CrossRef] [Green Version]
- Maezawa, I.; Hong, H.S.; Liu, R.; Wu, C.Y.; Cheng, R.H.; Kung, M.P.; Kung, H.F.; Lam, K.S.; Oddo, S.; LaFerla, F.M. Congo red and thioflavin-T analogs detect Aβ oligomers. J. Neurochem. 2008, 104, 457–468. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Insight into amyloid structure using chemical probes. Chem. Biol. Drug Des. 2011, 77, 399–411. [Google Scholar] [CrossRef]
- Lindgren, M.; Hammarström, P. Amyloid oligomers: Spectroscopic characterization of amyloidogenic protein states. FEBS J. 2010, 277, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Mostaert, A.S.; Higgins, M.J.; Fukuma, T.; Rindi, F.; Jarvis, S.P. Nanoscale mechanical characterisation of amyloid fibrils discovered in a natural adhesive. J. Biol. Phys. 2006, 32, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Hawe, A.; Sutter, M.; Jiskoot, W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm. Res. 2008, 25, 1487–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howie, A.J.; Brewer, D.B. Optical properties of amyloid stained by Congo red: History and mechanisms. Micron 2009, 40, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Şen, S.; Başdemir, G. Diagnosis of renal amyloidosis using Congo red fluorescence. Pathol. Int. 2003, 53, 534–538. [Google Scholar] [CrossRef]
- Setti, S.E.; Raymick, J.; Hanig, J.; Sarkar, S. In vivo demonstration of Congo Red labeled amyloid plaques via perfusion in the Alzheimer disease rat model. J. Neurosci. Methods 2021, 353, 109082. [Google Scholar] [CrossRef]
- Hamley, I.W.; Castelletto, V.; Moulton, C.; Myatt, D.; Siligardi, G.; Oliveira, C.L.P.; Pedersen, J.S.; Abutbul, I.; Danino, D. Self-assembly of a modified amyloid peptide fragment: pH-responsiveness and nematic phase formation. Macromol. Biosci. 2010, 10, 40–48. [Google Scholar] [CrossRef]
- Haris, P.I.; Severcan, F. FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. J. Mol. Catal. B Enzym. 1999, 7, 207–221. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolym. Orig. Res. Biomol. 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Evaluation of the information content in infrared spectra for protein secondary structure determination. Biophys. J. 2006, 90, 2946–2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with β-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 2006, 155, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Choo, L.-P.i.; Wetzel, D.L.; Halliday, W.C.; Jackson, M.; LeVine, S.M.; Mantsch, H.H. In situ characterization of beta-amyloid in Alzheimer’s diseased tissue by synchrotron Fourier transform infrared microspectroscopy. Biophys. J. 1996, 71, 1672–1679. [Google Scholar] [CrossRef] [Green Version]
- Rak, M.; Del Bigio, M.R.; Mai, S.; Westaway, D.; Gough, K. Dense-core and diffuse Aβ plaques in TgCRND8 mice studied with synchrotron FTIR microspectroscopy. Biopolym. Orig. Res. Biomol. 2007, 87, 207–217. [Google Scholar] [CrossRef]
- Li, H.; Rahimi, F.; Sinha, S.; Maiti, P.; Bitan, G.; Murakami, K. Amyloids and protein aggregation–analytical methods. Encycl. Anal. Chem. 2009, 1–32. [Google Scholar] [CrossRef]
- Annamalai, K.; Gührs, K.H.; Koehler, R.; Schmidt, M.; Michel, H.; Loos, C.; Gaffney, P.M.; Sigurdson, C.J.; Hegenbart, U.; Schönland, S. Polymorphism of amyloid fibrils in vivo. Angew. Chem. Int. Ed. 2016, 55, 4822–4825. [Google Scholar] [CrossRef] [Green Version]
- Linke, W.A.; Grützner, A. Pulling single molecules of titin by AFM—Recent advances and physiological implications. Pflügers Arch.-Eur. J. Physiol. 2008, 456, 101–115. [Google Scholar] [CrossRef]
- Li, Q.; Song, J.; Saura-Múzquiz, M.; Besenbacher, F.; Christensen, M.; Dong, M. Magnetic properties of strontium hexaferrite nanostructures measured with magnetic force microscopy. Sci. Rep. 2016, 6, 25985. [Google Scholar] [CrossRef] [Green Version]
- Serem, W.K.; Bett, C.K.; Ngunjiri, J.N.; Garno, J.C. Studies of the growth, evolution, and self-aggregation of β-amyloid fibrils using tapping-mode atomic force microscopy. Microsc. Res. Tech. 2011, 74, 699–708. [Google Scholar] [CrossRef]
- Tycko, R. Characterization of amyloid structures at the molecular level by solid state nuclear magnetic resonance spectroscopy. Methods Enzymol. 2006, 413, 103–122. [Google Scholar]
- Petkova, A.T.; Ishii, Y.; Balbach, J.J.; Antzutkin, O.N.; Leapman, R.D.; Delaglio, F.; Tycko, R. A structural model for Alzheimer’s β-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. USA 2002, 99, 16742–16747. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Takio, K.; Ogawara, M.; Selkoe, D.J. Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J. Biol. Chem. 1992, 267, 17082–17086. [Google Scholar] [CrossRef]
- Nettleton, E.J.; Tito, P.; Sunde, M.; Bouchard, M.; Dobson, C.M.; Robinson, C.V. Characterization of the oligomeric states of insulin in self-assembly and amyloid fibril formation by mass spectrometry. Biophys. J. 2000, 79, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Zandomeneghi, G.; Krebs, M.R.H.; McCammon, M.G.; Fändrich, M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, A.; Yuan, M.; Zhang, Z.; Paganetti, P.A.; Sturchler-Pierrat, C.; Staufenbiel, M.; Mautino, J.; Vigo, F.S.; Sommer, B.; Yankner, B.A. Amyloid β interacts with the amyloid precursor protein: A potential toxic mechanism in Alzheimer’s disease. Nat. Neurosci. 2000, 3, 460–464. [Google Scholar] [CrossRef]
- Zheng, H.; Koo, E.H. Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 2011, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.V. The human β-amyloid precursor protein: Biomolecular and epigenetic aspects. Biomol. Concepts 2015, 6, 11–32. [Google Scholar] [CrossRef] [Green Version]
- Müller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Radzimanowski, J.; Simon, B.; Sattler, M.; Beyreuther, K.; Sinning, I.; Wild, K. Structure of the intracellular domain of the amyloid precursor protein in complex with Fe65-PTB2. EMBO Rep. 2008, 9, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.N.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of Amyloid Precursor Protein (APP) and Its Effects in Cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef]
- Baumkötter, F.; Schmidt, N.; Vargas, C.; Schilling, S.; Weber, R.; Wagner, K.; Fiedler, S.; Klug, W.; Radzimanowski, J.; Nickolaus, S. Amyloid precursor protein dimerization and synaptogenic function depend on copper binding to the growth factor-like domain. J. Neurosci. 2014, 34, 11159–11172. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Yu, C.-E.; Bird, T.D.; Tsuang, D. The Genetics of Alzheimer’s Disease and Parkinson’s Disease. Neurochem. Mech. Dis. 2011, 695–755. [Google Scholar] [CrossRef]
- Julia, T.C.W.; Goate, A.M. Genetics of β-amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb. Perspect. Med. 2017, 7, a024539. [Google Scholar]
- Chow, V.W.; Mattson, M.P.; Wong, P.C.; Gleichmann, M. An overview of APP processing enzymes and products. Neuromol. Med. 2010, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Song, W. The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimer Res. Ther. 2013, 5, 46. [Google Scholar] [CrossRef]
- Hussain, I.; Powell, D.J.; Howlett, D.R.; Chapman, G.A.; Gilmour, L.; Murdock, P.R.; Tew, D.G.; Meek, T.D.; Chapman, C.; Schneider, K. ASP1 (BACE2) cleaves the amyloid precursor protein at the β-secretase site. Mol. Cell. Neurosci. 2000, 16, 609–619. [Google Scholar] [CrossRef]
- Farzan, M.; Schnitzler, C.E.; Vasilieva, N.; Leung, D.; Choe, H. BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc. Natl. Acad. Sci. USA 2000, 97, 9712–9717. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Xu, X. γ-Secretase catalyzes sequential cleavages of the AβPP transmembrane domain. J. Alzheimer Dis. 2009, 16, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Steiner, H.; Fukumori, A.; Tagami, S.; Okochi, M. Making the final cut: Pathogenic amyloid-β peptide generation by γ-secretase. Cell Stress 2018, 2, 292. [Google Scholar] [CrossRef] [Green Version]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Coronel, R.; Palmer, C.; Bernabeu-Zornoza, A.; Monteagudo, M.; Rosca, A.; Zambrano, A.; Liste, I. Physiological effects of amyloid precursor protein and its derivatives on neural stem cell biology and signaling pathways involved. Neural Regen. Res. 2019, 14, 1661. [Google Scholar] [PubMed]
- Willem, M.; Tahirovic, S.; Busche, M.A.; Ovsepian, S.V.; Chafai, M.; Kootar, S.; Hornburg, D.; Evans, L.D.B.; Moore, S.; Daria, A. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 2015, 526, 443–447. [Google Scholar] [CrossRef]
- Reinhard, C.; Hébert, S.S.; De Strooper, B. The amyloid-β precursor protein: Integrating structure with biological function. EMBO J. 2005, 24, 3996–4006. [Google Scholar] [CrossRef] [Green Version]
- Becker-Pauly, C.; Pietrzik, C.U. The metalloprotease meprin β is an alternative β-secretase of APP. Front. Mol. Neurosci. 2017, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, T.; Čaušević, M.; Auf dem Keller, U.; Schilling, O.; Isbert, S.; Geyer, R.; Maier, W.; Tschickardt, S.; Jumpertz, T.; Weggen, S. Metalloprotease meprin β generates nontoxic N-terminal amyloid precursor protein fragments in vivo. J. Biol. Chem. 2011, 286, 27741–27750. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.A.; Biette, K.M.; Dolios, G.; Seth, D.; Wang, R.; Wolfe, M.S. Transmembrane substrate determinants for γ-secretase processing of APP CTFβ. Biochemistry 2016, 55, 5675–5688. [Google Scholar] [CrossRef] [Green Version]
- Banwait, S.; Galvan, V.; Zhang, J.; Gorostiza, O.F.; Ataie, M.; Huang, W.; Crippen, D.; Koo, E.H.; Bredesen, D.E. C-terminal cleavage of the amyloid-β protein precursor at Asp664: A switch associated with Alzheimer’s disease. J. Alzheimer Dis. 2008, 13, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Q.; Zhang, Y.W.; Xu, H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. REVIEW 2012, 120, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Nhan, H.S.; Chiang, K.; Koo, E.H. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: Friends and foes. Acta Neuropathol. 2015, 129, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggert, S.; Thomas, C.; Kins, S.; Hermey, G. Trafficking in Alzheimer’s disease: Modulation of APP transport and processing by the transmembrane proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin. Mol. Neurobiol. 2018, 55, 5809–5829. [Google Scholar] [CrossRef]
- Haskins, J.W.; Nguyen, D.X.; Stern, D.F. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal. 2014, 7, ra116. [Google Scholar] [CrossRef] [Green Version]
- Seubert, P.; Vigo-Pelfrey, C.; Esch, F.; Lee, M.; Dovey, H.; Davis, D.; Sinha, S.; Schiossmacher, M.; Whaley, J.; Swindlehurst, C. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature 1992, 359, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, F.; Schmidt, S.; Althoff, V.; Munter, L.M.; Jin, S.; Rosqvist, S.; Lendahl, U.; Multhaup, G.; Lundkvist, J. Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions. J Biol Chem 2014, 289, 1540–1550. [Google Scholar] [CrossRef] [Green Version]
- Takami, M.; Nagashima, Y.; Sano, Y.; Ishihara, S.; Morishima-Kawashima, M.; Funamoto, S.; Ihara, Y. γ-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J. Neurosci. 2009, 29, 13042–13052. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Wirthensohn, D.C.; Flagmeier, P.; Hughes, C.; Aprile, F.A.; Ruggeri, F.S.; Whiten, D.R.; Emin, D.; Xia, Z.; Varela, J.A. Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms. Nat. Commun. 2019, 10, 1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Wang, C. Aβ42 is more rigid than Aβ40 at the C terminus: Implications for Aβ aggregation and toxicity. J. Mol. Biol. 2006, 364, 853–862. [Google Scholar] [CrossRef]
- Kim, W.; Hecht, M.H. Sequence determinants of enhanced amyloidogenicity of Alzheimer Aβ42 peptide relative to Aβ40. J. Biol. Chem. 2005, 280, 35069–35076. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.G.; Forman, M.S.; Sung, J.C.; Leight, S.; Kolson, D.L.; Iwatsubo, T.; Lee, V.M.Y.; Doms, R.W. Alzheimer’s Aβ(1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat. Med. 1997, 3, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.P.; Tsai, J.; Gouras, G.K.; Hai, B.; Thinakaran, G.; Checler, F.; Sisodia, S.S.; Greengard, P.; Xu, H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer β-amyloid peptides. Proc. Natl. Acad. Sci. USA 1999, 96, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Marr, R.A.; Guan, H.; Rockenstein, E.; Kindy, M.; Gage, F.H.; Verma, I.; Masliah, E.; Hersh, L.B. Neprilysin regulates amyloid β peptide levels. J. Mol. Neurosci. 2004, 22, 5–11. [Google Scholar] [CrossRef]
- Eckman, E.A.; Reed, D.K.; Eckman, C.B. Degradation of the Alzheimer’s amyloid β peptide by endothelin-converting enzyme. J. Biol. Chem. 2001, 276, 24540–24548. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.Q.; Walsh, D.M.; Ye, Z.; Vekrellis, K.; Zhang, J.; Podlisny, M.B.; Rosner, M.R.; Safavi, A.; Hersh, L.B.; Selkoe, D.J. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 1998, 273, 32730–32738. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.-C.; Van Nostrand, W.E. Degradation of soluble and fibrillar amyloid β-protein by matrix metalloproteinase (MT1-MMP) in vitro. Biochemistry 2010, 49, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Oberstein, T.J.; Utz, J.; Spitzer, P.; Klafki, H.W.; Wiltfang, J.; Lewczuk, P.; Kornhuber, J.; Maler, J.M. The role of Cathepsin B in the degradation of Aβ and in the production of Aβ peptides starting with Ala2 in cultured astrocytes. Front. Mol. Neurosci. 2021, 13, 615740. [Google Scholar] [CrossRef]
- Tucker, H.M.; Kihiko, M.; Caldwell, J.N.; Wright, S.; Kawarabayashi, T.; Price, D.; Walker, D.; Scheff, S.; McGillis, J.P.; Rydel, R.E. The plasmin system is induced by and degrades amyloid-β aggregates. J. Neurosci. 2000, 20, 3937–3946. [Google Scholar] [CrossRef] [Green Version]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-β peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2009, 8, 16–30. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Yamada, K.; Hashimoto, T.; Yabuki, C.; Nagae, Y.; Tachikawa, M.; Strickland, D.K.; Liu, Q.; Bu, G.; Basak, J.M.; Holtzman, D.M. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid β peptides in an in vitro model of the blood-brain barrier cells. J. Biol. Chem. 2008, 283, 34554–34562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, Z.A.; Larini, L.; LaPointe, N.E.; Feinstein, S.C.; Shea, J.-E. Regulation and aggregation of intrinsically disordered peptides. Proc. Natl. Acad. Sci. USA 2015, 112, 2758–2763. [Google Scholar] [CrossRef]
- Wei, G.; Shea, J.-E. Effects of solvent on the structure of the Alzheimer amyloid-β (25–35) peptide. Biophys. J. 2006, 91, 1638–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, K. Amyloid β conformation in aqueous environment. Curr. Alzheimer Res. 2008, 5, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Sgourakis, N.G.; Merced-Serrano, M.; Boutsidis, C.; Drineas, P.; Du, Z.; Wang, C.; Garcia, A.E. Atomic-level characterization of the ensemble of the Aβ(1-42) monomer in water using unbiased molecular dynamics simulations and spectral algorithms. J. Mol. Biol. 2011, 405, 570–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Das, P. Emergence of alternative structures in amyloid beta 1-42 monomeric landscape by N-terminal hexapeptide amyloid inhibitors. Sci. Rep. 2017, 7, 9941. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-W.; Chang, C.-F.; Chang, Y.-J.; Liao, Y.-H.; Yu, H.-M.; Chen, Y.-R. Alzheimer’s amyloid-β A2T variant and its N-terminal peptides inhibit amyloid-β fibrillization and rescue the induced cytotoxicity. PLoS ONE 2017, 12, e0174561. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, P.; Prajapati, K.P.; Anand, B.G.; Dubey, K.; Kar, K. Amyloid cross-seeding raises new dimensions to understanding of amyloidogenesis mechanism. Ageing Res. Rev. 2019, 56, 100937. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Edwards Iii, G.; Salvadores, N.; Shahnawaz, M.; Diaz-Espinoza, R.; Soto, C. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol Psychiatry 2017, 22, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Thacker, D.; Sanagavarapu, K.; Frohm, B.; Meisl, G.; Knowles, T.P.J.; Linse, S. The role of fibril structure and surface hydrophobicity in secondary nucleation of amyloid fibrils. Proc. Natl. Acad. Sci. USA 2020, 117, 25272–25283. [Google Scholar] [CrossRef]
- Tjernberg, L.O.; Callaway, D.J.E.; Tjernberg, A.; Hahne, S.; Lilliehöök, C.; Terenius, L.; Thyberg, J.; Nordstedt, C. A Molecular Model of Alzheimer Amyloid β-Peptide Fibril Formation*. J. Biol. Chem. 1999, 274, 12619–12625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbach, J.J.; Ishii, Y.; Antzutkin, O.N.; Leapman, R.D.; Rizzo, N.W.; Dyda, F.; Reed, J.; Tycko, R. Amyloid fibril formation by Aβ16-22, a seven-residue fragment of the Alzheimer’s β-amyloid peptide, and structural characterization by solid state NMR. Biochemistry 2000, 39, 13748–13759. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, J.; Korn, A.; Surendran, D.; Krueger, M.; Scheidt, H.A.; Mote, K.R.; Madhu, P.K.; Maiti, S.; Huster, D. Probing the Influence of Single-Site Mutations in the Central Cross-β Region of Amyloid β (1–40) Peptides. Biomolecules 2021, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Lopes, D.H.J.; Bitan, G. A key role for lysine residues in amyloid β-protein folding, assembly, and toxicity. ACS Chem. Neurosci. 2012, 3, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, S.L.; Wyttenbach, T.; Baumketner, A.; Shea, J.-E.; Bitan, G.; Teplow, D.B.; Bowers, M.T. Amyloid β-protein: Monomer structure and early aggregation states of Aβ42 and its Pro19 alloform. J. Am. Chem. Soc. 2005, 127, 2075–2084. [Google Scholar] [CrossRef]
- Xie, L.; Luo, Y.; Wei, G. Aβ(16–22) Peptides Can Assemble into Ordered β-Barrels and Bilayer β-Sheets, while Substitution of Phenylalanine 19 by Tryptophan Increases the Population of Disordered Aggregates. J. Phys. Chem. B 2013, 117, 10149–10160. [Google Scholar] [CrossRef]
- Tjernberg, L.O.; Näslund, J.; Lindqvist, F.; Johansson, J.; Karlström, A.R.; Thyberg, J.; Terenius, L.; Nordstedt, C. Arrest of-Amyloid Fibril Formation by a Pentapeptide Ligand (∗). J. Biol. Chem. 1996, 271, 8545–8548. [Google Scholar] [CrossRef] [Green Version]
- Gazit, E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef]
- Davis, J.; Van Nostrand, W.E. Enhanced pathologic properties of Dutch-type mutant amyloid beta-protein. Proc. Natl. Acad. Sci. USA 1996, 93, 2996–3000. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.; Kim, N.H.; Jin, G.Y.; Kim, Y.S.; Kim, Y.H.; Eom, K. Sequence-dependent aggregation-prone conformations of islet amyloid polypeptide. Phys. Chem. Chem. Phys. 2021, 23, 22532–22542. [Google Scholar] [CrossRef]
- Baumketner, A.; Krone, M.G.; Shea, J.-E. Role of the familial Dutch mutation E22Q in the folding and aggregation of the 15–28 fragment of the Alzheimer amyloid-β protein. Proc. Natl. Acad. Sci. USA 2008, 105, 6027–6032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravastu, A.K.; Petkova, A.T.; Tycko, R. Polymorphic fibril formation by residues 10–40 of the Alzheimer’s β-amyloid peptide. Biophys. J. 2006, 90, 4618–4629. [Google Scholar] [CrossRef] [PubMed]
- Ulamec, S.M.; Brockwell, D.J.; Radford, S.E. Looking beyond the core: The role of flanking regions in the aggregation of amyloidogenic peptides and proteins. Front. Neurosci. 2020, 14, 611285. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.C.; Kitt, C.A.; Schwam, E.; Buckwald, B.; Garcia, F.; Sepinwall, J.; Price, D.L. Senile plaques in aged squirrel monkeys. Neurobiol. Aging 1987, 8, 291–296. [Google Scholar] [CrossRef]
- Rosen, R.F.; Farberg, A.S.; Gearing, M.; Dooyema, J.; Long, P.M.; Anderson, D.C.; Davis-Turak, J.; Coppola, G.; Geschwind, D.H.; Paré, J.F.; et al. Tauopathy with paired helical filaments in an aged chimpanzee. J. Comp. Neurol. 2008, 509, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Satou, T.; Cummings, B.J.; Head, E.; Nielson, K.A.; Hahn, F.F.; Milgram, N.W.; Velazquez, P.; Cribbs, D.H.; Tenner, A.J.; Cotman, C.W. The progression of β-amyloid deposition in the frontal cortex of the aged canine. Brain Res. 1997, 774, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Cummings, B.J.; Head, E.; Afagh, A.J.; Milgram, N.W.; Cotman, C.W. β-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol. Learn. Mem. 1996, 66, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Cummings, B.J.; Satou, T.; Head, E.; Milgram, N.W.; Cole, G.M.; Savage, M.J.; Podlisny, M.B.; Selkoe, D.J.; Siman, R.; Greenberg, B.D. Diffuse plaques contain C-terminal Aβ42 and not Aβ40: Evidence from cats and dogs. Neurobiol. Aging 1996, 17, 653–659. [Google Scholar] [CrossRef]
- Batra-Safferling, R.; Granzin, J.; Mödder, S.; Hoffmann, S.; Willbold, D. Structural studies of the phosphatidylinositol 3-kinase (PI3K) SH3 domain in complex with a peptide ligand: Role of the anchor residue in ligand binding. Biol. Chem. 2010, 391, 33–42. [Google Scholar] [CrossRef]
- Koyama, S.; Yu, H.; Dalgarno, D.C.; Shin, T.B.; Zydowsky, L.D.; Schreiber, S.L. Structure of the Pl3K SH3 domain and analysis of the SH3 family. Cell 1993, 72, 945–952. [Google Scholar] [CrossRef]
- Röder, C.; Vettore, N.; Mangels, L.N.; Gremer, L.; Ravelli, R.B.G.; Willbold, D.; Hoyer, W.; Buell, A.K.; Schröder, G.F. Atomic structure of PI3-kinase SH3 amyloid fibrils by cryo-electron microscopy. Nat. Commun. 2019, 10, 3754. [Google Scholar] [CrossRef] [PubMed]
- Orte, A.; Birkett, N.R.; Clarke, R.W.; Devlin, G.L.; Dobson, C.M.; Klenerman, D. Direct Characterization of Amyloidogenic Oligomers by Single-Molecule Fluorescence. Proc. Natl. Acad. Sci. USA 2008, 105, 14424–14429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Webster, P.; Taddei, N.; Clark, A.; Stefani, M.; Ramponi, G.; Dobson, C.M. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl. Acad. Sci. USA 1999, 96, 3590–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBrasseur, N. Polyamino acids take amyloid form. J. Cell Biol. 2002, 159, 538–539. [Google Scholar] [CrossRef] [Green Version]
- Tomar, D.; Chaudhary, S.; Jena, K.C. Self-assembly of L-phenylalanine amino acid: Electrostatic induced hindrance of fibril formation. RSC Adv. 2019, 9, 12596–12605. [Google Scholar] [CrossRef] [Green Version]
- Dill, K.A.; Ozkan, S.B.; Shell, M.S.; Weikl, T.R. The protein folding problem. Annu. Rev. Biophys. 2008, 37, 289. [Google Scholar] [CrossRef]
- Karplus, M. Behind the folding funnel diagram. Nat. Chem. Biol. 2011, 7, 401–404. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Lett. 2020, 594, 2770–2781. [Google Scholar] [CrossRef]
- Anfinsen, C.B. Principles that govern the protein folding chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Sivertsson, E.M.; Itzhaki, L.S. When ribosomes pick the structure. Nat. Chem. 2014, 6, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Shortle, D. Propensities, probabilities, and the Boltzmann hypothesis. Protein Sci. 2003, 12, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Buell, A.K.; Hung, P.; Salvatella, X.; Welland, M.E.; Dobson, C.M.; Knowles, T.P.J. Electrostatic effects in filamentous protein aggregation. Biophys. J. 2013, 104, 1116–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beerten, J.; Schymkowitz, J.; Rousseau, F. Aggregation prone regions and gatekeeping residues in protein sequences. Curr. Top. Med. Chem. 2012, 12, 2470–2478. [Google Scholar] [CrossRef]
- March, D.; Bianco, V.; Franzese, G. Protein unfolding and aggregation near a hydrophobic interface. Polymers 2021, 13, 156. [Google Scholar] [CrossRef]
- Forsdyke, D.R. Entropy-driven protein self-aggregation as the basis for self/not-self discrimination in the crowded cytosol. J. Biol. Syst. 1995, 3, 273–287. [Google Scholar] [CrossRef]
- Pekar, A.H.; Frank, B.H. Conformation of proinsulin. Comparison of insulin and proinsulin self-association at neutral pH. Biochemistry 1972, 11, 4013–4016. [Google Scholar] [CrossRef]
- Alford, J.R.; Kendrick, B.S.; Carpenter, J.F.; Randolph, T.W. High concentration formulations of recombinant human interleukin-1 receptor antagonist: II. Aggregation kinetics. J. Pharm. Sci. 2008, 97, 3005–3021. [Google Scholar] [CrossRef]
- Levine, P.M.; Galesic, A.; Balana, A.T.; Mahul-Mellier, A.-L.; Navarro, M.X.; De Leon, C.A.; Lashuel, H.A.; Pratt, M.R. α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2019, 116, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-Z.; Xu, H.-H.; Ju, T.-T.; Zhao, X.-H. The effect of limited proteolysis by different proteases on the formation of whey protein fibrils. J. Dairy Sci. 2013, 96, 7383–7392. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Li, L. The effect of limited proteolysis by trypsin on the formation of soy protein isolate nanofibrils. J. Chem. 2020, 2020, 8185037. [Google Scholar] [CrossRef]
- Ledward, D.A. Effects of pressure on protein structure. Int. J. High Press. Res. 2000, 19, 1–10. [Google Scholar] [CrossRef]
- Kendrick, B.S.; Carpenter, J.F.; Cleland, J.L.; Randolph, T.W. A transient expansion of the native state precedes aggregation of recombinant human interferon-γ. Proc. Natl. Acad. Sci. USA 1998, 95, 14142–14146. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Chi, E.Y.; Webb, J.N.; Chang, B.S.; Shan, D.; Goldenberg, M.; Manning, M.C.; Randolph, T.W.; Carpenter, J.F. Aggregation of granulocyte colony stimulating factor under physiological conditions: Characterization and thermodynamic inhibition. Biochemistry 2002, 41, 6422–6431. [Google Scholar] [CrossRef] [PubMed]
- Raso, S.W.; Abel, J.; Barnes, J.M.; Maloney, K.M.; Pipes, G.; Treuheit, M.J.; King, J.; Brems, D.N. Aggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state. Protein Sci. 2005, 14, 2246–2257. [Google Scholar] [CrossRef]
- Linse, S.; Cabaleiro-Lago, C.; Xue, W.-F.; Lynch, I.; Lindman, S.; Thulin, E.; Radford, S.E.; Dawson, K.A. Nucleation of protein fibrillation by nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 8691–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, E.Y.; Weickmann, J.; Carpenter, J.F.; Manning, M.C.; Randolph, T.W. Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation. J. Pharm. Sci. 2005, 94, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Szczepankiewicz, O.; Linse, S. The effect of nanoparticles on amyloid aggregation depends on the protein stability and intrinsic aggregation rate. Langmuir 2012, 28, 1852–1857. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pittman, J.M.; Zerweck, J.; Venkata, B.S.; Moore, P.C.; Sachleben, J.R.; Meredith, S.C. β-Amyloid aggregation and heterogeneous nucleation. Protein Sci. 2019, 28, 1567–1581. [Google Scholar] [CrossRef]
- Sethuraman, A.; Belfort, G. Protein structural perturbation and aggregation on homogeneous surfaces. Biophys. J. 2005, 88, 1322–1333. [Google Scholar] [CrossRef] [Green Version]
- Co, N.T.; Li, M.S. Effect of Surface Roughness on Aggregation of Polypeptide Chains: A Monte Carlo Study. Biomolecules 2021, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.R.G.; Wolf Pérez, A.-M.; Zucca, M.V.; Capasso Palmiero, U.; Friedrichsen, B.; Lorenzen, N.; Arosio, P. An accelerated surface-mediated stress assay of antibody instability for developability studies. MAbs 2020, 12, 1815995. [Google Scholar] [CrossRef] [PubMed]
- Vargo, K.B.; Stahl, P.; Hwang, B.; Hwang, E.; Giordano, D.; Randolph, P.; Celentano, C.; Hepler, R.; Amin, K. Surfactant impact on interfacial protein aggregation and utilization of surface tension to predict surfactant requirements for biological formulations. Mol. Pharm. 2020, 18, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hansted, J.G.; Wejse, P.L.; Bertelsen, H.; Otzen, D.E. Effect of protein–surfactant interactions on aggregation of β-lactoglobulin. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Oosawa, F.; Asakura, S.; Hotta, K.; Imai, N.; Ooi, T. G-F transformation of actin as a fibrous condensation. J. Polym. Sci. 1959, 37, 323–336. [Google Scholar] [CrossRef]
- Oosawa, F.; Kasai, M. A theory of linear and helical aggregations of macromolecules. J. Mol. Biol. 1962, 4, 10–21. [Google Scholar] [CrossRef]
- Auer, S.; Dobson, C.M.; Vendruscolo, M. Characterization of the nucleation barriers for protein aggregation and amyloid formation. HFSP J. 2007, 1, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.M.; Watzky, M.A.; Agar, J.N.; Finke, R.G. Fitting neurological protein aggregation kinetic data via a 2-step, Minimal/“Ockham’s Razor” Model: The Finke− Watzky mechanism of nucleation followed by autocatalytic surface growth. Biochemistry 2008, 47, 2413–2427. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Transition metal nanocluster formation kinetic and mechanistic studies. A new mechanism when hydrogen is the reductant: Slow, continuous nucleation and fast autocatalytic surface growth. J. Am. Chem. Soc. 1997, 119, 10382–10400. [Google Scholar] [CrossRef]
- Serio, T.R.; Cashikar, A.G.; Kowal, A.S.; Sawicki, G.J.; Moslehi, J.J.; Serpell, L.; Arnsdorf, M.F.; Lindquist, S.L. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 2000, 289, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, T.J.; Murphy, R.M. Inhibition of insulin fibrillogenesis with targeted peptides. Protein Sci. 2006, 15, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Waudby, C.A.; Devlin, G.L.; Cohen, S.I.A.; Aguzzi, A.; Vendruscolo, M.; Terentjev, E.M.; Welland, M.E.; Dobson, C.M. An Analytical Solution to the Kinetics of Breakable Filament Assembly. Science 2009, 326, 1533–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padrick, S.B.; Miranker, A.D. Islet amyloid: Phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry 2002, 41, 4694–4703. [Google Scholar] [CrossRef]
- Ramachandran, G.; Udgaonkar, J.B. Evidence for the existence of a secondary pathway for fibril growth during the aggregation of tau. J. Mol. Biol. 2012, 421, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.A.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef] [Green Version]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, T.; Khan, H. Direct interaction between the β-amyloid core and tau facilitates cross-seeding: A novel target for therapeutic intervention. Biochemistry 2020, 59, 341–342. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.-F.; Hellewell, A.L.; Hewitt, E.W.; Radford, S.E. Fibril fragmentation in amyloid assembly and cytotoxicity: When size matters. Prion 2010, 4, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Modler, A.J.; Gast, K.; Lutsch, G.; Damaschun, G. Assembly of amyloid protofibrils via critical oligomers—A novel pathway of amyloid formation. J. Mol. Biol. 2003, 325, 135–148. [Google Scholar] [CrossRef]
- Schor, M.; Mey, A.S.J.S.; Noe, F.; MacPhee, C.E. Shedding light on the Dock–Lock mechanism in amyloid fibril growth using Markov State Models. J. Phys. Chem. Lett. 2015, 6, 1076–1081. [Google Scholar] [CrossRef]
- Chiricotto, M.; Melchionna, S.; Derreumaux, P.; Sterpone, F. Multiscale aggregation of the amyloid Aβ16–22 peptide: From disordered coagulation and lateral branching to Amorphous prefibrils. J. Phys. Chem. Lett. 2019, 10, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.; Yagi, H.; Manno, M.; Martorana, V.; Ban, T.; Christiansen, G.; Otzen, D.E.; Goto, Y.; Rischel, C. Branching in amyloid fibril growth. Biophys. J. 2009, 96, 1529–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dear, A.J.; Meisl, G.; Šarić, A.; Michaels, T.C.T.; Kjaergaard, M.; Linse, S.; Knowles, T.P.J. Identification of on-and off-pathway oligomers in amyloid fibril formation. Chem. Sci. 2020, 11, 6236–6247. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Yu, W.-C.; Shih, Y.-H.; Chen, C.-Y.; Guo, Z.-H.; Huang, S.-J.; Chan, J.C.C.; Chen, Y.-R. Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 2018, 8, 4772. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; LaBrenz, S.R.; Woo, L.; Serpell, L.C.; Kelly, J.W. Protofilaments, filaments, ribbons, and fibrils from peptidomimetic self-assembly: Implications for amyloid fibril formation and materials science. J. Am. Chem. Soc. 2000, 122, 5262–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, P.A.; Carreño, L.J.; Coombs, D.; Mora, J.E.; Palmieri, E.; Goldstein, B.; Nathenson, S.G.; Kalergis, A.M. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. Proc. Natl. Acad. Sci. USA 2005, 102, 4824–4829. [Google Scholar] [CrossRef] [Green Version]
- Conway, K.A.; Lee, S.-J.; Rochet, J.-C.; Ding, T.T.; Williamson, R.E.; Lansbury, P.T. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson’s disease: Implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Jordens, S.; Adamcik, J.; Amar-Yuli, I.; Mezzenga, R. Disassembly and reassembly of amyloid fibrils in Water—Ethanol mixtures. Biomacromolecules 2011, 12, 187–193. [Google Scholar] [CrossRef]
- Thorn, D.C.; Meehan, S.; Sunde, M.; Rekas, A.; Gras, S.L.; MacPhee, C.E.; Dobson, C.M.; Wilson, M.R.; Carver, J.A. Amyloid fibril formation by bovine milk κ-casein and its inhibition by the molecular chaperones αS-and β-casein. Biochemistry 2005, 44, 17027–17036. [Google Scholar] [CrossRef]
- Meehan, S.; Knowles, T.P.; Baldwin, A.J.; Smith, J.F.; Squires, A.M.; Clements, P.; Treweek, T.M.; Ecroyd, H.W.; Tartaglia, G.; Vendruscolo, M. Characterisation of amyloid fibril formation by small heat-shock proteins, human alphaA-, alphaB-and R120G alphaB-crystallins. J. Mol. Biol. 2007, 372, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Ecroyd, H.; Carver, J.A. Crystallin proteins and amyloid fibrils. Cell. Mol. Life Sci. 2009, 66, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Hatters, D.M.; MacPhee, C.E.; Lawrence, L.J.; Sawyer, W.H.; Howlett, G.J. Human apolipoprotein C-II forms twisted amyloid ribbons and closed loops. Biochemistry 2000, 39, 8276–8283. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Griffin, M.D.W.; Binger, K.J.; Schuck, P.; Howlett, G.J. An equilibrium model for linear and closed-loop amyloid fibril formation. J. Mol. Biol. 2012, 421, 364–377. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.I.A.; Vendruscolo, M.; Welland, M.E.; Dobson, C.M.; Terentjev, E.M.; Knowles, T.P.J. Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. J. Chem. Phys. 2011, 135, 08B615. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.I.A.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Nucleated polymerization with secondary pathways. II. Determination of self-consistent solutions to growth processes described by non-linear master equations. J. Chem. Phys. 2011, 135, 08B611. [Google Scholar] [CrossRef] [Green Version]
- Scheidt, T.; Łapińska, U.; Kumita, J.R.; Whiten, D.R.; Klenerman, D.; Wilson, M.R.; Cohen, S.I.A.; Linse, S.; Vendruscolo, M.; Dobson, C.M.; et al. Secondary nucleation and elongation occur at different sites on Alzheimer’s amyloid-β aggregates. Sci. Adv. 2019, 5, eaau3112. [Google Scholar] [CrossRef] [Green Version]
- Gillam, J.E.; MacPhee, C.E. Modelling amyloid fibril formation kinetics: Mechanisms of nucleation and growth. J. Phys. Condens. Matter 2013, 25, 373101. [Google Scholar] [CrossRef]
- Karamanos, T.K.; Kalverda, A.P.; Thompson, G.S.; Radford, S.E. Mechanisms of amyloid formation revealed by solution NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2015, 88, 86–104. [Google Scholar] [CrossRef] [Green Version]
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease. Redox Biol. 2019, 22, 101133. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H. Aggregation drives “misfolding” in protein amyloid fiber formation. Amyloid-J. Protein Fold. Disord. 2007, 14, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palladini, G.; Sachchithanantham, S.; Milani, P.; Gillmore, J.; Foli, A.; Lachmann, H.; Basset, M.; Hawkins, P.; Merlini, G.; Wechalekar, A.D. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood J. Am. Soc. Hematol. 2015, 126, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Hazenberg, B.P.C. Amyloidosis: A clinical overview. Rheum. Dis. Clin. 2013, 39, 323–345. [Google Scholar] [CrossRef] [Green Version]
- Westermark, P. Localized AL amyloidosis: A suicidal neoplasm? Upsala J. Med. Sci. 2012, 117, 244–250. [Google Scholar] [CrossRef]
- Rh, F.; Rl, C.; Skinner, M. The systemic amyloidoses. N. Engl. J. Med. 1997, 337, 898–909. [Google Scholar]
- Nordberg, A. Amyloid plaque imaging in vivo: Current achievement and future prospects. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.; Dickson, D.W.; Lin, W.-L.; Chisholm, L.; Corral, A.; Jones, G.; Yen, S.-H.; Sahara, N.; Skipper, L.; Yager, D. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 2001, 293, 1487–1491. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.A. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimer Dement. 2014, 10, 372–380. [Google Scholar] [CrossRef]
- Serpell, L.C. Alzheimer’s amyloid fibrils: Structure and assembly. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2000, 1502, 16–30. [Google Scholar] [CrossRef]
- Kuner, P.; Bohrmann, B.; Tjernberg, L.O.; Näslund, J.; Huber, G.; Celenk, S.; Grüninger-Leitch, F.; Richards, J.G.; Jakob-Rœtne, R.; Kemp, J.A. Controlling polymerization of β-amyloid and prion-derived peptides with synthetic small molecule ligands. J. Biol. Chem. 2000, 275, 1673–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, V.M.Y.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R.W.; Bullock, R.; Love, S.; Neal, J.W. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008, 372, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Holtzman, D.M. Moving towards a vaccine. Nature 2008, 454, 419–420. [Google Scholar] [CrossRef]
- Alzheimer’s-Association. Beta-Amyloid and the Amyloid Hypothesis; Alzheimer’s-Association: Chicago, IL, USA, 2017. [Google Scholar]
- Tampi, R.R.; Forester, B.P.; Agronin, M. Aducanumab: Evidence from clinical trial data and controversies. Drugs Context 2021, 10. [Google Scholar] [CrossRef]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Alzheimer’s Disease and Protein Kinases. In Protein Kinase-Mediated Decisions Between Life and Death; Springer: Berlin/Heidelberg, Germany, 2021; pp. 285–321. [Google Scholar]
- Kauwe, G.; Tracy, T.E. Amyloid beta emerges from below the neck to disable the brain. PLoS Biol. 2021, 19, e3001388. [Google Scholar] [CrossRef]
- Schwartz, M.F.; Buxbaum, L.J.; Montgomery, M.W.; Fitzpatrick-DeSalme, E.; Hart, T.; Ferraro, M.; Lee, S.S.; Coslett, H.B. Naturalistic action production following right hemisphere stroke. Neuropsychologia 1998, 37, 51–66. [Google Scholar] [CrossRef]
- Weggen, S.; Eriksen, J.L.; Das, P.; Sagi, S.A.; Wang, R.; Pietrzik, C.U.; Findlay, K.A.; Smith, T.E.; Murphy, M.P.; Bulter, T. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 2001, 414, 212–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brothers, H.M.; Gosztyla, M.L.; Robinson, S.R. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Urbanc, B. Cross-Linked Amyloid β-Protein Oligomers: A Missing Link in Alzheimer’s Disease Pathology? J. Phys. Chem. B 2021, 125, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ułamek-Kozioł, M. Tau protein-targeted therapies in Alzheimer’s disease: Current state and future perspectives. Exon Publ. 2020, 69–82. [Google Scholar] [CrossRef]
- Ashe, K.H.; Aguzzi, A. Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion 2013, 7, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Colin, M.; Dujardin, S.; Schraen-Maschke, S.; Meno-Tetang, G.; Duyckaerts, C.; Courade, J.-P.; Buee, L. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 2020, 139, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Rafii, M.S. Targeting tau protein in Alzheimer’s disease. Lancet 2016, 388, 2842–2844. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—A critical review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Uversky, V.N.; Fink, A.L. Pathways to amyloid fibril formation: Partially folded intermediates in the fibrillation of natively unfolded proteins. Amyloid Proteins Beta Sheet Conform. Dis. 2005, 246–273. [Google Scholar] [CrossRef]
- Westergard, L.; Christensen, H.M.; Harris, D.A. The cellular prion protein (PrPC): Its physiological function and role in disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2007, 1772, 629–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, K.; Woerman, A.L.; Berry, D.B.; Prusiner, S.B. Bioassays and inactivation of prions. Cold Spring Harb. Perspect. Biol. 2017, 9, a023499. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, C.; Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Haass, C. Games played by rogue proteins in prion disorders and Alzheimer’s disease. Science 2003, 302, 814–818. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Coomaraswamy, J.; Bolmont, T.; Kaeser, S.; Schaefer, C.; Kilger, E.; Neuenschwander, A.; Abramowski, D.; Frey, P.; Jaton, A.L. Exogenous induction of cerebral ß-amyloidogenesis is governed by agent and host. Science 2006, 313, 1781–1784. [Google Scholar] [CrossRef]
- Sinha, N.; Thakur, A.K. Likelihood of amyloid formation in COVID-19-induced ARDS. Trends Microbiol 2021, 29, 967–969. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Földvári-Nagy, L.; Schnabel, T.; Dörnyei, G.; Korcsmáros, T.; Lenti, K. On the role of bacterial metalloproteases in COVID-19 associated cytokine storm. Cell Commun. Signal. 2021, 19, 7. [Google Scholar] [CrossRef]
- Wang, W.; Khatua, P.; Hansmann, U.H.E. Cleavage, downregulation, and aggregation of serum amyloid A. J. Phys. Chem. B 2020, 124, 1009–1019. [Google Scholar] [CrossRef]
- Skinner, M.; Stone, P.; Shirahama, T.; Connors, L.H.; Calore, J.; Cohen, A.S. The association of an elastase with amyloid fibrils. Proc. Soc. Exp. Biol. Med. 1986, 181, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Sait, A.; Angeli, C.; Doig, A.J.; Day, P.J.R. Viral involvement in Alzheimer’s disease. ACS Chem. Neurosci. 2021, 12, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Miwata, H.; Yamada, T.; Okada, M.; Kudo, T.; Kimura, H.; Morishima, T. Serum amyloid A protein in acute viral infections. Arch. Dis. Child. 1993, 68, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Pal, I.; Nath, A.K.; Dey, S.G. Peroxidase activity of heme bound amyloid β peptides associated with Alzheimer’s disease. Chem. Commun. 2020, 56, 4505–4518. [Google Scholar] [CrossRef]
- Maji, S.K.; Schubert, D.; Rivier, C.; Lee, S.; Rivier, J.E.; Riek, R. Amyloid as a depot for the formulation of long-acting drugs. PLoS Biol. 2008, 6, e17. [Google Scholar] [CrossRef]
- Pal, S.; Goswami, S.; Das, D. Cross β amyloid assemblies as complex catalytic machinery. Chem. Commun. 2021, 57, 7597–7609. [Google Scholar] [CrossRef]
- Kapil, N.; Singh, A.; Singh, M.; Das, D. Efficient MoS2 Exfoliation by Cross-β-Amyloid Nanotubes for Multistimuli-Responsive and Biodegradable Aqueous Dispersions. Angew. Chem. Int. Ed. 2016, 55, 7772–7776. [Google Scholar] [CrossRef]
- Das, S.; Jacob, R.S.; Patel, K.; Singh, N.; Maji, S.K. Amyloid fibrils: Versatile biomaterials for cell adhesion and tissue engineering applications. Biomacromolecules 2018, 19, 1826–1839. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef]
- Zeng, H.; Zhai, L.; Qiao, T.; Yu, Y.; Zhang, J.; Li, D. Efficient removal of As (V) from aqueous media by magnetic nanoparticles prepared with Iron-containing water treatment residuals. Sci. Rep. 2020, 10, 9335. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate use recommendations. J. Prev. Alzheimer Dis. 2021, 8, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Pradhan, S.; Ojha, D.P.; Das, A.; Hosmane, N.S.; Das, S. The role of tau protein in diseases. Ann. Adv. Chem. 2018, 2, 001–016. [Google Scholar]

| Variable | Description |
|---|---|
| Total fibril mass at a time t | |
| Final fibril mass | |
| Rate constant for primary nucleation | |
| Rate constant for monomer addition and elongation | |
| Rate constant for depolymerization | |
| Rate constant for fragmentation | |
| Rate constant for secondary nucleation | |
| Initial concentration of soluble monomers in solution | |
| Rate order with respect to monomer (primary pathway) | |
| Rate order with respect to monomer (secondary pathway) | |
| S.No. | Disease | Protein/Peptide | Structure |
|---|---|---|---|
| 1. | AD (Alzheimer’s Disease) | Aβ (β-Amyloid peptide) | Natively unfolded |
| 2. | HCHWA (Dutch Hereditary Cerebral Hemorrhage With Amyloidosis) | Aβ (β-Amyloid peptide) | Natively unfolded |
| 3. | (CAA) Cerebral Amyloid Angiopathy | Aβ (β-Amyloid peptide) | Natively unfolded |
| 4. | Familial British dementia | Cystine Cross-linked Amyloid Bri | Natively unfolded |
| 5. | (HD) Huntington Disease | Huntingtin | Unfolded exon 1 which forms fibrils |
| 6. | SBMA (Spinal and Bulbar DNA-binding Muscular Atrophy) | Androgen receptor protein | Ligand-binding and domains α-helical; N-terminal—natively unfolded |
| 7. | NIID (Neuronal Intranuclear Inclusion Disease) | Ataxin-1 | Natively unfolded |
| 8. | SCA (Spinocerebellar Ataxia) | Ataxin-1 | Natively unfolded |
| 9. | DRPLA (hereditary Dentatorubral Pallidoluysian Atrophy) | Atrophin-1 | Probably natively unfolded |
| 10. | Type II Diabetes Mellitus | Amylin | Natively unfolded |
| 11. | MCT (Medullary Carcinoma of Thyroid) | Calcitonin | Natively unfolded |
| 12. | PD (Parkinson’s disease) | α-Synuclein | Natively unfolded |
| 13. | DLBD (Diffuse Lewy Bodies Disease) | α-Synuclein | Natively unfolded |
| 14. | LBVAD (Lewy Bodies Variant of Alzheimer’s Disease) | α-Synuclein | Natively unfolded |
| 15. | DLB (Dementia with Lewy Bodies) | α-Synuclein | Natively unfolded |
| 16. | MSA (Multiple System Atrophy) | α-Synuclein | Natively unfolded |
| 17. | Pick’s Disease | Tau protein | Natively unfolded |
| 18. | PSP (Progressive Supranuclear Palsy) | Tau protein | Natively unfolded |
| 19. | CJD (Creutzfeldt–Jakob Disease) | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 20. | GSS (Gerstmann–Straussler Schneiker Syndrome) | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 21. | FII (Fatal Familial Insomnia) | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 22. | Kuru | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 23. | BSE (Bovine Spongiform) | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 24. | Scrapie | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 25. | Spongiform Encephalopathy | Prion protein | N-terminal fragment— natively unfolded; C-terminal domain: α-helical |
| 26. | Amyotrophic lateral sclerosis | Superoxide dismutase 1 | β-sheet and Ig-like |
| 27. | Familial Amyloidotic Polyneuropathy | Transthyretin mutants | β-sheet |
| 28. | Amyloid light chain (AL) amyloidosis | Immunoglobulin (Ig) light chains | β-sheet and Ig-like |
| 29. | Senile systemic amyloidosis | Wild-type transthyretin | β-sheet |
| 30. | Haemodialysis-related amyloidosis | β2-microglobulin | β-sheet and Ig-like |
| 31. | Lysozyme amyloidosis | Lysozyme mutants | α-helical and β-sheet |
| 32. | Apolipoprotein A1 amyloidosis | Apo A-1 fragments | Intrinsically disordered |
| 33. | Injection-localized amyloidosis | Insulin | α-helical and insulin-like |
| 34. | Progressive supranuclear palsy | Tau protein | Intrinsically disordered |
| 35. | Argyrophilic grain disease | Tau protein | Intrinsically disordered |
| 36. | Tangle predominant dementia | Tau protein | Intrinsically disordered |
| 37. | Chronic traumatic encephalopathy | Tau protein | Intrinsically disordered |
| 38. | Ganglioglioma | Tau protein | Intrinsically disordered |
| 39. | Meningioangiomatosis | Tau protein | Intrinsically disordered |
| 40. | Subacute sclerosing panencephalitis | Tau protein | Intrinsically disordered |
| 41. | Lead encephalopathy | Tau protein | Intrinsically disordered |
| 42. | Tuberous sclerosis | Tau protein | Intrinsically disordered |
| 43. | Hallervorden–Spatz disease | Tau protein | Intrinsically disordered |
| 44. | Lipofuscinosis | Tau protein | Intrinsically disordered |
| 45. | Familial Danish dementia | Tau protein | Intrinsically disordered |
| 46. | Heavy-chain amyloidosis | Fragments of immunoglobulin heavy chain | All-β, Ig-like |
| 47. | AA amyloidosis | Full or N-terminal fragments of serum amyloid A protein (SAA) | All-α, SA-like four-helix bundle |
| 48. | Hereditary visceral amyloidosis | β2-microglobulin | All-β, Ig-like |
| 49. | ApoAII amyloidosis (mainly renal) | C-term extended apolipoprotein A-II | Unknown |
| 50. | ApoAIV amyloidosis (many organs) | N-term fragments of apolipoprotein A-IV (ApoAIV) | Unknown |
| 51. | ApoCII amyloidosis | Apolipoprotein C-II | All-α, unknown fold |
| 52. | Fibrinogen amyloidosis | Fragments of fibrinogenα-chain | Unknown |
| 53. | Atrial amyloidosis | Atrial natriuretic factor(ANF) | Intrinsically disordered |
| 54. | Pituitary prolactinoma | N-term fragments of prolactin (PRL) | Unknown |
| 55. | Aortic medial amyloidosis | Medin | Intrinsically disordered |
| 56. | Gelatinous drop-like corneal dystrophy | Lactotransferrin | α + β, periplasmic binding protein-like II |
| 57. | Calcifying epithelial odontogenic tumors | Odontogenic Ameloblast Associated protein (ODAM) | Unknown |
| 58. | Pulmonary alveolar proteinosis | Pulmonary surfactant-associated protein C (SP-C) | All α-transmembrane helical fragment |
| 59. | Renal amyloidosis | Leukocyte cell-derived chemotaxin-2 (LECT-2) | Unknown |
| 60. | Lattice corneal dystrophy, type 1 | C-term fragments of kerato-epithelin | Unknown |
| 61. | Lattice corneal dystrophy, type 3A | C-term fragments of kerato-epithelin | Unknown |
| 62. | Lattice corneal dystrophy, Avellino type | C-term fragments of kerato-epithelin | Unknown |
| 63. | Seminal vesicle amyloidosis | Semenogelin-1 (SGI) | Unknown |
| 64. | Prostate cancer | All-α, EF hand-like | Proteins S100A8/A9 |
| 65. | Injection-localized amyloidosis | Enfuvirtide | Unknown |
| 66. | Frontotemporal dementia with Parkinsonism | Tau protein | Natively unfolded |
| 67. | Icelandic hereditary cerebral amyloid angiopathy | Mutant of cystatin C | A + β, cystatin-like |
| 68. | Spinocerebellar ataxia 17 | TATA box-binding protein with polyQ expansion | A + β, TBP-like |
| 69. | Hereditary cerebral haemorrhage with amyloidosis | Mutants of amyloid β | Natively unfolded |
| 70. | Cataract | γ-Crystallins | All-β, γ-crystallin-like |
| 71. | Pulmonary alveolar proteinosis | Lung surfactant protein C | Unknown |
| 72. | Inclusion-body myositis | Amyloid β peptide | Natively unfolded |
| 73. | Cutaneous lichen amyloidosis | Keratins | Unknown |
| 74. | Secondary systemic amyloidosis | Serum amyloid A | Unknown |
| 75. | Familial Amyloid AApoA1 | Polyneuropathy II | Unknown |
| 76. | Hereditary nonneuropathic systemic amyloidosis | ALys | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alraawi, Z.; Banerjee, N.; Mohanty, S.; Kumar, T.K.S. Amyloidogenesis: What Do We Know So Far? Int. J. Mol. Sci. 2022, 23, 13970. https://doi.org/10.3390/ijms232213970
Alraawi Z, Banerjee N, Mohanty S, Kumar TKS. Amyloidogenesis: What Do We Know So Far? International Journal of Molecular Sciences. 2022; 23(22):13970. https://doi.org/10.3390/ijms232213970
Chicago/Turabian StyleAlraawi, Zeina, Nayan Banerjee, Srujana Mohanty, and Thallapuranam Krishnaswamy Suresh Kumar. 2022. "Amyloidogenesis: What Do We Know So Far?" International Journal of Molecular Sciences 23, no. 22: 13970. https://doi.org/10.3390/ijms232213970





