New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis
Abstract
:1. Introduction
2. Results and Discussion
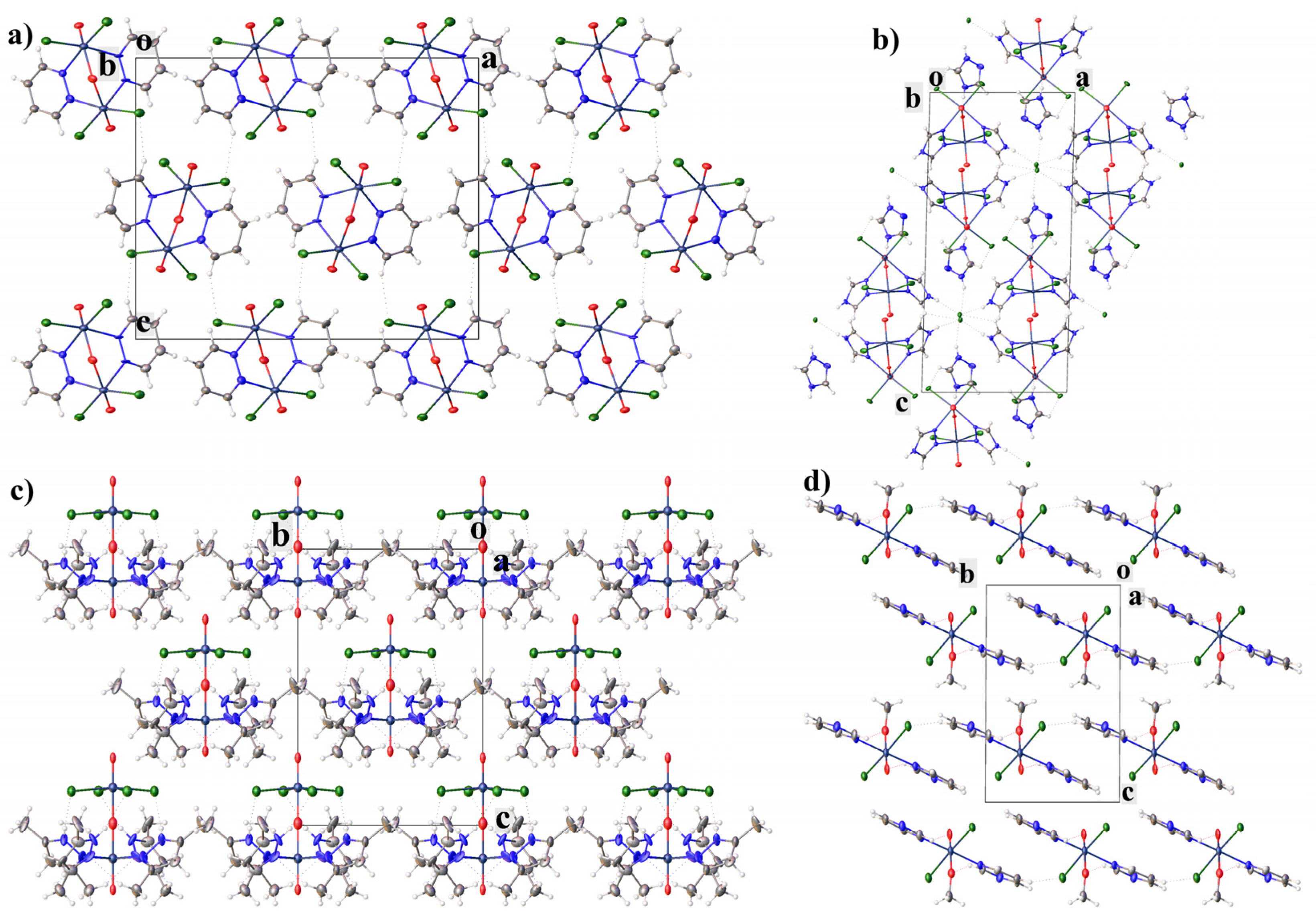
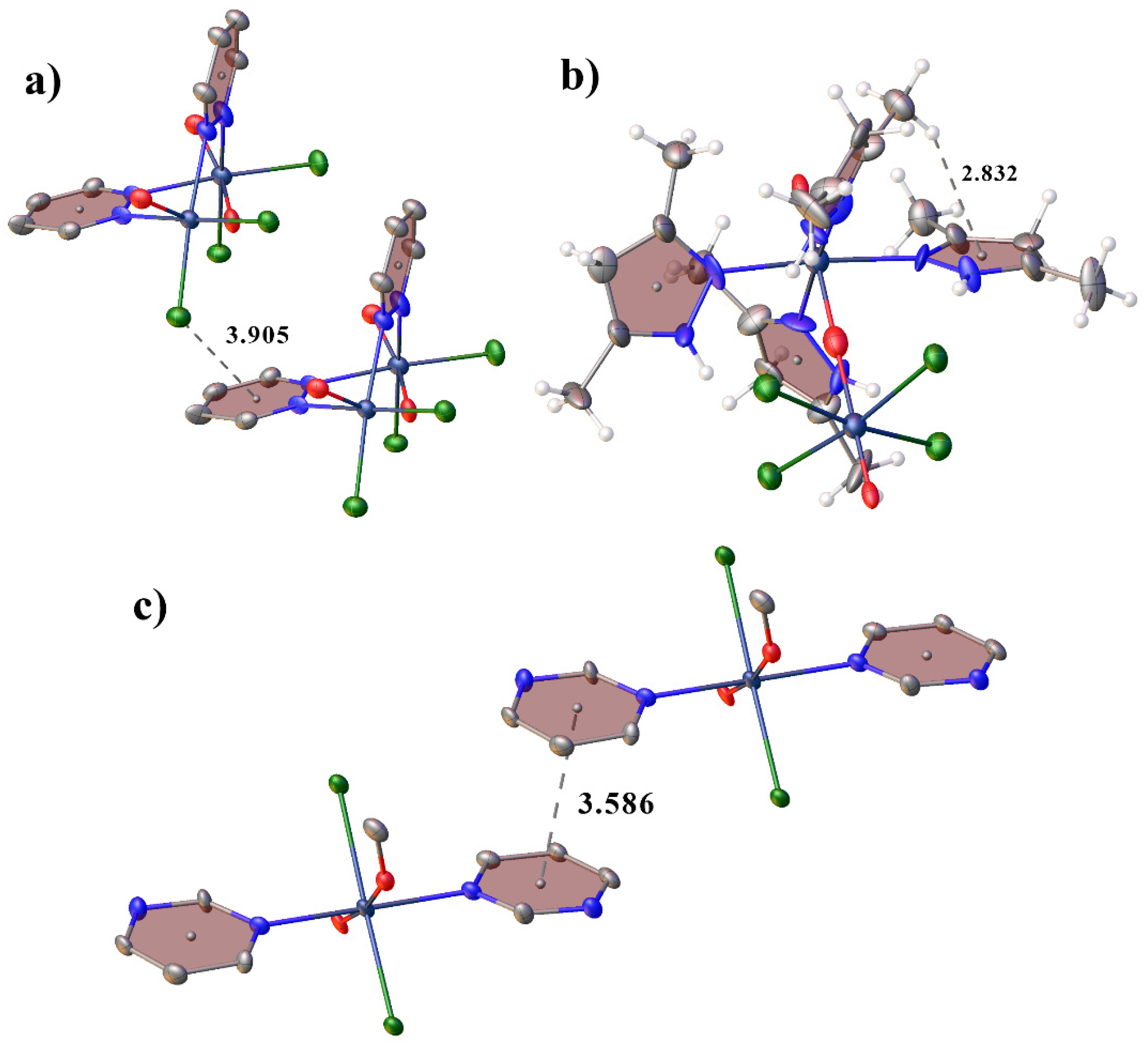
2.1. Structural Description of Tc(V) Complexes
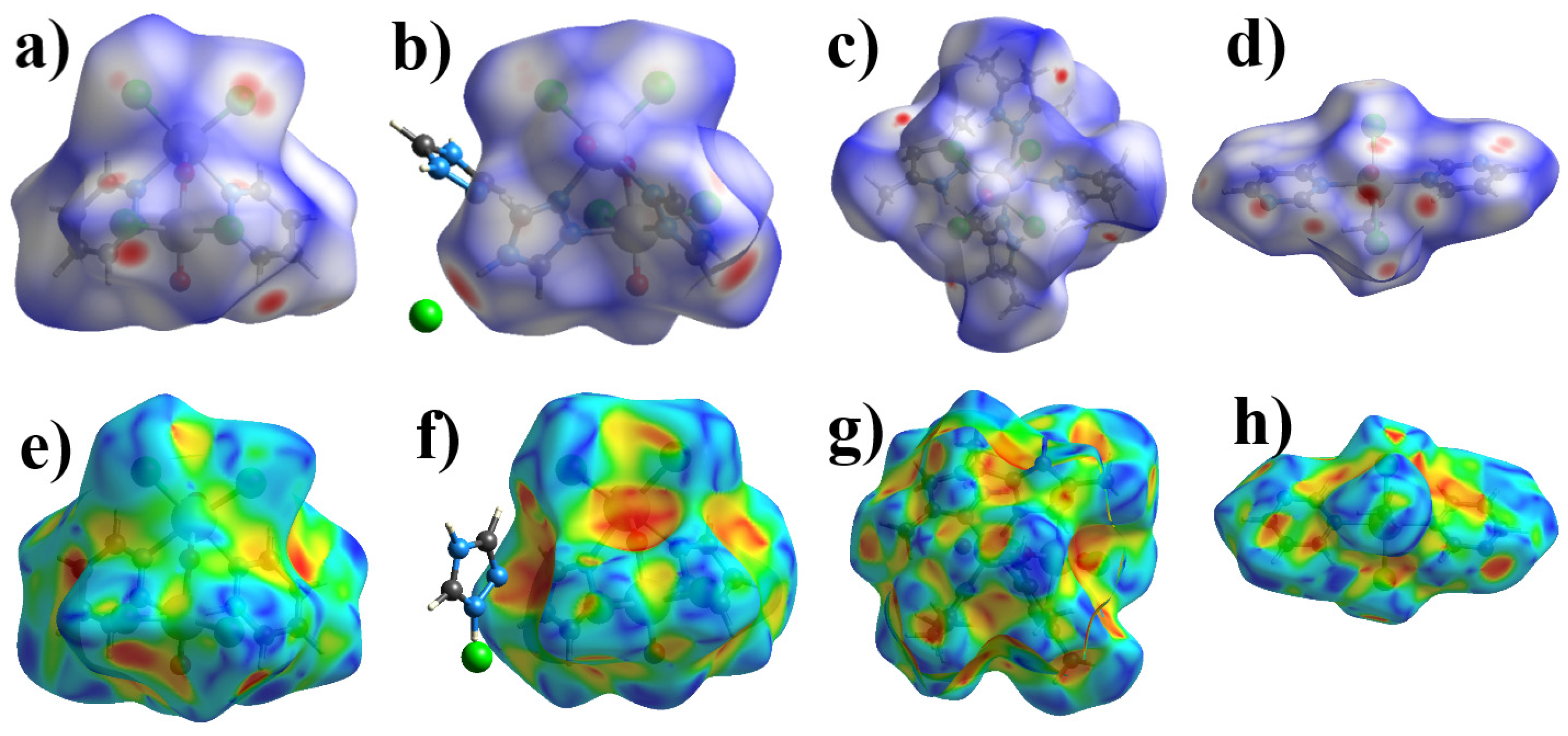
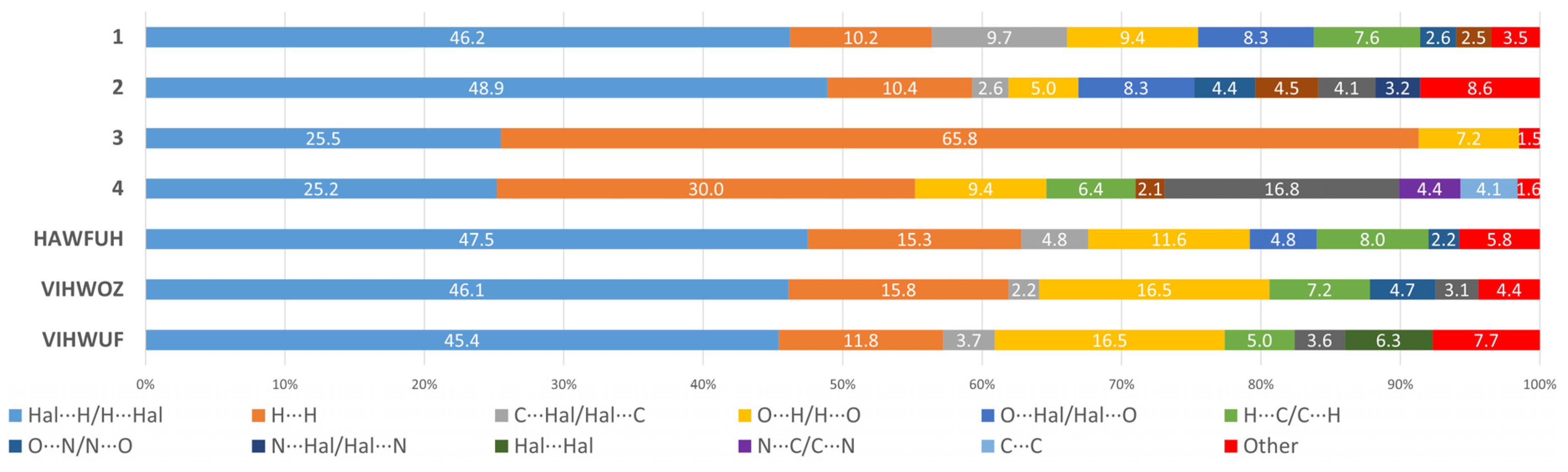
2.2. Hirshfeld Surface Analysis
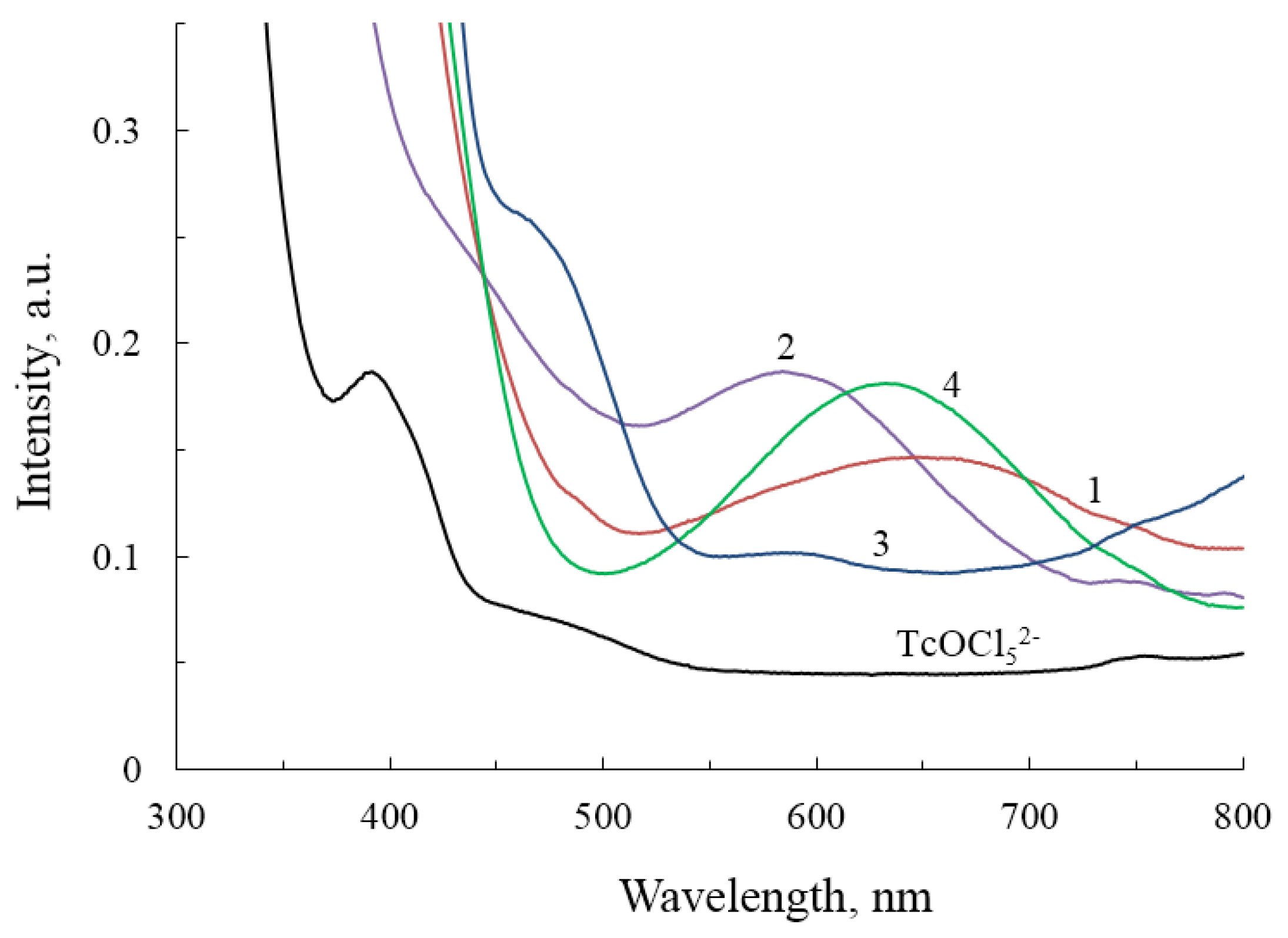
2.3. Spectroscopic Studies
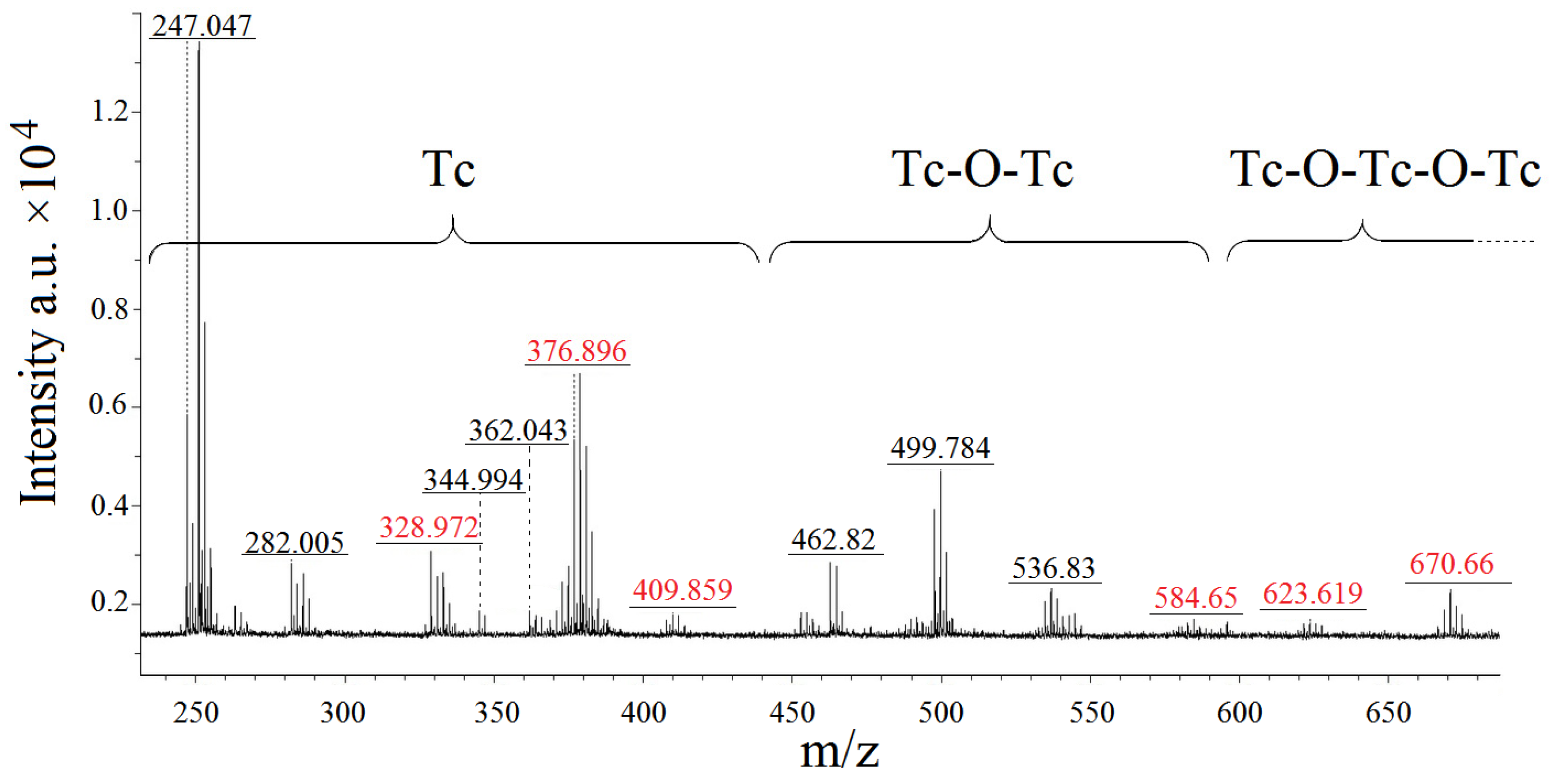
2.4. MALDI-ToF Analysis of the Mother Liquor of 1
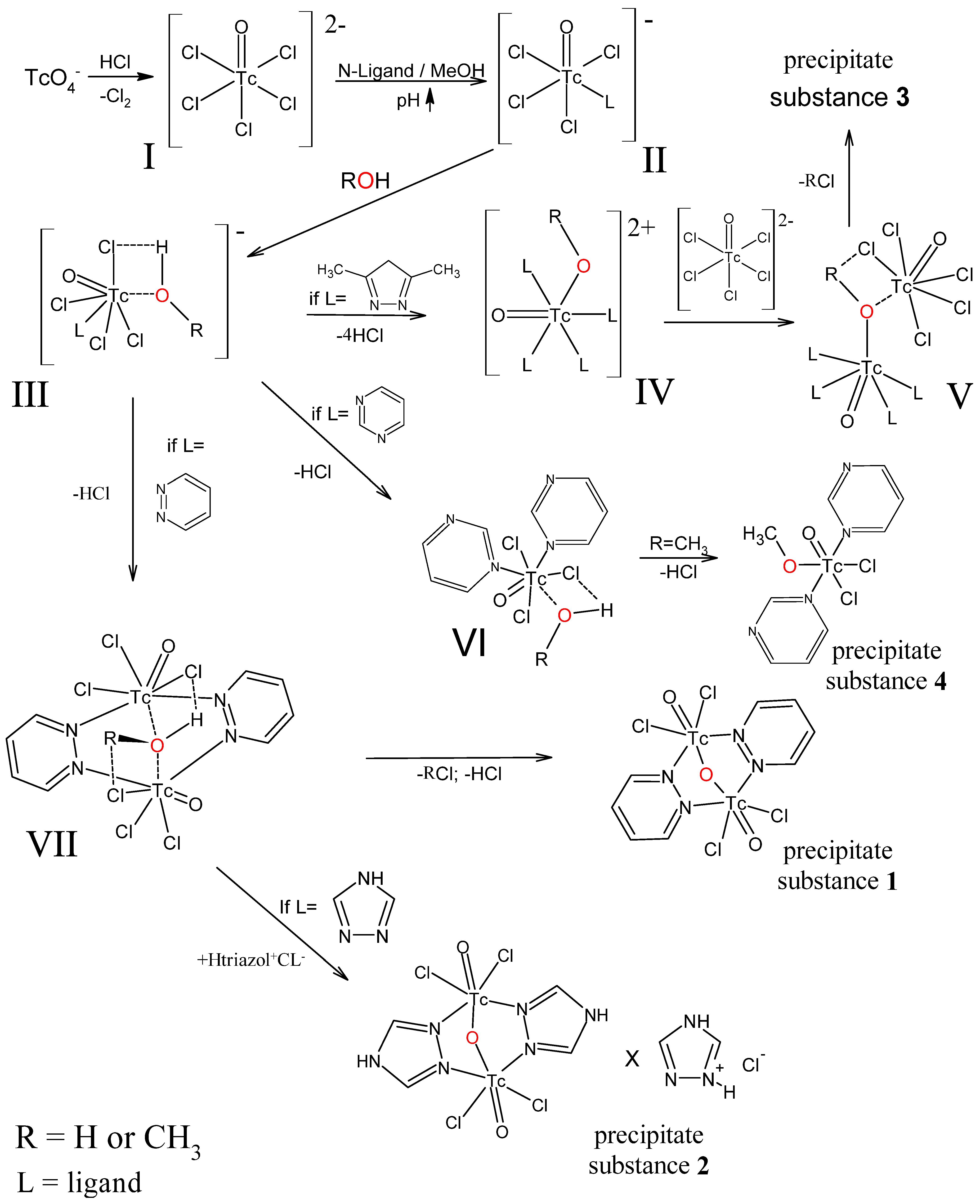
2.5. Proposed Mechanism for the Formation of Complexes
2.6. DTA-Analysis
3. Materials and Methods
3.1. Synthesis of Complexes
3.2. Single-Crystal XRD Analysis
3.3. Spectroscopic Analysis
3.4. MALDI-ToF—Analysis
3.5. Thermal Analysis
3.6. Elemental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkov, M.A.; Fedoseev, A.M.; Krivoborodov, E.G.; Toropygin, I.Y.; German, K.E.; Grigoriev, M.S.; Kuznetsov, V.V.; Budantseva, N.A.; Novikov, A.P.; Mezhuev, Y.O. A new method for the synthesis of polynuclear carboxylate complexes of technetium (II, III). J. Organomet. Chem. 2022, 957, 122146. [Google Scholar] [CrossRef]
- Alberto, R. From oxo to carbonyl and arene complexes; A journey through technetium chemistry. J. Organomet. Chem. 2018, 869, 264–269. [Google Scholar] [CrossRef]
- Sidorenko, G.V.; Miroslavov, A.E. Higher Technetium(I) Carbonyls and Possibility of Using Them in Nuclear Medicine: Problems and Prospects. Radiochemistry 2021, 63, 253–262. [Google Scholar] [CrossRef]
- Zegke, M.; Grödler, D.; Roca Jungfer, M.; Haseloer, A.; Kreuter, M.; Neudörfl, J.M.; Sittel, T.; James, C.M.; Rothe, J.; Altmaier, M.; et al. Ammonium Pertechnetate in Mixtures of Trifluoromethanesulfonic Acid and Trifluoromethanesulfonic Anhydride. Angew. Chem. Int. Ed. 2022, 61, e202113777. [Google Scholar] [CrossRef] [PubMed]
- German, K.E.; Fedoseev, A.M.; Grigoriev, M.S.; Kirakosyan, G.A.; Dumas, T.; Den Auwer, C.; Moisy, P.; Lawler, K.V.; Forster, P.M.; Poineau, F. A 70-Year-Old Mystery in Technetium Chemistry Explained by the New Technetium Polyoxometalate [H7O3]4[Tc20O68]⋅4H2O. Chem. Eur. J. 2021, 27, 13624–13631. [Google Scholar] [CrossRef] [PubMed]
- Abram, U.; Beyer, R.; Münze, R.; Stach, J.; Kaden, L.; Lorenz, B.; Findeisen, M. Mixed-ligand complexes of technetium-III. Synthesis and characterization of [bis(diphenylphosphino)ethane]tetrakis(trimethylphosphite)technetium(I) hexafluorophosphate, [Tc(DPPE)(TMP)4]PF6. Polyhedron 1989, 8, 1201–1204. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef]
- Jürgens, S.; Herrmann, W.A.; Kühn, F.E. Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J. Organomet. Chem. 2014, 751, 83–89. [Google Scholar] [CrossRef]
- Melent’ev, A.B.; Mashkin, A.N.; German, K.E. The influence of deviations in process parameters on the purification of uranium from different radionuclides. Theor. Found. Chem. Eng. 2016, 50, 554–561. [Google Scholar] [CrossRef]
- Fackler, P.H.; Lindsay, M.J.; Clarke, M.J.; Kastner, M.E. Synthesis and structure of trans-[O2(Im)4Tc]Cl·2H2O, trans-[O2(1-meIm)4Tc]Cl·3H2O and related compounds. Inorg. Chim. Acta 1985, 109, 39–49. [Google Scholar] [CrossRef]
- Welch, M.J.; Redvanly, C.S. Handbook of Radiopharmaceuticals: Radiochemistry and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-0-470-84638-4. [Google Scholar]
- Abram, U.; Alberto, R. Technetium and rhenium: Coordination chemistry and nuclear medical applications. J. Braz. Chem. Soc. 2006, 17, 1486–1500. [Google Scholar] [CrossRef]
- Liu, S. The role of coordination chemistry in the development of target-specific radiopharmaceuticals. Chem. Soc. Rev. 2004, 33, 445–461. [Google Scholar] [CrossRef]
- Maruk, A.Y.; Bruskin, A.B.; Kodina, G.E. Novel 99m Tc radiopharmaceuticals with bifunctional chelating agents. Radiochemistry 2011, 53, 341–353. [Google Scholar] [CrossRef]
- Günther, T.; Konrad, M.; Stopper, L.; Kunert, J.P.; Fischer, S.; Beck, R.; Casini, A.; Wester, H.J. Optimization of the Pharmacokinetic Profile of [99mTc]Tc-N4-Bombesin Derivatives by Modification of the Pharmacophoric Gln-Trp Sequence. Pharmaceuticals 2022, 15, 1133. [Google Scholar] [CrossRef]
- Volkov, M.A.; Novikov, A.P.; Grigoriev, M.S.; Fedoseev, A.M.; German, K.E. Novel Synthesis Methods of New Imidazole-Containing Coordination Compounds Tc(IV, V, VII)—Reaction Mechanism, Xrd and Hirshfeld Surface Analysis. Int. J. Mol. Sci. 2022, 23, 9461. [Google Scholar] [CrossRef]
- Sergienko, V.S.; Churakov, A.V. Specific features of technetium mononuclear octahedral oxo complexes: A review. Crystallogr. Rep. 2013, 58, 1–25. [Google Scholar] [CrossRef]
- Kastner, M.E.; Lindsay, M.J.; Clarke, M.J. Synthesis and Structure of trans-[O2(en)2Tcv]+. Inorg. Chem. 1982, 21, 2037–2040. [Google Scholar] [CrossRef]
- Zuckman, S.A.; Freeman, G.M.; Troutner, D.E.; Volkert, W.A.; Holmes, R.A.; Van Derveer, D.G.; Barefield, E.K. Preparation and X-ray Structure of trans-Dioxo(1,4,8,11-tetraazacyclotetradecane)technetium(V) Perchlorate Hydrate. Inorg. Chem. 1981, 20, 2386–2389. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Trieu, T.N.; Abram, U. Syntheses and Structures of Nitridorhenium(V) and Nitridotechnetium(V) Complexes with N,N-[(Dialkylamino)(thiocarbonyl)]-N′-(2-hydroxyphenyl)benzamidines. Z. Anorg. Allg. Chem. 2011, 637, 1330–1333. [Google Scholar] [CrossRef]
- Clarke, M.J.; Lu, J. Synthesis and Spectra of Cis-[NCl(phen)2Tc]Cl-H2O and c/s-[NCl(phen)2Tc]PF6 and Considerations of their Structural Distortions. Inorg. Chem. 1992, 31, 2476–2480. [Google Scholar] [CrossRef]
- Rue, K.L.; McLachlan, J.R.; Cazzaniga, J.A.; Chakraborty, I.; Dares, C.J.; Raptis, R.G. Redox-active dinuclear oxorhenium(V) pyrazolate complexes. Inorg. Chim. Acta 2021, 516, 120126. [Google Scholar] [CrossRef]
- MacHura, B.; Dziȩgielewski, J.O.; Kruszynski, R.; Bartczak, T.J.; Kusz, J. Linear and bent oxo-bridged dinuclear rhenium(V) complexes with terminal and bridging pyrazole ligands. Inorg. Chim. Acta 2004, 357, 1011–1022. [Google Scholar] [CrossRef]
- Machura, B.; Kruszynski, R.; Jaworska, M.; Lodowski, P. Synthesis, spectroscopic characterization, crystal, molecular and electronic structure of the [{ReOBr2(pyz)2}2(μ-O)] and [{ReOBr2}2(μ-O)(μ-pyd)2] complexes. Polyhedron 2005, 24, 1893–1906. [Google Scholar] [CrossRef]
- Machura, B.; Kruszynski, R.; Kusz, J. Reactivity of [ReOX3(PPh3)2] complexes towards pyridazine. X-ray structures of [{ReOCl2}2(μ-O)(μ-pyd)2]·C6H6 and [{ReOBr2}2(μ-O)(μ-pyd)2]·CH3CN, and DFT calculations for [{ReOCl2}2(μ-O)(μ-pyd)2]. Polyhedron 2007, 26, 3054–3062. [Google Scholar] [CrossRef]
- Machura, B.; Wolff, M.; Palion, J.; Benoist, E. Synthesis, spectroscopic characterization and X-ray crystal structures of mononuclear and binuclear oxidorhenium(V) complexes containing indazolyl moieties. Inorg. Chim. Acta 2013, 404, 144–154. [Google Scholar] [CrossRef]
- Fackler, P.H.; Kastner, M.E.; Clarke, M.J. Synthesis, spectra, and structure of af-dibromo-b-ethoxo-d-oxo-ce-bis(4-nitropyridine)technetium(V) and related. Inorg. Chem. 1984, 23, 3968–3972. [Google Scholar] [CrossRef]
- Lock, C.J.L.; Turner, G. Studies of the rhenium-oxygen bond. III. The crystal and molecular structure of 1-oxo-6-ethoxo-2,4-dichloro-3,5-dipyridinerhenium(V). Can. J. Chem. 1972, 55, 333–339. [Google Scholar] [CrossRef]
- Fortin, S.; Beauchamp, A.L. Synthesis and crystal structure of a new isomer of the μ-oxo-bis[dichlorooxobis(pyridine)rhenium(V)] complex {OReCl2py2}2O. Inorg. Chim. Acta 1998, 279, 159–164. [Google Scholar] [CrossRef]
- Iengo, E.; Zangrando, E.; Mestroni, S.; Fronzoni, G.; Stener, M.; Alessio, E. Complexed bridging ligands: Oxorhenium(V) compounds with mono-coordinated pyrazine or pyrimidine as possible building blocks for the construction of polynuclear architectures. J. Chem. Soc. Dalton Trans. 2001, 1338–1346. [Google Scholar] [CrossRef]
- Daolio, A.; Pizzi, A.; Terraneo, G.; Frontera, A.; Resnati, G. Anion⋅Anion Interactions Involving σ-Holes of Perrhenate, Pertechnetate and Permanganate Anions. ChemPhysChem 2021, 22, 2281–2285. [Google Scholar] [CrossRef]
- Novikov, A.P.; German, K.E.; Safonov, A.V.; Grigoriev, M.S. Cation Protonation Degree Influence on the Formation of Anion⋅⋅⋅Anion and Other Non-Valent Interactions in Guaninium Perrhenates and Pertechnetate. ChemistrySelect 2022, 7, e202202814. [Google Scholar] [CrossRef]
- Xie, R.; Shen, N.; Chen, X.; Li, J.; Wang, Y.; Zhang, C.; Xiao, C.; Chai, Z.; Wang, S. 99TcO4-Separation through Selective Crystallization Assisted by Polydentate Benzene-Aminoguanidinium Ligands. Inorg. Chem. 2021, 60, 6463–6471. [Google Scholar] [CrossRef]
- Zhu, L.; Sheng, D.; Xu, C.; Dai, X.; Silver, M.A.; Li, J.; Li, P.; Wang, Y.; Wang, Y.; Chen, L.; et al. Identifying the Recognition Site for Selective Trapping of 99TcO4- in a Hydrolytically Stable and Radiation Resistant Cationic Metal-Organic Framework. J. Am. Chem. Soc. 2017, 139, 14873–14876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xiao, C.; Dai, X.; Li, J.; Gui, D.; Sheng, D.; Chen, L.; Zhou, R.; Chai, Z.; Albrecht-Schmitt, T.E.; et al. Exceptional perrhenate/pertechnetate uptake and subsequent immobilization by a low-dimensional cationic coordination polymer: Overcoming the hofmeister bias selectivity. Environ. Sci. Technol. Lett. 2017, 4, 316–322. [Google Scholar] [CrossRef]
- Sheng, D.; Zhu, L.; Dai, X.; Xu, C.; Li, P.; Pearce, C.I.; Xiao, C.; Chen, J.; Zhou, R.; Duan, T.; et al. Successful Decontamination of 99TcO4− in Groundwater at Legacy Nuclear Sites by a Cationic Metal-Organic Framework with Hydrophobic Pockets. Angew. Chem. 2019, 131, 5022–5026. [Google Scholar] [CrossRef]
- Artemjev, A.A.; Novikov, A.P.; Burkin, G.M.; Sapronov, A.A.; Kubasov, A.S.; Nenajdenko, V.G.; Khrustalev, V.N.; Borisov, A.V.; Kirichuk, A.A.; Kritchenkov, A.S.; et al. Towards Anion Recognition and Precipitation with Water-Soluble 1,2,4-Selenodiazolium Salts: Combined Structural and Theoretical Study. Int. J. Mol. Sci. 2022, 23, 6372. [Google Scholar] [CrossRef] [PubMed]
- Chotkowski, M.; Wrzosek, B.; Grdeń, M. Intermediate oxidation states of technetium in concentrated sulfuric acid solutions. J. Electroanal. Chem. 2018, 814, 83–90. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shen, N.; Chen, L.; Guo, Q.; Chen, L.; He, L.; Dai, X.; Chai, Z.; Wang, S. Task-Specific Tailored Cationic Polymeric Network with High Base-Resistance for Unprecedented 99TcO4-Cleanup from Alkaline Nuclear Waste. ACS Cent. Sci. 2021, 7, 1441–1450. [Google Scholar] [CrossRef]
- Machura, B.; Kruszynski, R.; Kusz, J. A novel oxo-bridged rhenium complex with Re centers in different coordination environments–Synthesis, spectroscopic characterization and X-ray structure. Inorg. Chem. Commun. 2007, 10, 494–497. [Google Scholar] [CrossRef]
- Pervukhina, N.V.; Sokolov, M.N.; Fedorova, N.; Fedorov, V.E. Crystal Structures of Two Re(V) Complexes of 3,5-Dimethylpyrazole with Linear and Bent [OReOReO]4+ Fragments. J. Struct. Chem. 2001, 42, 833–837. [Google Scholar] [CrossRef]
- Kückmann, T.I.; Abram, U. Oxorhenium(V)- and Tricarbonylrhenium(I) Complexes with Substituted Pyrazoles as Products of the Degradation of Hydrotrispyrazolylborates. Z. Anorg. Allg. Chem. 2004, 630, 783–785. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Fedorova, N.E.; Pervukhina, N.V.; Peresypkina, E.V.; Virovets, A.V.; Pätow, R.; Fedorov, V.E.; Fenske, D. Rhenium(IV) and rhenium(V) complexes with 3,5-dimethylpyrazole. Russ. Chem. Bull. 2006, 55, 53–61. [Google Scholar] [CrossRef]
- Novikov, A.P.; Volkov, M.A.; Safonov, A.V.; Grigoriev, M.S. Synthesis, Crystal Structure, and Hirshfeld Surface Analysis of Hexachloroplatinate and Tetraclorouranylate of 3-Carboxypyridinium—Halogen Bonds and π-Interactions vs. Hydrogen Bonds. Crystals 2022, 12, 271. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalt. Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiwok, V. CH/π Interactions in Carbohydrate Recognition. Molecules 2017, 22, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hao, J.; Zhai, L.X.; Zhang, Y.; Dong, W.K. Synthesis, Crystal Structure, Luminescence, Electrochemical and Antimicrobial Properties of Bis(salamo)-Based Co(II) Complex. Crystals 2017, 7, 277. [Google Scholar] [CrossRef] [Green Version]
- Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in organic reactions. Tetrahedron 2005, 61, 6923–6950. [Google Scholar] [CrossRef]
- Zhuo, H.; Li, Q.; Li, W.; Cheng, J. Is π halogen bonding or lone pair⋯π interaction formed between borazine and some halogenated compounds? Phys. Chem. Chem. Phys. 2013, 16, 159–165. [Google Scholar] [CrossRef]
- Shah, M.B.; Liu, J.; Zhang, Q.; Stout, C.D.; Halpert, J.R. Halogen-π Interactions in the Cytochrome P450 Active Site: Structural Insights into Human CYP2B6 Substrate Selectivity. ACS Chem. Biol. 2017, 12, 1210. [Google Scholar] [CrossRef]
- Mitra, D.; Bankoti, N.; Michael, D.; Sekar, K.; Row, T.N.G. C-halogen…pi interactions in nucleic acids: A database study. J. Chem. Sci. 2020, 132, 93. [Google Scholar] [CrossRef]
- Riley, K.E.; Tran, K.A. Strength and Character of R–X···π Interactions Involving Aromatic Amino Acid Sidechains in Protein-Ligand Complexes Derived from Crystal Structures in the Protein Data Bank. Crystals 2017, 7, 273. [Google Scholar] [CrossRef] [Green Version]
- Dumitrescu, D.; Shova, S.; Man, I.C.; Caira, M.R.; Popa, M.M.; Dumitrascu, F. 5-Iodo-1-Arylpyrazoles as Potential Benchmarks for Investigating the Tuning of the Halogen Bonding. Crystals 2020, 10, 1149. [Google Scholar] [CrossRef]
- Novikov, A.P.; Volkov, M.A.; Safonov, A.V.; Grigoriev, M.S.; Abkhalimov, E.V. Synthesis and Characterization of New Guanine Complexes of Pt(IV) and Pd(II) by X-Ray Diffraction and Hirshfeld Surface Analysis. Crystals 2021, 11, 1417. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Scheiner, S. Noncovalent Forces; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 9783319141633. [Google Scholar]
- Psycharis, V.; Dermitzaki, D.; Raptopoulou, C.P. The Use of Hirshfeld Surface Analysis Tools to Study the Intermolecular Interactions in Single Molecule Magnets. Crystals 2021, 11, 1246. [Google Scholar] [CrossRef]
- Liu, M.; Yin, C.; Chen, P.; Zhang, M.; Parkin, S.; Zhou, P.; Li, T.; Yu, F.; Long, S. sp2CH⋯Cl hydrogen bond in the conformational polymorphism of 4-chloro-phenylanthranilic acid. CrystEngComm 2017, 19, 4345–4354. [Google Scholar] [CrossRef]
- Marek, P.H.; Urban, M.; Madura, I.D. The study of interactions with a halogen atom: Influence of NH2 group insertion on the crystal structures of meta-bromonitrobenzene derivatives. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 1509–1517. [Google Scholar] [CrossRef]
- Novikov, A.P.; Bezdomnikov, A.A.; Grigoriev, M.S.; German, K.E. Synthesis, crystal structure and Hirshfeld surface analysis of 2-(perfluorophenyl)acetamide in comparison with some related compounds. Acta Crystallogr. Sect. E Crystallogr. Commun. 2022, 78, 80–83. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dirghangi, B.K.; Menon, M.; Pramanik, A.; Chakravorty, A. A Triad of Variable-Valent Rhenium Aldimine and Amide Systems Interrelated by Successive Oxygen Atom Transfer. Inorg. Chem. 1997, 36, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, G.K.; Goswami, S.; Falvello, L.R.; Chakravorty, A. A New Family of Semibent Rhenium(V) Arylimides Formed by Azo Splitting: Structure, Bonding, and Electrooxidation to Rhenium(VI) Congeners. Inorg. Chem. 1987, 26, 3365–3370. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Gerber, T.I.A.; Fourie, P.J.; Van Wyk, A.J. The Chemistry of rhenium and technetium. Part 1. Syntheses and characterisation of new dioxo technetium(V) complexes with schiff base type ligands. Inorg. Chim. Acta 1984, 82, 201–205. [Google Scholar] [CrossRef]
- L’Annunziata, M.F. (Ed.) Handbook of Radioactivity Analysis: Volume 2. Radioanalytical Applications; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-814395-7. [Google Scholar]
- Bruker AXS Inc.: Madison, WI, USA. SAINT, V8.40B, Elsevier: Edinburgh, UK, 2020.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Identification Code | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C8H8Cl4N4O3Tc2 | C6H10Cl5N9O3Tc2 | C20H36Cl4N8O3Tc2 | C9H11Cl2N4O2Tc |
| Formula weight | 545.98 | 631.30 | 774.37 | 376.12 |
| Temperature/K | 100(2) | |||
| Crystal system | orthorhombic | monoclinic | tetragonal | triclinic |
| Space group | Pna21 | P21/n | I4 | P-1 |
| a/Å | 17.929(2) | 10.4766(9) | 10.059(6) | 6.168(3) |
| b/Å | 6.1567(9) | 8.4060(7) | 10.059(6) | 8.617(4) |
| c/Å | 14.726(2) | 21.6695(18) | 15.055(13) | 13.054(6) |
| α/° | 90 | 90 | 90 | 89.553(17) |
| β/° | 90 | 91.572(3) | 90 | 89.505(19) |
| γ/° | 90 | 90 | 90 | 69.338(16) |
| Volume/Å3 | 1625.5(4) | 1907.6(3) | 1523(2) | 649.2(5) |
| Z | 4 | 4 | 2 | 2 |
| ρcalcg/cm3 | 2.231 | 2.198 | 1.688 | 1.924 |
| μ/mm−1 | 2.368 | 2.176 | 1.294 | 1.519 |
| F(000) | 1048.0 | 1216.0 | 780.0 | 372.0 |
| Crystal size/mm3 | 0.3 × 0.12 × 0.05 | 0.22 × 0.09 × 0.06 | 0.06 × 0.05 × 0.04 | 0.39 × 0.12 × 0.04 |
| Radiation | MoKα (λ = 0.71073) | |||
| 2Θ range for data collection/° | 8.494 to 54.986 | 8.326 to 59.998 | 9.078 to 59.994 | 9.37 to 59.982 |
| Index ranges | −23 ≤ h ≤ 23, −7 ≤ k ≤ 7, −19 ≤ l ≤ 18 | −14 ≤ h ≤ 14, −11 ≤ k ≤ 9, −30 ≤ l ≤ 30 | −14 ≤ h ≤ 13, −13 ≤ k ≤ 13, −21 ≤ l ≤ 20 | −7 ≤ h ≤ 8, −12 ≤ k ≤ 11, −18 ≤ l ≤ 18 |
| Reflections collected | 18191 | 32465 | 3234 | 10884 |
| Independent reflections | 3664 [Rint = 0.1657, Rsigma = 0.1277] | 5544 [Rint = 0.0925, Rsigma = 0.0779] | 1764 [Rint = 0.1930, Rsigma = 0.3190] | 3691 [Rint = 0.1534, Rsigma = 0.2125] |
| Data/restraints/parameters | 3664/1/162 | 5544/0/239 | 1764/1/83 | 3691/0/165 |
| Goodness-of-fit on F2 | 1.025 | 1.023 | 0.941 | 0.986 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0691, wR2 = 0.1416 | R1 = 0.0426, wR2 = 0.0734 | R1 = 0.0971, wR2 = 0.1829 | R1 = 0.1046, wR2 = 0.2348 |
| Final R indexes [all data] | R1 = 0.1262, wR2 = 0.1680 | R1 = 0.0659, wR2 = 0.0805 | R1 = 0.2371, wR2 = 0.2429 | R1 = 0.1907, wR2 = 0.2894 |
| Largest diff. peak/hole/e Å−3 | 2.42/−1.45 | 0.97/−0.88 | 1.39/−1.28 | 3.04/−2.23 |
| Flack parameter | 0.5(2) | 0.06(19) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikov, A.P.; Volkov, M.A. New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis. Int. J. Mol. Sci. 2022, 23, 14034. https://doi.org/10.3390/ijms232214034
Novikov AP, Volkov MA. New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis. International Journal of Molecular Sciences. 2022; 23(22):14034. https://doi.org/10.3390/ijms232214034
Chicago/Turabian StyleNovikov, Anton Petrovich, and Mikhail Alexandrovich Volkov. 2022. "New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis" International Journal of Molecular Sciences 23, no. 22: 14034. https://doi.org/10.3390/ijms232214034





