Phytochemical Targeting of Mitochondria for Breast Cancer Chemoprevention, Therapy, and Sensitization
Abstract
:1. Introduction
2. The Landscape of Breast Cancer
2.1. Epidemiology
2.2. Treatment and Prevention
3. Phytochemicals for Breast Cancer Chemoprevention, Therapy, and Sensitization
3.1. Phytochemical Anticancer Agents and Application in Breast Cancer Therapy
3.2. Mitochondria: Essential Organelles for Breast Cancer Development and Progression
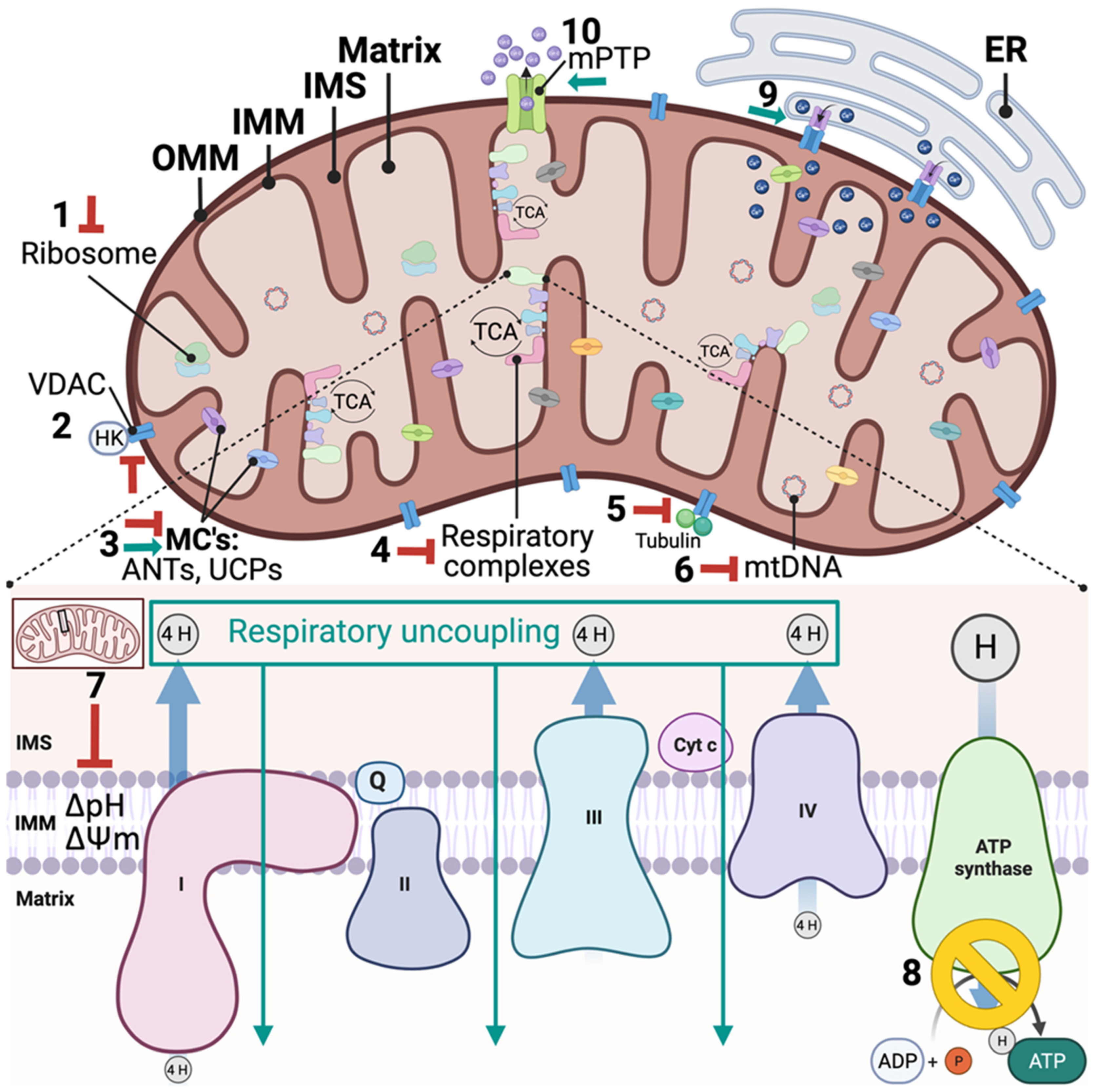
3.3. Phytochemically Targeting Mitochondria
4. Challenges and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Available online: https://seer.cancer.gov/explorer/ (accessed on 1 September 2022).
- Lv, L.; Yang, S.; Zhu, Y.; Zhai, X.; Li, S.; Tao, X.; Dong, D. Relationship between metabolic reprogramming and drug resistance in breast cancer. Front. Oncol. 2022, 12, 942064. [Google Scholar] [CrossRef] [PubMed]
- Germann, U.A.; Harding, M.W. Chemosensitizers to Overcome and Prevent Multidrug Resistance? JNCI J. Natl. Cancer Inst. 1995, 87, 1573–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woelfel, I.A.; Fernandez, L.J.; Idowu, M.O.; Takabe, K. A high burden of comorbid conditions leads to decreased survival in breast cancer. Gland Surg. 2018, 7, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, S.L.; Cahyono, R.; Prabowo, D.; Avanti, W.S.; Choridah, L.; Dwianingsih, E.K.; Harahap, W.A.; Aryandono, T. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer 2021, 21, 590. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Suzuki, R.; Orsini, N.; Saji, S.; Key, T.J.; Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int. J. Cancer 2009, 124, 698–712. [Google Scholar] [CrossRef]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef]
- Pruthi, S.; Heisey, R.E.; Bevers, T.B. Chemoprevention for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 3230–3235. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.S.; Renehan, A.G.; Saxton, J.M.; Bell, J.; Cade, J.; Cross, A.J.; King, A.; Riboli, E.; Sniehotta, F.; Treweek, S.; et al. Cancer prevention through weight control—Where are we in 2020? Br. J. Cancer 2021, 124, 1049–1056. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Telukunta, K.K.; Fobofou, S.A.T.; Chukwudi Osamor, V.; Egieyeh, S.A.; Valli, M.; Djoumbou-Feunang, Y.; Sorokina, M.; Stork, C.; Mathai, N.; et al. Computational Applications in Secondary Metabolite Discovery (CAiSMD): An online workshop. J. Cheminformatics 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, A.R.; Abdel-Azim, N.S.; Shams, K.A.; Hammouda, F.M. Targeting multidrug resistance in cancer by natural chemosensitizers. Bull. Natl. Res. Cent. 2019, 43, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, D.D.; Lakhawat, S.S.; Yasmeen, N.; Pandey, A.; Singla, R.K. Biogenic Phytochemicals Modulating Obesity: From Molecular Mechanism to Preventive and Therapeutic Approaches. Evid. Based Complement. Altern. Med. 2022, 2022, 6852276. [Google Scholar] [CrossRef]
- Shankar G, M.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharm. 2021, 12, 809308. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Gopalan, V.; Holland, O.; Neuzil, J. Mitocans Revisited: Mitochondrial Targeting as Efficient Anti-Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 7941. [Google Scholar] [CrossRef]
- Ferlay, J.L.M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow (accessed on 1 September 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bogdanova, N.; Helbig, S.; Dörk, T. Hereditary breast cancer: Ever more pieces to the polygenic puzzle. Hered. Cancer Clin. Pract. 2013, 11, 12. [Google Scholar] [CrossRef]
- Parkin, D.M. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105, S77–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Powers, S.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016, 529, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satariano, W.A. Comorbidity and functional status in older women with breast cancer: Implications for screening, treatment, and prognosis. J. Gerontol. 1992, 47, 24–31. [Google Scholar] [PubMed]
- Knobf, M.T. Psychosocial responses in breast cancer survivors. Semin. Oncol. Nurs. 2007, 23, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.R.; Axelrod, D.; Guth, A.A.; Cleland, C.M.; Ryan, C.E.; Weaver, K.R.; Qiu, J.M.; Kleinman, R.; Scagliola, J.; Palamar, J.J.; et al. Comorbidities and Quality of Life among Breast Cancer Survivors: A Prospective Study. J. Pers. Med. 2015, 5, 229–242. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.S.; Vitry, A.; Koczwara, B.; Roder, D.; McBride, M.L. Patterns of comorbidities in women with breast cancer: A Canadian population-based study. Cancer Causes Control 2019, 30, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Trivers, K.F.; Lund, M.J.; Porter, P.L.; Liff, J.M.; Flagg, E.W.; Coates, R.J.; Eley, J.W. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control 2009, 20, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Díaz, B.; Spínola-Maroño, H.; Reynoso, N.; González-Aguilar, A.; Mohar-Betancourt, A. Role of Overweight, Obesity, and Comorbidities in the Prognosis of Patients with Breast Cancer with Brain Metastases. Clin. Breast Cancer 2019, 19, e394–e398. [Google Scholar] [CrossRef]
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, H.P. Poverty, culture, and social injustice: Determinants of cancer disparities. CA Cancer J. Clin. 2004, 54, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.H.; Neuhouser, M.L.; Agurs-Collins, T.; Zanetti, K.A.; Cadmus-Bertram, L.; Dean, L.T.; Drake, B.F. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J. Natl. Cancer Inst. 2013, 105, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, M.J.; Neumann, S.; Campbell, K.; Pattison, S.T.; Young, S.L. The Effects of Obesity on Anti-Cancer Immunity and Cancer Immunotherapy. Cancers 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Faguet, G.B. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int. J. Cancer 2015, 136, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Friese, C.R.; Harrison, J.M.; Janz, N.K.; Jagsi, R.; Morrow, M.; Li, Y.; Hamilton, A.S.; Ward, K.C.; Kurian, A.W.; Katz, S.J.; et al. Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer 2017, 123, 1925–1934. [Google Scholar] [CrossRef] [Green Version]
- Gallo, M.; Adinolfi, V.; Barucca, V.; Prinzi, N.; Renzelli, V.; Barrea, L.; Di Giacinto, P.; Ruggeri, R.M.; Sesti, F.; Arvat, E.; et al. Expected and paradoxical effects of obesity on cancer treatment response. Rev. Endocr. Metab. Disord. 2020, 22, 681–702. [Google Scholar] [CrossRef]
- FDA Administration. Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed on 9 September 2022).
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dees, S.; Ganesan, R.; Singh, S.; Grewal, I.S. Emerging CAR-T Cell Therapy for the Treatment of Triple-Negative Breast Cancer. Mol. Cancer Ther. 2020, 19, 2409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, W.; He, D.; Wang, S.; Lou, J.; Jiang, Y.; Wang, S. Recent Progress on Immunotherapy for Breast Cancer: Tumor Microenvironment, Nanotechnology and More. Front. Bioeng. Biotechnol. 2021, 9, 680315. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, M.; Wang, B.; Zhang, L.; Fang, M.; Zhou, F. Chemoresistance and Metastasis in Breast Cancer Molecular Mechanisms and Novel Clinical Strategies. Front. Oncol. 2021, 11, 658552. [Google Scholar] [CrossRef]
- Taylor, D.J.; Parsons, C.E.; Han, H.; Jayaraman, A.; Rege, K. Parallel screening of FDA-approved antineoplastic drugs for identifying sensitizers of TRAIL-induced apoptosis in cancer cells. BMC Cancer 2011, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs with P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Force, U.P.S.T. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 857–867. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Bonanni, B. Breast cancer chemoprevention: Old and new approaches. J. Biomed. Biotechnol. 2012, 2012, 985620. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.L. Topoisomerase I inhibitors: Review and update. Ann. Oncol. 1997, 8, 837–855. [Google Scholar] [CrossRef]
- Fresno, M.; Jiménez, A.; Vázquez, D. Inhibition of translation in eukaryotic systems by harringtonine. Eur. J. Biochem. 1977, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Rowe, T.; Glisson, B.; Yalowich, J.; Liu, L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984, 44, 5857–5860. [Google Scholar] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Hamel, S.; McNair, D.S.; Birkett, N.J.; Mattison, D.R.; Krantis, A.; Krewski, D. Off-label use of cancer therapies in women diagnosed with breast cancer in the United States. Springerplus 2015, 4, 209. [Google Scholar] [CrossRef] [Green Version]
- Coussy, F.; El-Botty, R.; Château-Joubert, S.; Dahmani, A.; Montaudon, E.; Leboucher, S.; Morisset, L.; Painsec, P.; Sourd, L.; Huguet, L.; et al. BRCAness, SLFN11, and RB1 loss predict response to topoisomerase I inhibitors in triple-negative breast cancers. Sci. Transl. Med. 2020, 12, eaax2625. [Google Scholar] [CrossRef] [Green Version]
- Segar, J.M.; Reed, D.; Stopeck, A.; Livingston, R.B.; Chalasani, P. A Phase II Study of Irinotecan and Etoposide as Treatment for Refractory Metastatic Breast Cancer. Oncologist 2019, 24, 1512-e1267. [Google Scholar] [CrossRef] [Green Version]
- Yakhni, M.; Briat, A.; El Guerrab, A.; Furtado, L.; Kwiatkowski, F.; Miot-Noirault, E.; Cachin, F.; Penault-Llorca, F.; Radosevic-Robin, N. Homoharringtonine, an approved anti-leukemia drug, suppresses triple negative breast cancer growth through a rapid reduction of anti-apoptotic protein abundance. Am. J. Cancer Res. 2019, 9, 1043–1060. [Google Scholar]
- Wang, L.B.; Wang, D.N.; Wu, L.G.; Cao, J.; Tian, J.H.; Liu, R.; Ma, R.; Yu, J.J.; Wang, J.; Huang, Q.; et al. Homoharringtonine inhibited breast cancer cells growth via miR-18a-3p/AKT/mTOR signaling pathway. Int. J. Biol. Sci. 2021, 17, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.P.; Ding, G.X.; Gao, H.Y.; Shen, Z.Z.; Li, K.Y. A clinical trial of homoharringtonine in the treatment of advanced breast cancer. Tumori 1986, 72, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Milani, A.; Ghisoni, E.; Genta, S.; Mittica, G.; Montemurro, F.; Valabrega, G. Oral etoposide in heavily pre-treated metastatic breast cancer: A retrospective series. Breast 2018, 38, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.R.; Muthusamy, G.; Shanmugam, M.; Ambudkar, S.V. South Asian Medicinal Compounds as Modulators of Resistance to Chemotherapy and Radiotherapy. Cancers 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkham, A.A.; King, K.; Joy, A.A.; Pelletier, A.B.; Mackey, J.R.; Young, K.; Zhu, X.; Meza-Junco, J.; Basi, S.K.; Hiller, J.P.; et al. Rationale and design of the Diet Restriction and Exercise-induced Adaptations in Metastatic breast cancer (DREAM) study: A 2-arm, parallel-group, phase II, randomized control trial of a short-term, calorie-restricted, and ketogenic diet plus exercise during intravenous chemotherapy versus usual care. BMC Cancer 2021, 21, 1093. [Google Scholar] [CrossRef]
- Muttiah, C.; Whittle, J.R.; Oakman, C.; Lindeman, G.J. PALVEN: Phase Ib trial of palbociclib, letrozole and venetoclax in estrogen receptor- and BCL2-positive advanced breast cancer. Future Oncol. 2022, 18, 1805–1816. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Manouchehri, J.M.; Kalafatis, M. Ursolic Acid Promotes the Sensitization of rhTRAIL-resistant Triple-negative Breast Cancer. Anticancer Res. 2018, 38, 6789–6795. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Gu, J.; Wang, S.; Wang, N.; Yang, B.; Zhang, F.; Wang, D.; Fu, W.; Wang, Z. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78. Cell Death Dis. 2018, 9, 636. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Chowdhury, N.; Singh, M. Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Sci. Rep. 2017, 7, 15824. [Google Scholar] [CrossRef] [PubMed]
- Pai Bellare, G.; Sankar Patro, B. Resveratrol sensitizes breast cancer to PARP inhibitor, talazoparib through dual inhibition of AKT and autophagy flux. Biochem. Pharmacol. 2022, 199, 115024. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wu, J.; Han, S.; Liu, Y.; Su, Z.; Zhu, H.-L.; Liu, H.-K.; Qian, Y. Biotinylated curcumin as a novel chemosensitizer enhances naphthalimide-induced autophagic cell death in breast cancer cells. Eur. J. Med. Chem. 2022, 228, 114029. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Choi, C.-H. Characterization of SN38-resistant T47D breast cancer cell sublines overexpressing BCRP, MRP1, MRP2, MRP3, and MRP4. BMC Cancer 2022, 22, 446. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Dorsey, J.; Griffith, J.K.; Bisoffi, M.; Trujillo, K.A. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int. J. Cancer 2013, 133, 2504–2510. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Haritha, N.H.; Nawab, A.; Vijayakurup, V.; Anto, N.P.; Liju, V.B.; Alex, V.V.; Amrutha, A.N.; Aiswarya, S.U.; Swetha, M.; Vinod, B.S.; et al. Targeting Thymidylate Synthase Enhances the Chemosensitivity of Triple-Negative Breast Cancer Towards 5-FU-Based Combinatorial Therapy. Front. Oncol. 2021, 11, 656804. [Google Scholar] [CrossRef]
- Reddy, C.A.; Somepalli, V.; Golakoti, T.; Kanugula, A.K.; Karnewar, S.; Rajendiran, K.; Vasagiri, N.; Prabhakar, S.; Kuppusamy, P.; Kotamraju, S.; et al. Mitochondrial-Targeted Curcuminoids: A Strategy to Enhance Bioavailability and Anticancer Efficacy of Curcumin. PLoS ONE 2014, 9, e89351. [Google Scholar] [CrossRef] [Green Version]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.W.; Lim, H.Y.; Wong, K.P. Uncoupling of oxidative phosphorylation by curcumin: Implication of its cellular mechanism of action. Biochem. Biophys. Res. Commun. 2009, 389, 187–192. [Google Scholar] [CrossRef]
- Moustapha, A.; Pérétout, P.A.; Rainey, N.E.; Sureau, F.; Geze, M.; Petit, J.M.; Dewailly, E.; Slomianny, C.; Petit, P.X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015, 1, 15017. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Phenolic Compounds Cannabidiol, Curcumin and Quercetin Cause Mitochondrial Dysfunction and Suppress Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 204. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Ronghe, A.; Padhye, S.B.; Spade, D.A.; Bhat, N.K.; Bhat, H.K. Antioxidant activities of novel resveratrol analogs in breast cancer. J. Biochem. Mol. Toxicol. 2018, 32, e21925. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evid.-Based Complement. Altern. Med. 2015, 2015, 527209. [Google Scholar] [CrossRef] [Green Version]
- Aghazadeh, T.; Bakhtiari, N.; Rad, I.A.; Ramezani, F. Formulation of Kaempferol in Nanostructured Lipid Carriers (NLCs): A Delivery Platform to Sensitization of MDA-MB468 Breast Cancer Cells to Paclitaxel. Biointerface Res. Appl. Chem. 2021, 11, 14591–14601. [Google Scholar]
- Nandi, S.K.; Roychowdhury, T.; Chattopadhyay, S.; Basu, S.; Chatterjee, K.; Choudhury, P.; Banerjee, N.; Saha, P.; Mukhopadhyay, S.; Mukhopadhyay, A.; et al. Deregulation of the CD44-NANOG-MDR1 associated chemoresistance pathways of breast cancer stem cells potentiates the anti-cancer effect of Kaempferol in synergism with Verapamil. Toxicol. Appl. Pharm. 2022, 437, 115887. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef]
- Verma, A.K.; Johnson, J.A.; Gould, M.N.; Tanner, M.A. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988, 48, 5754–5758. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xue, L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(-)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Choi, Y.K.; Woo, J.K.; Kim, M.; Kim, I.; Na, C.H.; Hur, H.; Jang, B.H.; et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncol. Rep. 2016, 36, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Prado, I.M.R.; Helena, A.F.C.; Uyemura, S.A.; Santos, A.C.; Curti, C. The interaction of flavonoids with mitochondria: Effects on energetic processes. Chem.-Biol. Interact. 2005, 152, 67–78. [Google Scholar] [CrossRef]
- Ortega, R.; García, N. The flavonoid quercetin induces changes in mitochondrial permeability by inhibiting adenine nucleotide translocase. J. Bioenerg. Biomembr. 2009, 41, 41–47. [Google Scholar] [CrossRef]
- Lang, D.R.; Racker, E. Effects of quercetin and F1 inhibitor on mitochondrial ATPase and energy-linked reactions in submitochondrial particles. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1974, 333, 180–186. [Google Scholar] [CrossRef]
- Baptista Moreno Martin, A.C.; Tomasin, R.; Luna-Dulcey, L.; Graminha, A.E.; Araújo Naves, M.; Teles, R.H.G.; da Silva, V.D.; da Silva, J.A.; Vieira, P.C.; Annabi, B.; et al. [10]-Gingerol improves doxorubicin anticancer activity and decreases its side effects in triple negative breast cancer models. Cell Oncol. (Dordr) 2020, 43, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Wala, K.; Szlasa, W.; Sauer, N.; Kasperkiewicz-Wasilewska, P.; Szewczyk, A.; Saczko, J.; Rembiałkowska, N.; Kulbacka, J.; Baczyńska, D. Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma. Molecules 2022, 27, 2693. [Google Scholar] [CrossRef]
- Konmun, J.; Danwilai, K.; Ngamphaiboon, N.; Sripanidkulchai, B.; Sookprasert, A.; Subongkot, S. A phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med. Oncol. 2017, 34, 69. [Google Scholar] [CrossRef]
- Martin, A.; Fuzer, A.M.; Becceneri, A.B.; da Silva, J.A.; Tomasin, R.; Denoyer, D.; Kim, S.H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B.; et al. [10]-Gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget 2017, 8, 72260–72271. [Google Scholar] [CrossRef] [Green Version]
- Sp, N.; Kang, D.Y.; Lee, J.M.; Bae, S.W.; Jang, K.J. Potential Antitumor Effects of 6-Gingerol in p53-Dependent Mitochondrial Apoptosis and Inhibition of Tumor Sphere Formation in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4660. [Google Scholar] [CrossRef]
- Bernard, M.M.; McConnery, J.R.; Hoskin, D.W. [10]-Gingerol, a major phenolic constituent of ginger root, induces cell cycle arrest and apoptosis in triple-negative breast cancer cells. Exp. Mol. Pathol. 2017, 102, 370–376. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Yu, S.T.; Chen, T.M.; Tseng, S.Y.; Chen, Y.H. Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2007, 358, 79–84. [Google Scholar] [CrossRef]
- Zeng, Q.; Luo, C.; Cho, J.; Lai, D.; Shen, X.; Zhang, X.; Zhou, W. Tryptanthrin exerts anti-breast cancer effects both in vitro and in vivo through modulating the inflammatory tumor microenvironment. Acta Pharm. 2021, 71, 245–266. [Google Scholar] [CrossRef]
- Qin, Q.P.; Zou, B.Q.; Hu, F.L.; Huang, G.B.; Wang, S.L.; Gu, Y.Q.; Tan, M.X. Platinum(ii) complexes with rutaecarpine and tryptanthrin derivatives induce apoptosis by inhibiting telomerase activity and disrupting mitochondrial function. Medchemcomm 2018, 9, 1639–1648. [Google Scholar] [CrossRef]
- Zhang, L.; Lau, Y.K.; Xia, W.; Hortobagyi, G.N.; Hung, M.C. Tyrosine kinase inhibitor emodin suppresses growth of HER-2/neu-overexpressing breast cancer cells in athymic mice and sensitizes these cells to the inhibitory effect of paclitaxel. Clin. Cancer Res. 1999, 5, 343–353. [Google Scholar]
- Li, B.; Zhao, X.; Zhang, L.; Cheng, W. Emodin Interferes with AKT1-Mediated DNA Damage and Decreases Resistance of Breast Cancer Cells to Doxorubicin. Front. Oncol. 2020, 10, 588533. [Google Scholar] [CrossRef]
- Fu, J.M.; Zhou, J.; Shi, J.; Xie, J.S.; Huang, L.; Yip, A.Y.; Loo, W.T.; Chow, L.W.; Ng, E.L. Emodin affects ERCC1 expression in breast cancer cells. J. Transl. Med. 2012, 10, S7. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Yu, F.; Wang, J.; Iwanowycz, S.; Saaoud, F.; Wang, Y.; Hu, J.; Wang, Q.; Fan, D. Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res. Treat. 2014, 148, 291–302. [Google Scholar] [CrossRef]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef]
- Dumit, V.I.; Zerbes, R.M.; Kaeser-Pebernard, S.; Rackiewicz, M.; Wall, M.T.; Gretzmeier, C.; Küttner, V.; van der Laan, M.; Braun, R.J.; Dengjel, J. Respiratory status determines the effect of emodin on cell viability. Oncotarget 2017, 8, 37478–37490. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, C.J.; Bacus, S.S.; Hung, M.C. Suppressed transformation and induced differentiation of HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res. 1995, 55, 3890–3896. [Google Scholar]
- Akkol, E.K.; Tatlı, I.I.; Karatoprak, G.; Ağar, O.T.; Yücel, Ç.; Sobarzo-Sánchez, E.; Capasso, R. Is Emodin with Anticancer Effects Completely Innocent? Two Sides of the Coin. Cancers 2021, 13, 2733. [Google Scholar] [CrossRef]
- Khan, S.A.; Chatterton, R.T.; Michel, N.; Bryk, M.; Lee, O.; Ivancic, D.; Heinz, R.; Zalles, C.M.; Helenowski, I.B.; Jovanovic, B.D.; et al. Soy isoflavone supplementation for breast cancer risk reduction: A randomized phase II trial. Cancer Prev. Res. (Phila) 2012, 5, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Mai, Z.; Blackburn, G.L.; Zhou, J.R. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog 2007, 46, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 106. [Google Scholar] [CrossRef]
- de Oliveira, M.R. Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 2016, 29, 35–44. [Google Scholar] [CrossRef]
- Wang, S.; Chang, X.; Zhang, J.; Li, J.; Wang, N.; Yang, B.; Pan, B.; Zheng, Y.; Wang, X.; Ou, H.; et al. Ursolic Acid Inhibits Breast Cancer Metastasis by Suppressing Glycolytic Metabolism via Activating SP1/Caveolin-1 Signaling. Front. Oncol. 2021, 11, 745584. [Google Scholar] [CrossRef]
- Feng, X.M.; Su, X.L. Anticancer effect of ursolic acid via mitochondria-dependent pathways. Oncol. Lett 2019, 17, 4761–4767. [Google Scholar] [CrossRef] [Green Version]
- Bazin, M.-A.; Lamer, A.-C.L.; Delcros, J.-G.; Rouaud, I.; Uriac, P.; Boustie, J.; Corbel, J.-C.; Tomasi, S. Synthesis and cytotoxic activities of usnic acid derivatives. Bioorganic Med. Chem. 2008, 16, 6860–6866. [Google Scholar] [CrossRef]
- Abo-Khatwa, A.N.; al-Robai, A.A.; al-Jawhari, D.A. Lichen acids as uncouplers of oxidative phosphorylation of mouse-liver mitochondria. Nat. Toxins 1996, 4, 96–102. [Google Scholar] [CrossRef]
- Fulda, S.; Scaffidi, C.; Susin, S.A.; Krammer, P.H.; Kroemer, G.; Peter, M.E.; Debatin, K.M. Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J. Biol. Chem. 1998, 273, 33942–33948. [Google Scholar] [CrossRef] [Green Version]
- Quisbert-Valenzuela, E.O.; Calaf, G.M. Apoptotic effect of noscapine in breast cancer cell lines. Int. J. Oncol. 2016, 48, 2666–2674. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, L.; Sert, M.A.; Kelmer-Bracht, A.M.; Bracht, A.; Ishii-Iwamoto, E.L. Effects of rutin and quercetin on mitochondrial metabolism and on ATP levels in germinating tissues of Glycine max. Plant Physiol. Biochem. 1998, 36, 495–501. [Google Scholar] [CrossRef]
- Lan, Y.; Sun, Y.; Yang, T.; Ma, X.; Cao, M.; Liu, L.; Yu, S.; Cao, A.; Liu, Y. Co-Delivery of Paclitaxel by a Capsaicin Prodrug Micelle Facilitating for Combination Therapy on Breast Cancer. Mol. Pharm. 2019, 16, 3430–3440. [Google Scholar] [CrossRef]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Haramizu, S.; Oki, K.; Ohnuki, K.; Watanabe, T.; Yazawa, S.; Kawada, T.; Hashizume, S.-i.; Fushiki, T. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J. Appl. Physiol. 2003, 95, 2408–2415. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Yan, C.-Y.; Zhou, Q.-Q.; Zhen, L.-L. Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomed. Pharmacother. 2017, 89, 845–856. [Google Scholar] [CrossRef]
- Weston Ainsley, H.C.C. Multistage Carcinogenesis. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W.P.R., Weichselbaum, R.R., Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Hlatky, L.; Hahnfeldt, P. Beyond the cancer cell: Progression-level determinants highlight the multiscale nature of carcinogenesis risk. Cancer Res. 2014, 74, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Nam, J.-S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef]
- Giampazolias, E.; Tait, S.W. Mitochondria and the hallmarks of cancer. FEBS J. 2016, 283, 803–814. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S.; LaFramboise, T. Mutational patterns in the breast cancer mitochondrial genome, with clinical correlates. Carcinogenesis 2014, 35, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Putignani, L.; Raffa, S.; Pescosolido, R.; Rizza, T.; Del Chierico, F.; Leone, L.; Aimati, L.; Signore, F.; Carrozzo, R.; Callea, F.; et al. Preliminary evidences on mitochondrial injury and impaired oxidative metabolism in breast cancer. Mitochondrion 2012, 12, 363–369. [Google Scholar] [CrossRef]
- Zunica, E.R.M.; Axelrod, C.L.; Cho, E.; Spielmann, G.; Davuluri, G.; Alexopoulos, S.J.; Beretta, M.; Hoehn, K.L.; Dantas, W.S.; Stadler, K.; et al. Breast cancer growth and proliferation is suppressed by the mitochondrial targeted furazano [3,4-b]pyrazine BAM15. Cancer Metab. 2021, 9, 36. [Google Scholar] [CrossRef]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial mutations in cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef] [Green Version]
- Moindjie, H.; Rodrigues-Ferreira, S.; Nahmias, C. Mitochondrial Metabolism in Carcinogenesis and Cancer Therapy. Cancers 2021, 13, 3311. [Google Scholar] [CrossRef]
- Marino, N.; German, R.; Rao, X.; Simpson, E.; Liu, S.; Wan, J.; Liu, Y.; Sandusky, G.; Jacobsen, M.; Stoval, M.; et al. Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. NPJ Breast Cancer 2020, 6, 50. [Google Scholar] [CrossRef]
- Li, Y.-J.; Fahrmann, J.F.; Aftabizadeh, M.; Zhao, Q.; Tripathi, S.C.; Zhang, C.; Yuan, Y.; Ann, D.; Hanash, S.; Yu, H. Fatty acid oxidation protects cancer cells from apoptosis by increasing mitochondrial membrane lipids. Cell Rep. 2022, 39, 110870. [Google Scholar] [CrossRef]
- Liu, H. Application of Immunohistochemistry in Breast Pathology: A Review and Update. Arch. Pathol. Lab. Med. 2014, 138, 1629–1642. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Iyengar, P.; Espina, V.; Williams, T.W.; Lin, Y.; Berry, D.; Jelicks, L.A.; Lee, H.; Temple, K.; Graves, R.; Pollard, J.; et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Investig. 2005, 115, 1163–1176. [Google Scholar] [CrossRef] [Green Version]
- Andarawewa, K.L.; Motrescu, E.R.; Chenard, M.P.; Gansmuller, A.; Stoll, I.; Tomasetto, C.; Rio, M.C. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005, 65, 10862–10871. [Google Scholar] [CrossRef] [Green Version]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Attané, C.; Milhas, D.; Dirat, B.; Dauvillier, S.; Guerard, A.; Gilhodes, J.; Lazar, I.; Alet, N.; Laurent, V.; et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2017, 2, 87489. [Google Scholar] [CrossRef] [Green Version]
- Dupuy, F.; Tabariès, S.; Andrzejewski, S.; Dong, Z.; Blagih, J.; Annis, M.G.; Omeroglu, A.; Gao, D.; Leung, S.; Amir, E.; et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015, 22, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Montes-Alvarado, J.B.; Ramírez-Ramírez, D.; Flores-Alonso, J.C.; Cortés-Hernández, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Haukaas, T.H.; Euceda, L.R.; Giskeødegård, G.F.; Lamichhane, S.; Krohn, M.; Jernström, S.; Aure, M.R.; Lingjærde, O.C.; Schlichting, E.; Garred, Ø.; et al. Metabolic clusters of breast cancer in relation to gene- and protein expression subtypes. Cancer Metab. 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [Green Version]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Gandhi, N.; Das, G.M. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 2019, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Giddings, E.L.; Champagne, D.P.; Wu, M.-H.; Laffin, J.M.; Thornton, T.M.; Valenca-Pereira, F.; Culp-Hill, R.; Fortner, K.A.; Romero, N.; East, J.; et al. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat. Commun. 2021, 12, 2804. [Google Scholar] [CrossRef]
- Tekedereli, I.; Alpay, S.N.; Akar, U.; Yuca, E.; Ayugo-Rodriguez, C.; Han, H.-D.; Sood, A.K.; Lopez-Berestein, G.; Ozpolat, B. Therapeutic Silencing of Bcl-2 by Systemically Administered siRNA Nanotherapeutics Inhibits Tumor Growth by Autophagy and Apoptosis and Enhances the Efficacy of Chemotherapy in Orthotopic Xenograft Models of ER (−) and ER (+) Breast Cancer. Mol. Ther.-Nucleic Acids 2013, 2, e121. [Google Scholar] [CrossRef]
- Ralph, S.J.; Low, P.; Dong, L.; Lawen, A.; Neuzil, J. Mitocans: Mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents. Recent Pat. Anticancer Drug Discov. 2006, 1, 327–346. [Google Scholar] [CrossRef]
- Neuzil, J.; Dong, L.F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208. [Google Scholar] [CrossRef]
- Rovini, A.; Savry, A.; Braguer, D.; Carré, M. Microtubule-targeted agents: When mitochondria become essential to chemotherapy. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef] [Green Version]
- Ferlini, C.; Cicchillitti, L.; Raspaglio, G.; Bartollino, S.; Cimitan, S.; Bertucci, C.; Mozzetti, S.; Gallo, D.; Persico, M.; Fattorusso, C.; et al. Paclitaxel Directly Binds to Bcl-2 and Functionally Mimics Activity of Nur77. Cancer Res. 2009, 69, 6906–6914. [Google Scholar] [CrossRef] [Green Version]
- Kidd, J.F.; Pilkington, M.F.; Schell, M.J.; Fogarty, K.E.; Skepper, J.N.; Taylor, C.W.; Thorn, P. Paclitaxel Affects Cytosolic Calcium Signals by Opening the Mitochondrial Permeability Transition Pore *. J. Biol. Chem. 2002, 277, 6504–6510. [Google Scholar] [CrossRef] [Green Version]
- Goffart, S.; Hangas, A.; Pohjoismäki, J.L.O. Twist and Turn-Topoisomerase Functions in Mitochondrial DNA Maintenance. Int. J. Mol. Sci. 2019, 20, 2041. [Google Scholar] [CrossRef]
- Lin, J.H.; Castora, F.J. DNA topoisomerase II from mammalian mitochondria is inhibited by the antitumor drugs, m-AMSA and VM-26. Biochem. Biophys. Res. Commun. 1991, 176, 690–697. [Google Scholar] [CrossRef]
- Robertson, J.D.; Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. Distinct Pathways for Stimulation of Cytochrome cRelease by Etoposide*. J. Biol. Chem. 2000, 275, 32438–32443. [Google Scholar] [CrossRef] [Green Version]
- de la Loza, M.C.D.; Wellinger, R.E. A novel approach for organelle-specific DNA damage targeting reveals different susceptibility of mitochondrial DNA to the anticancer drugs camptothecin and topotecan. Nucleic Acids Res. 2009, 37, e26. [Google Scholar] [CrossRef] [Green Version]
- Myasnikov, A.G.; Kundhavai Natchiar, S.; Nebout, M.; Hazemann, I.; Imbert, V.; Khatter, H.; Peyron, J.F.; Klaholz, B.P. Structure-function insights reveal the human ribosome as a cancer target for antibiotics. Nat. Commun. 2016, 7, 12856. [Google Scholar] [CrossRef] [Green Version]
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 2014, 7, 913–942. [Google Scholar] [CrossRef] [Green Version]
- Pratheeshkumar, P.; Sreekala, C.; Zhang, Z.; Budhraja, A.; Ding, S.; Son, Y.-O.; Wang, X.; Hitron, A.; Hyun-Jung, K.; Wang, L.; et al. Cancer prevention with promising natural products: Mechanisms of action and molecular targets. Anticancer Agents Med. Chem. 2012, 12, 1159–1184. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal 2018, 29, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, C.; Driessen, A.J.; Recourt, K. The uncoupling efficiency and affinity of flavonoids for vesicles. Biochem. Pharm. 2000, 60, 1593–1600. [Google Scholar] [CrossRef] [Green Version]
- Cocetta, V.; Quagliariello, V.; Fiorica, F.; Berretta, M.; Montopoli, M. Resveratrol as Chemosensitizer Agent: State of Art and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 2049. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wendorff, T.J.; Berger, J.M. Resveratrol: A novel type of topoisomerase II inhibitor. J. Biol. Chem. 2017, 292, 21011–21022. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, J.R.; Walker, J.E. Inhibition sites in F1-ATPase from bovine heart mitochondria. Biochem. J. 2005, 386, 591–598. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Parichatikanond, W.; Eroglu, E.; Klec, C.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Resveratrol Specifically Kills Cancer Cells by a Devastating Increase in the Ca2+; Coupling between the Greatly Tethered Endoplasmic Reticulum and Mitochondria. Cell. Physiol. Biochem. 2016, 39, 1404–1420. [Google Scholar] [CrossRef]
- Yu, H.; Sun, C.; Gong, Q.; Feng, D. Mitochondria-Associated Endoplasmic Reticulum Membranes in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 629669. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Dose-Dependent Interaction of trans-Resveratrol with Biomembranes: Effects on Antioxidant Property. J. Med. Chem. 2013, 56, 970–981. [Google Scholar] [CrossRef]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Colloids Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Sala de Oyanguren, F.J.; Rainey, N.E.; Moustapha, A.; Saric, A.; Sureau, F.; O’Connor, J.-E.; Petit, P.X. Highlighting Curcumin-Induced Crosstalk between Autophagy and Apoptosis as Supported by Its Specific Subcellular Localization. Cells 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Lv, L.; Shen, Y.; Hu, Z.; He, Q.; Chen, X. A nanoparticulate pre-chemosensitizer for efficacious chemotherapy of multidrug resistant breast cancer. Sci. Rep. 2016, 6, 21459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Langin, H.; Lefebvre, G.; Tresch-Bruneel, E.; Deley, M.-C.L.; Marliot, G.; Sakji, I.; Vanseymortier, M.; Reich, M.; Lartigau, E.; Bonneterre, J. Prevalence of herbal medicine (HM) use among breast cancer patients treated with chemotherapy, hormone therapy, or targeted therapy. J. Clin. Oncol. 2018, 36, e13108. [Google Scholar] [CrossRef]
- Morris, K.T.; Johnson, N.; Homer, L.; Walts, D. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am. J. Surg. 2000, 179, 407–411. [Google Scholar] [CrossRef]
- Damery, S.; Gratus, C.; Grieve, R.; Warmington, S.; Jones, J.; Routledge, P.; Greenfield, S.; Dowswell, G.; Sherriff, J.; Wilson, S. The use of herbal medicines by people with cancer: A cross-sectional survey. Br. J. Cancer 2011, 104, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Orfi, N.E.; Boutayeb, S.; Rahou, B.H.; Aitouma, A.; Souadka, A. Use of medicinal plants by cancer patients at the National Institute of Oncology, Rabat: A cross-sectional survey. Pan Afr. Med. J. 2021, 40, 18. [Google Scholar] [CrossRef]
- Mao, J.J.; Palmer, C.S.; Healy, K.E.; Desai, K.; Amsterdam, J. Complementary and alternative medicine use among cancer survivors: A population-based study. J. Cancer Surviv. 2011, 5, 8–17. [Google Scholar] [CrossRef]
| Secondary Metabolite Group | Type | Sub-Class | Phytochemical Example | Use in Breast Cancer |
|---|---|---|---|---|
| Nitrogen and/or Sulfur containing compounds | Alkaloid | Indole (commonly referred to by Genus: Vinca) | Vincristine, Vinblastin, Vinorelbine, Vindesine | Vinblastine Sulfate-FDA-approved to treat advanced-stage breast cancer Vinorelbine off-label use for metastatic breast cancer [60] |
| Monoterpene Indole (Camptothecan analogs) | Irinotecan, Topotecan | Under pre-clinical and clinical investigation: Irinotecan displayed therapeutic efficacy in preclinical patient-derived TNBC models [61] Displayed modest clinical efficacy and low tolerability in combination with etoposide in patients with metastatic breast cancer [62] | ||
| Isoquinoline | Homoharringtonine | Under preclinical investigation: Inhibited tumor growth in preclinical TNBC models [63] and HR+ model [64] No improved response rate or survival in combination with cyclophosphamide, methotrexate, and fluorouracil in patients with advanced-stage breast cancer [65] | ||
| Phenols | Lignan | Aryltetralin (Podophyllotoxins) | Etoposide, Tenisopide, Etopophos | Off-label clinical use for metastatic breast cancer [66] Under clinical investigation: Displayed modest clinical efficacy and low tolerability in combination with ironotecan in patients with metastatic breast cancer [62] |
| Terpenoids | Diterpene | Taxanes | Paclitaxel, Docetaxel | Paclitaxel derivatives and Docetaxel FDA-approved to treat chemoresistant and advanced breast cancer |
| Phytochemical | Examples of Anti-Cancer Evidence | Examples of Mitochondrial Mechanism of Action Evidence |
|---|---|---|
| Curcumin | Chemoprevention Pre-clinical
ClinicalPre-clinical In vitro | Mitochondrial respiratory uncoupling [84] Increased respiration, decreased ΔΨm, inhibited electron transfer at high concentrations [85] Rapid calcium influx into the mitochondria and sustained decrease in ΔΨm [86] |
| Resveratrol | Chemoprevention Clinical
In vitro and Pre-clinical
In vitro and Pre-clinical
| Indirect mitochondrial respiratory uncoupling through uncoupling proteins regulation [90] |
| Kaempferol | Chemosensitization In vitro In vitro and Pre-clinical | Modulated BcL-2 protein, decreased ΔΨm, increased caspase signaling [96] |
| Quercetin | Chemoprevention Pre-clinical
In vitro and Pre-clinical
| Mitochondrial respiratory uncoupling [99] Increased respiration, decreased ΔΨm, inhibited ANT, opened MPTP, increased cytochrome c release [100] Mitochondrial ATPase F1 inhibitor [101] |
| Gingerol | Chemosensitization Pre-clinical
Clinical
Pre-clinical
| Modulated BcL-2 protein, cytochrome c release, increased caspase signaling [106] Permeabilization of OMM and cytochrome c release [107] |
| Tryptanthrin | Chemosensitization In vitro
Pre-clinical
| In a platinum base complex, decreased ΔΨm, initiated cytochrome c-caspase cascade, addition of bromine bond increased localization to the mitochondria [111] |
| Emodin | Chemosensitization Pre-clinical
Pre-clinical | NADPH- dependent oxidoreductases, decreased complex I proteins, decrease ΔΨm, mitochondrial respiratory uncoupling [117] |
| Genistein | Chemoprevention Clinical
In vitro In vitro and Pre-clinical
| Modulatedrespiratory complex activity, decreased ΔΨm, cytochrome c-caspase cascade [124] |
| Ursolic Acid | Chemosensitization In vitro
In vitro
| Activated Cav-1, decreased mitochondrial respiration, decreased ΔΨm, increase apoptosis [125] Hexokinase 2 inhibitor, modulated p53 and mitochondrial ROS [126] |
| Usnic Acid | Direct anti-cancer action In vitro
| Direct mitochondrial respiratory uncoupler [128] |
| Bentulinic Acid | Chemosensitization In vitro
| Decreased ΔΨm, increased in MPTP [129] |
| Rutin | Chemosensitization In vitro
In vitro
| Decreased mitochondrial ATP, No effect on isolated mitochondria, needed to be hydrolyzed to quercetin [131] |
| Noscapine | Chemosensitization In vitro and Pre-clinical
| Decreased Bax/Bcl-2, induced apoptosis [130] |
| Capsaicin | Chemosensitization In vitro and Pre-clinical
In vitro
| Mitochondrial respiratory uncoupling via upregulation of uncoupling proteins [134] |
| Galangin | Direct anti-cancer action In vitro
| Mitochondrial uncoupling and decreased ATP [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zunica, E.R.M.; Axelrod, C.L.; Kirwan, J.P. Phytochemical Targeting of Mitochondria for Breast Cancer Chemoprevention, Therapy, and Sensitization. Int. J. Mol. Sci. 2022, 23, 14152. https://doi.org/10.3390/ijms232214152
Zunica ERM, Axelrod CL, Kirwan JP. Phytochemical Targeting of Mitochondria for Breast Cancer Chemoprevention, Therapy, and Sensitization. International Journal of Molecular Sciences. 2022; 23(22):14152. https://doi.org/10.3390/ijms232214152
Chicago/Turabian StyleZunica, Elizabeth R. M., Christopher L. Axelrod, and John P. Kirwan. 2022. "Phytochemical Targeting of Mitochondria for Breast Cancer Chemoprevention, Therapy, and Sensitization" International Journal of Molecular Sciences 23, no. 22: 14152. https://doi.org/10.3390/ijms232214152







