Lectin-Based Study Reveals the Presence of Disease-Relevant Glycoepitopes in Bladder Cancer Cells and Ectosomes
Abstract
:1. Introduction
2. Results
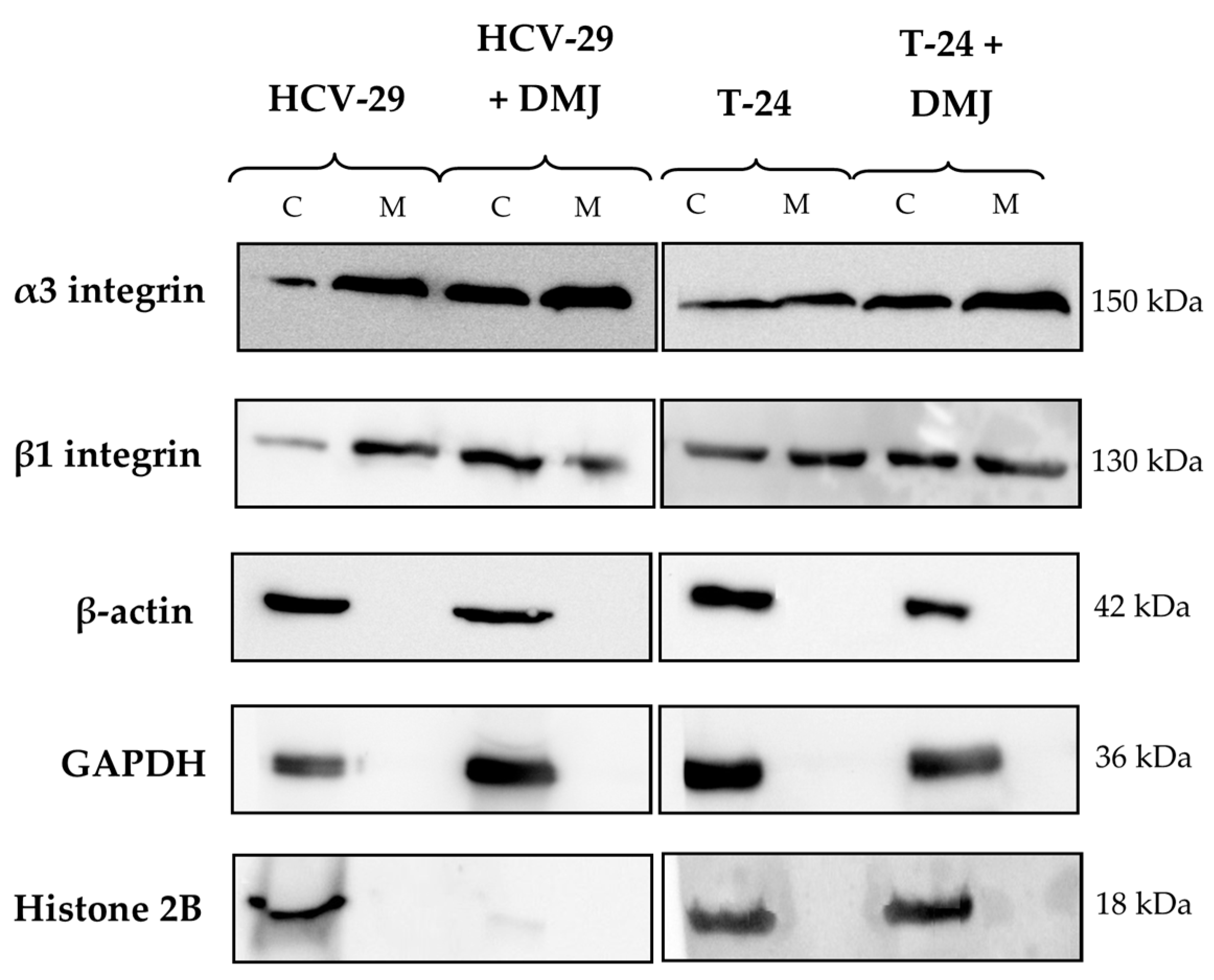
2.1. Characterization of Ectosome Samples
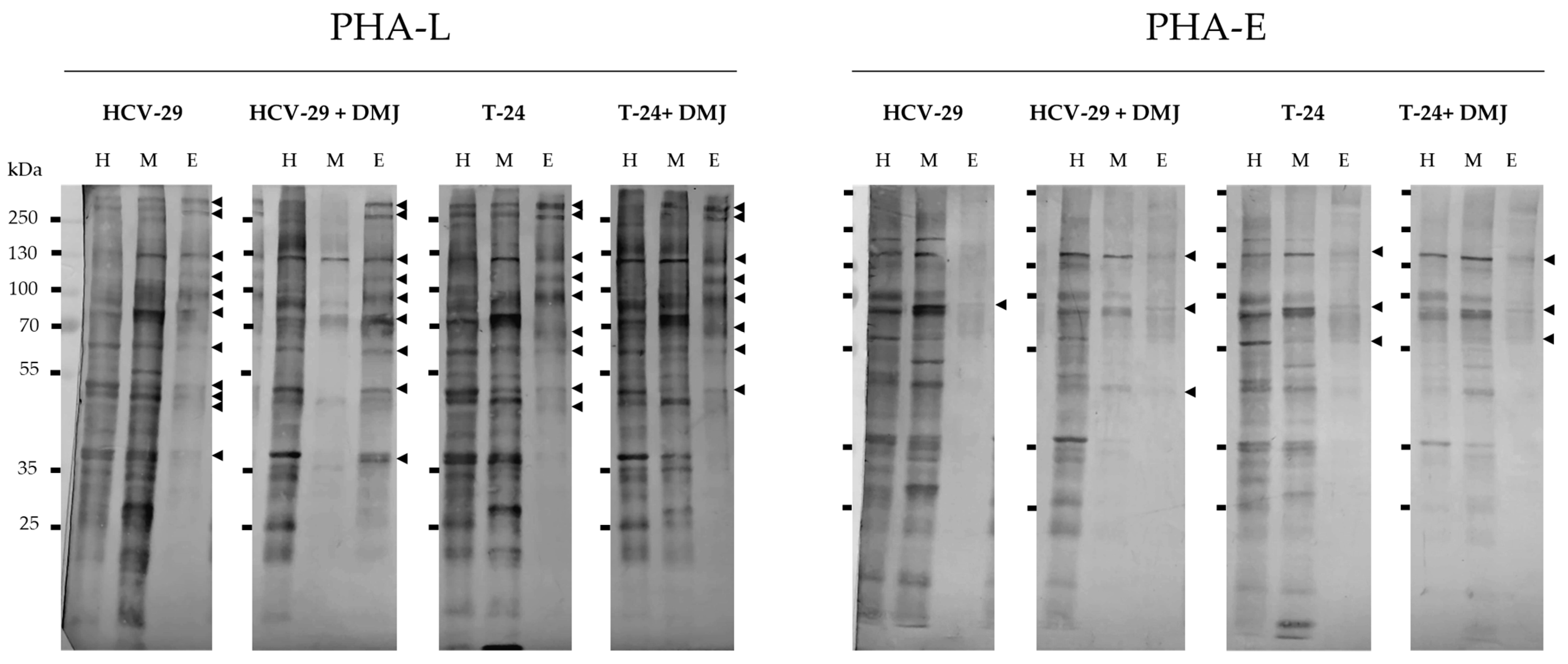
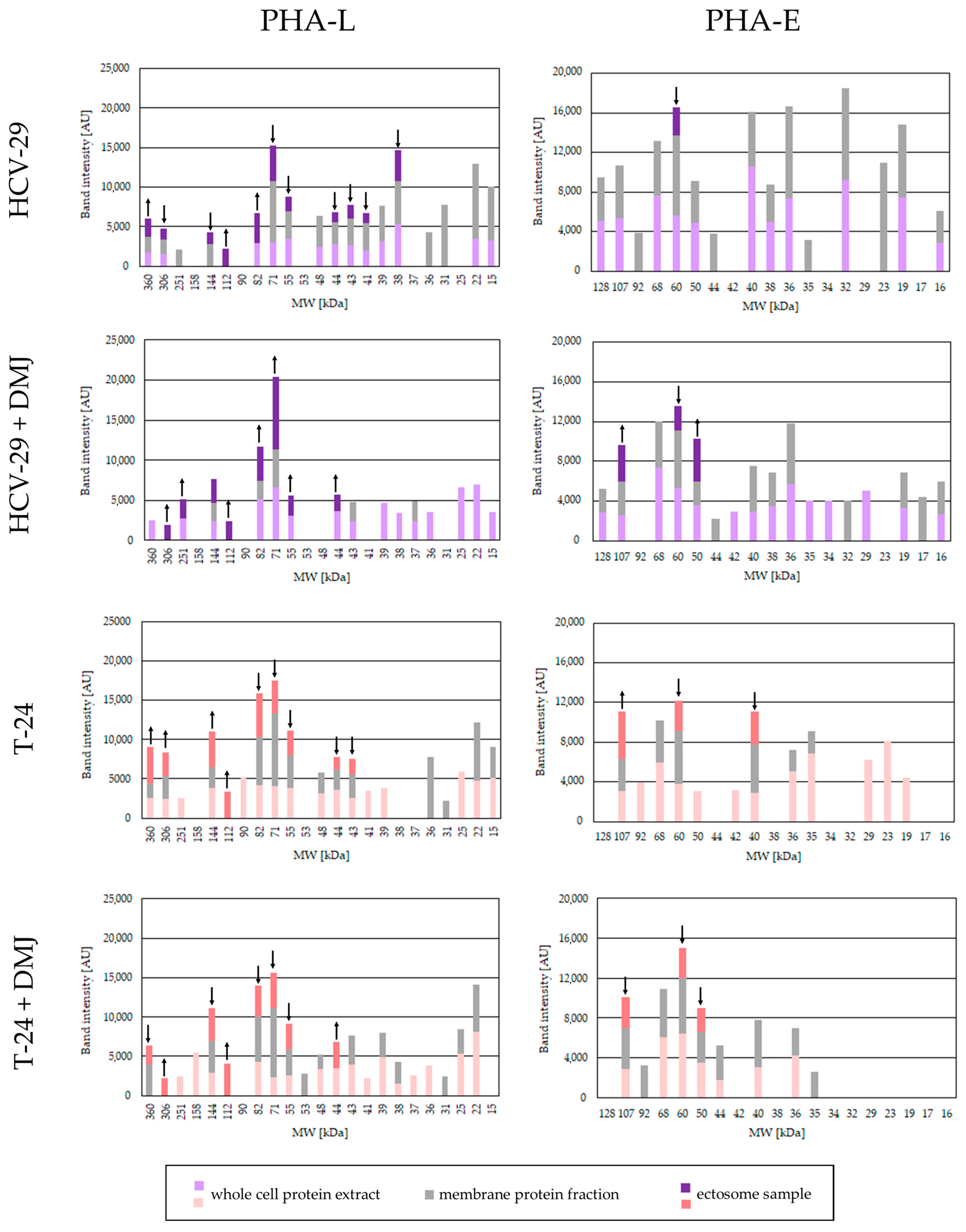
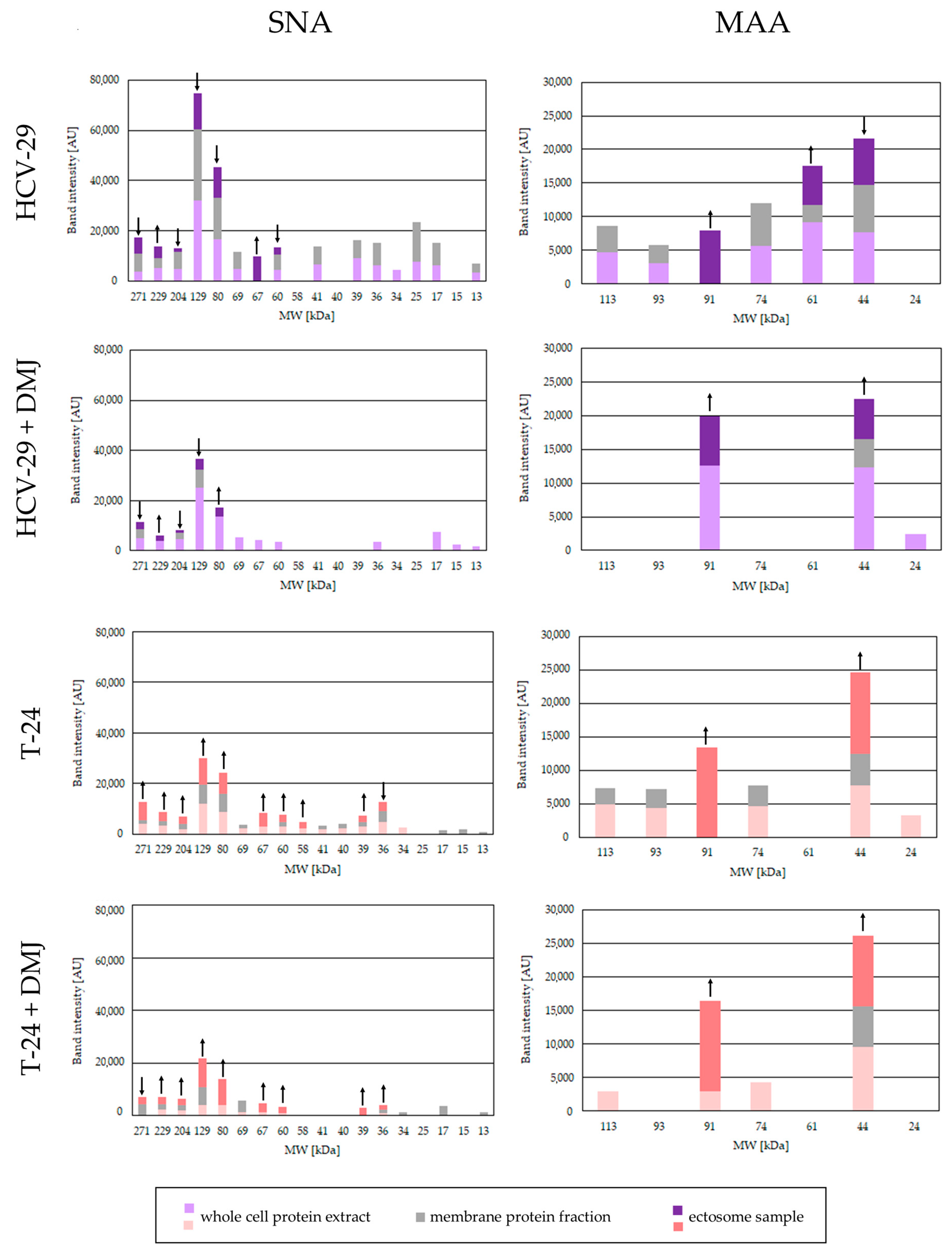
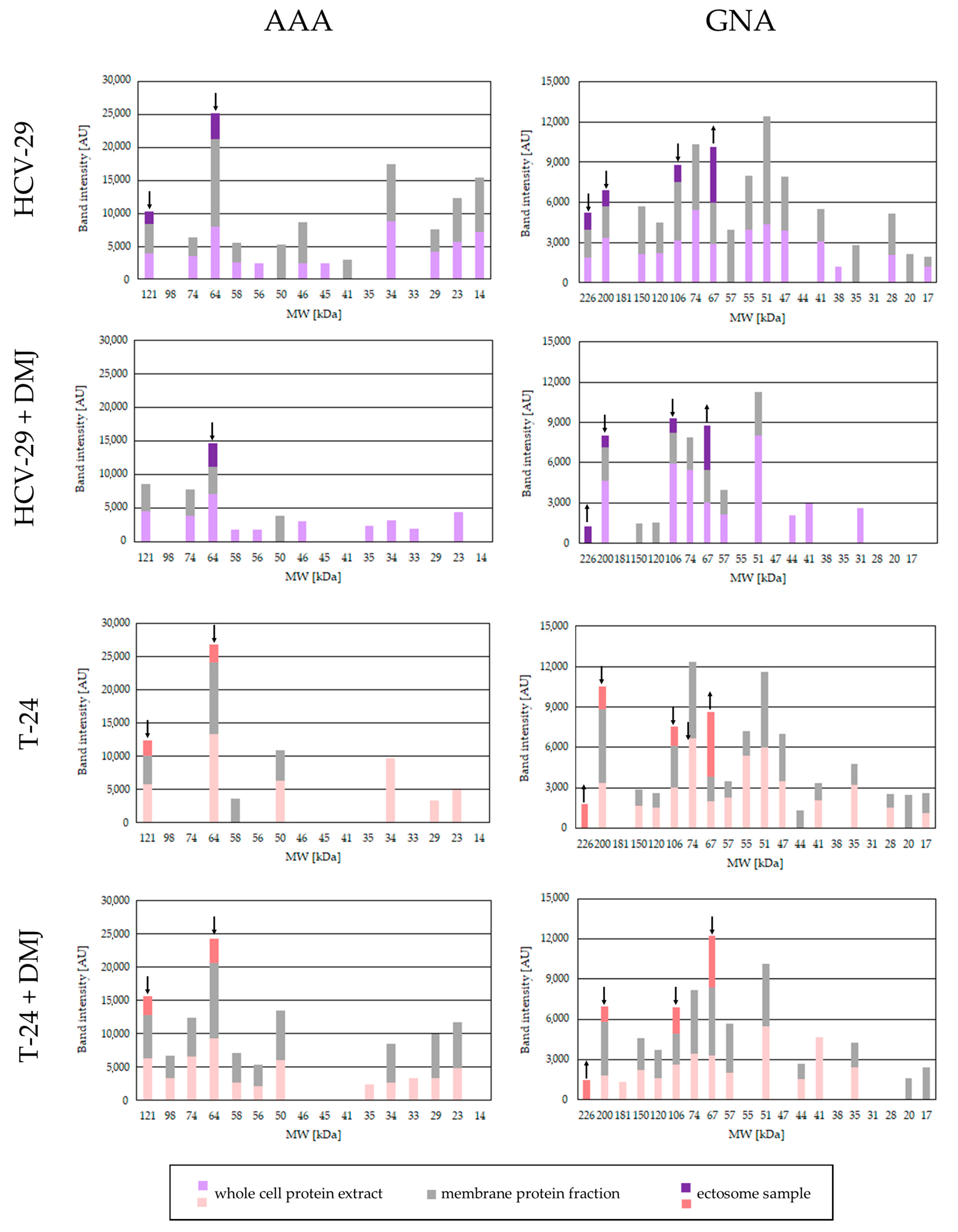
2.2. Lectin Blotting Analysis of Total Glycosylation of HCV-29 and T-24 Cells, Their Membrane Protein Fractions, and Released Ectosomes
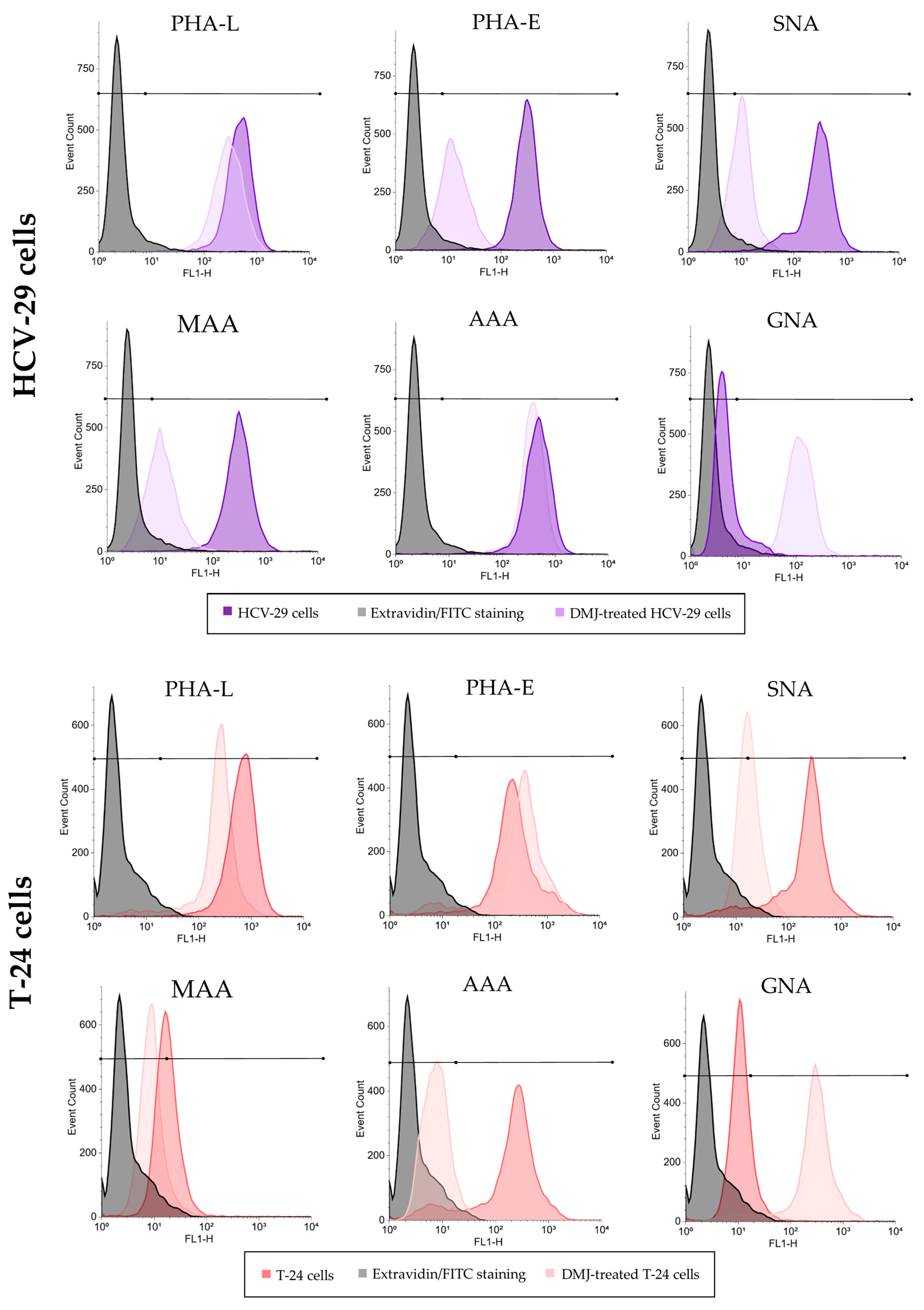
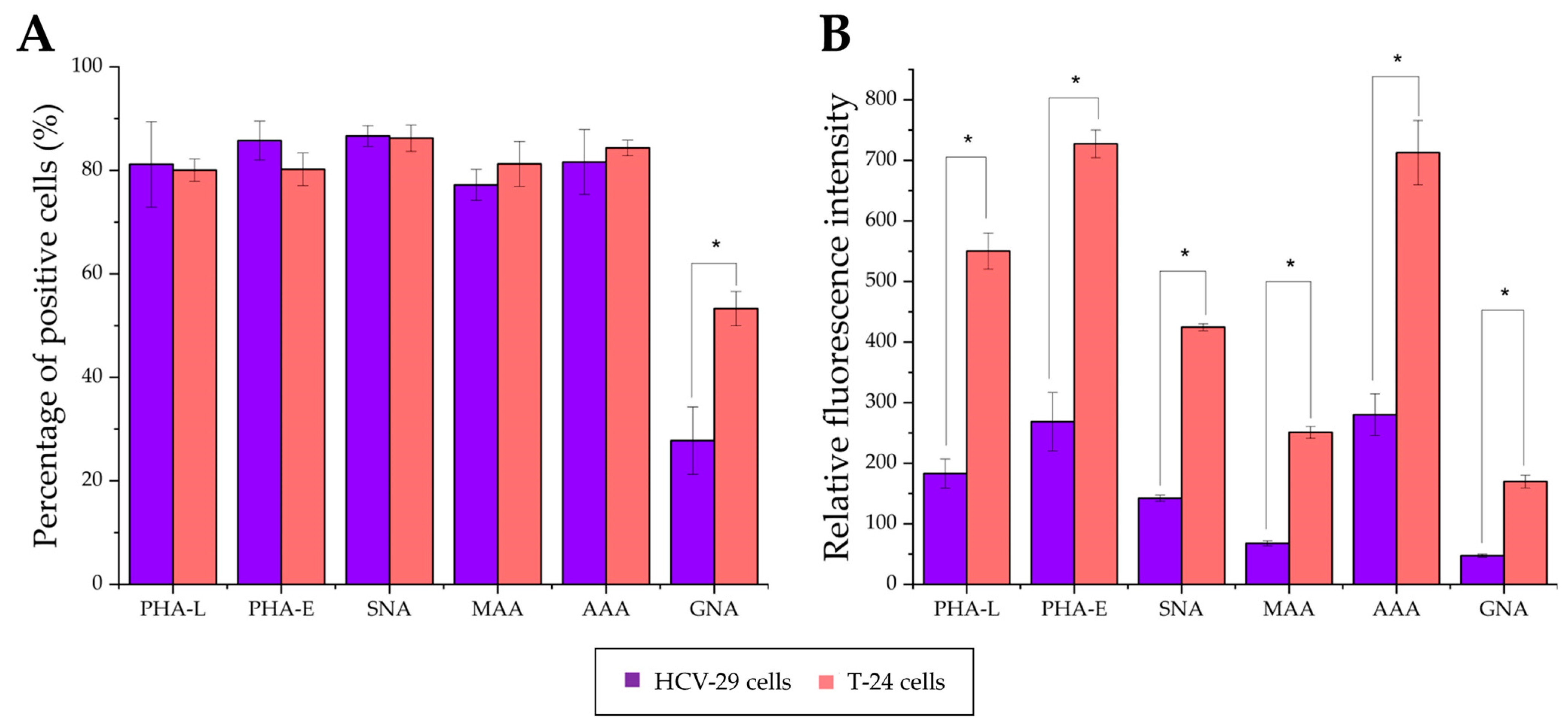
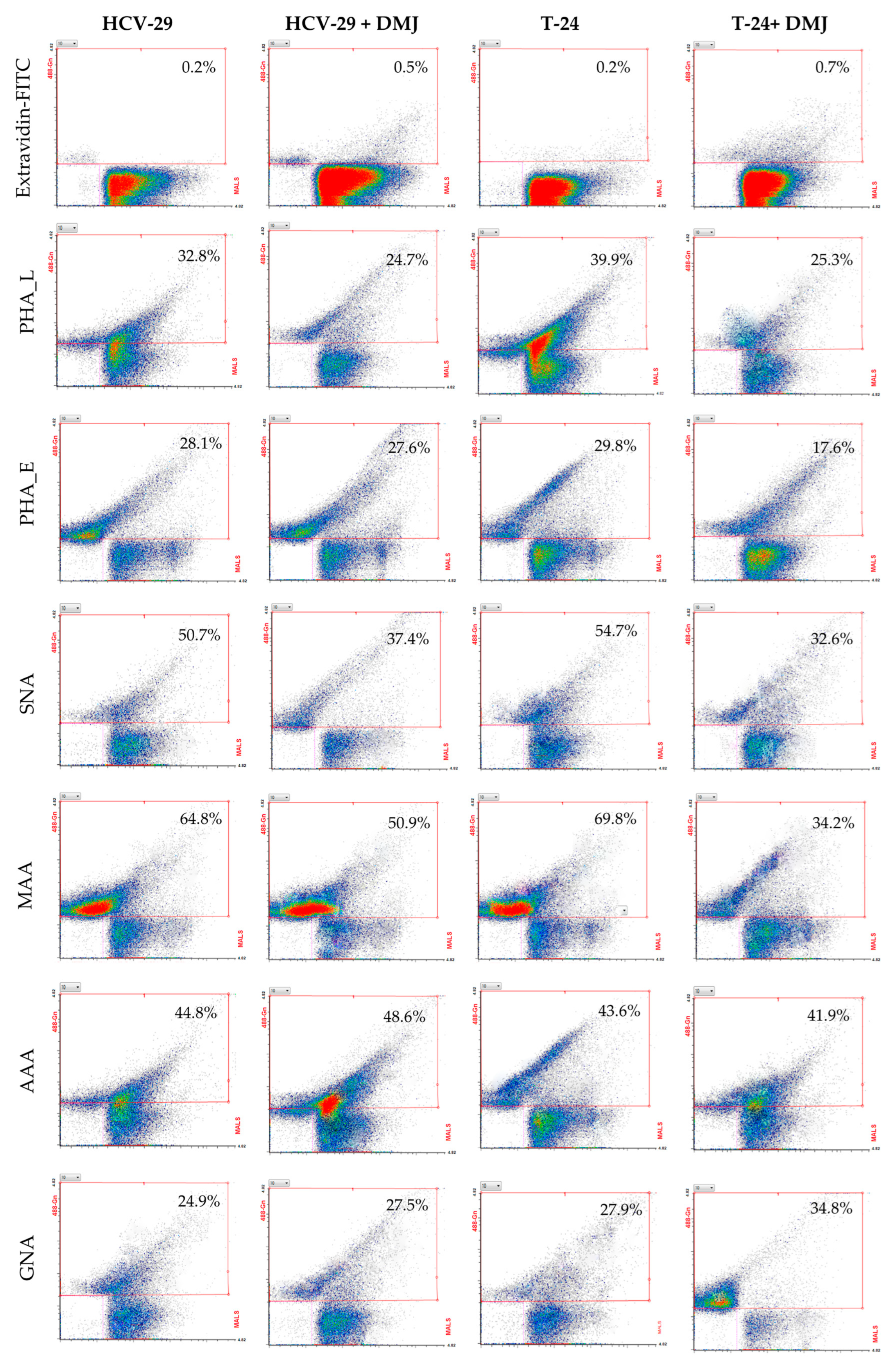
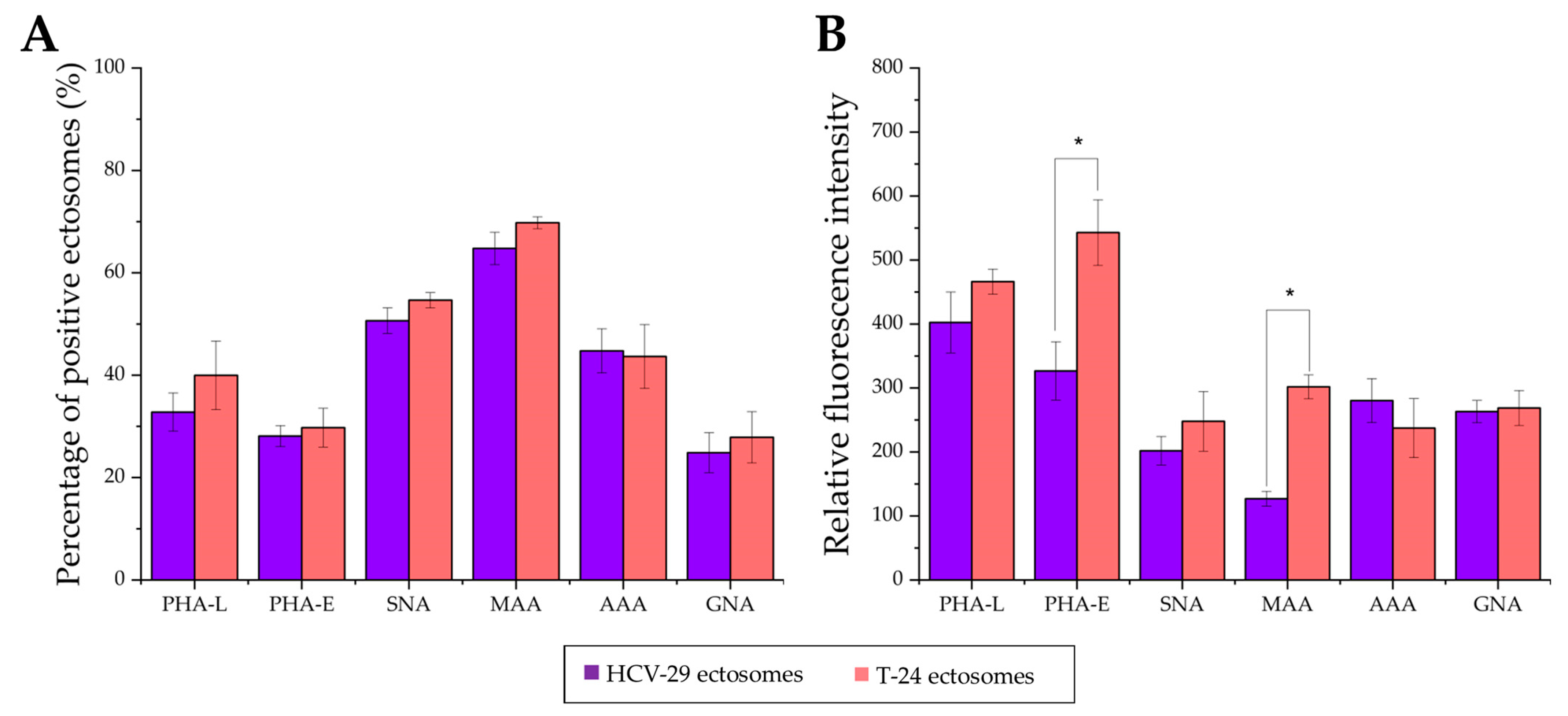
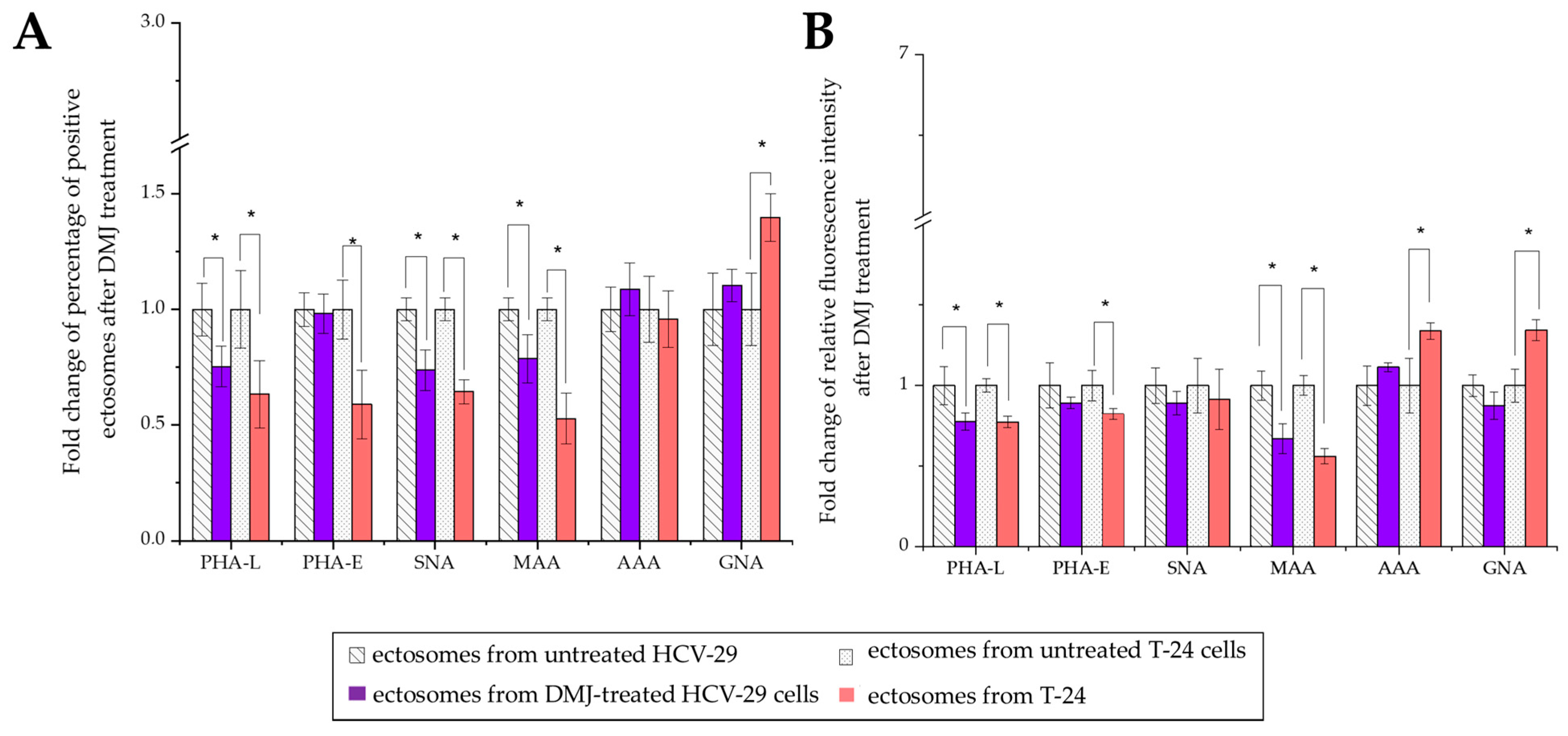
2.3. Surface Glycosylation of HCV-29 and T-24 Cells and Released Ectosomes
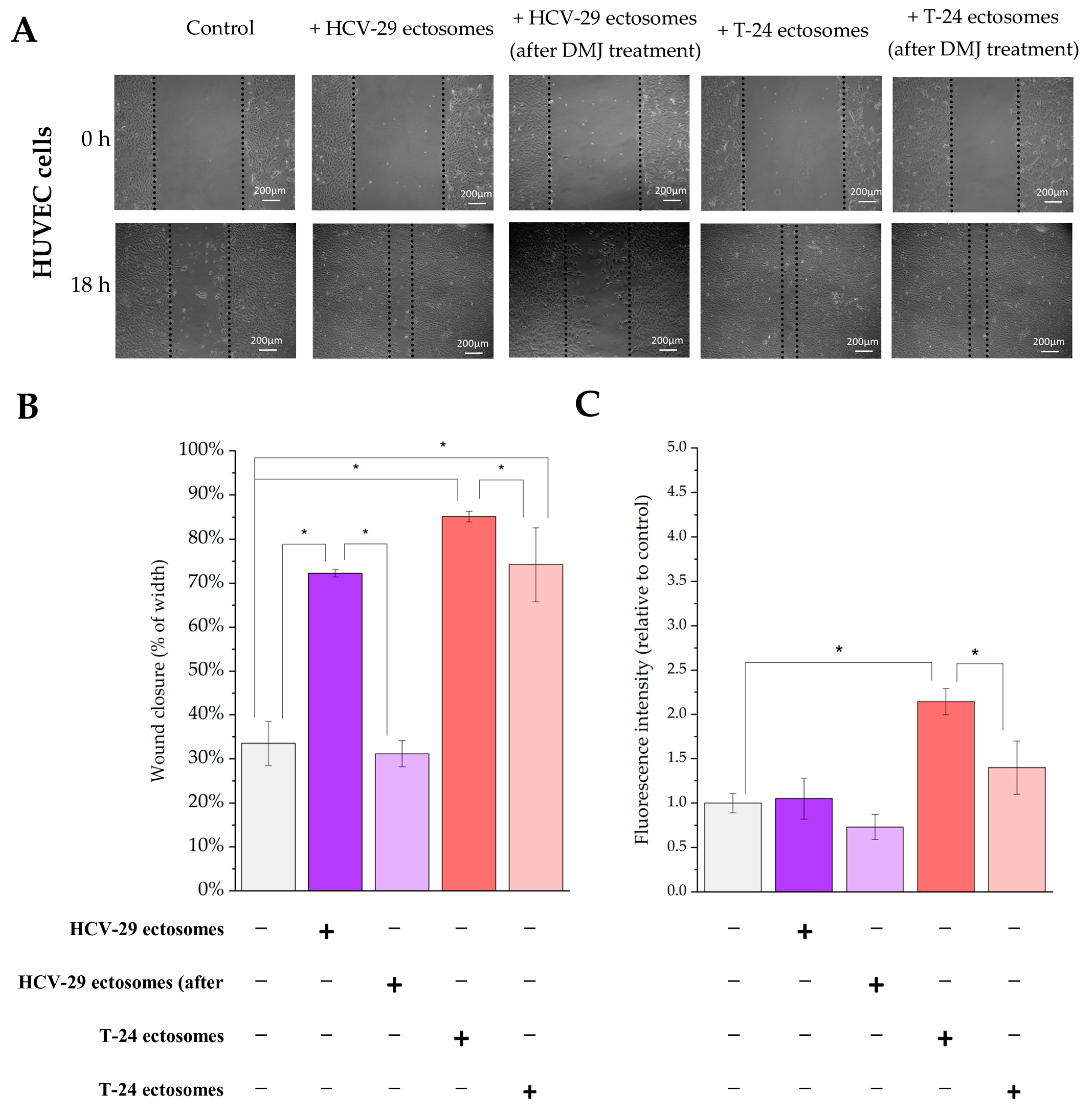
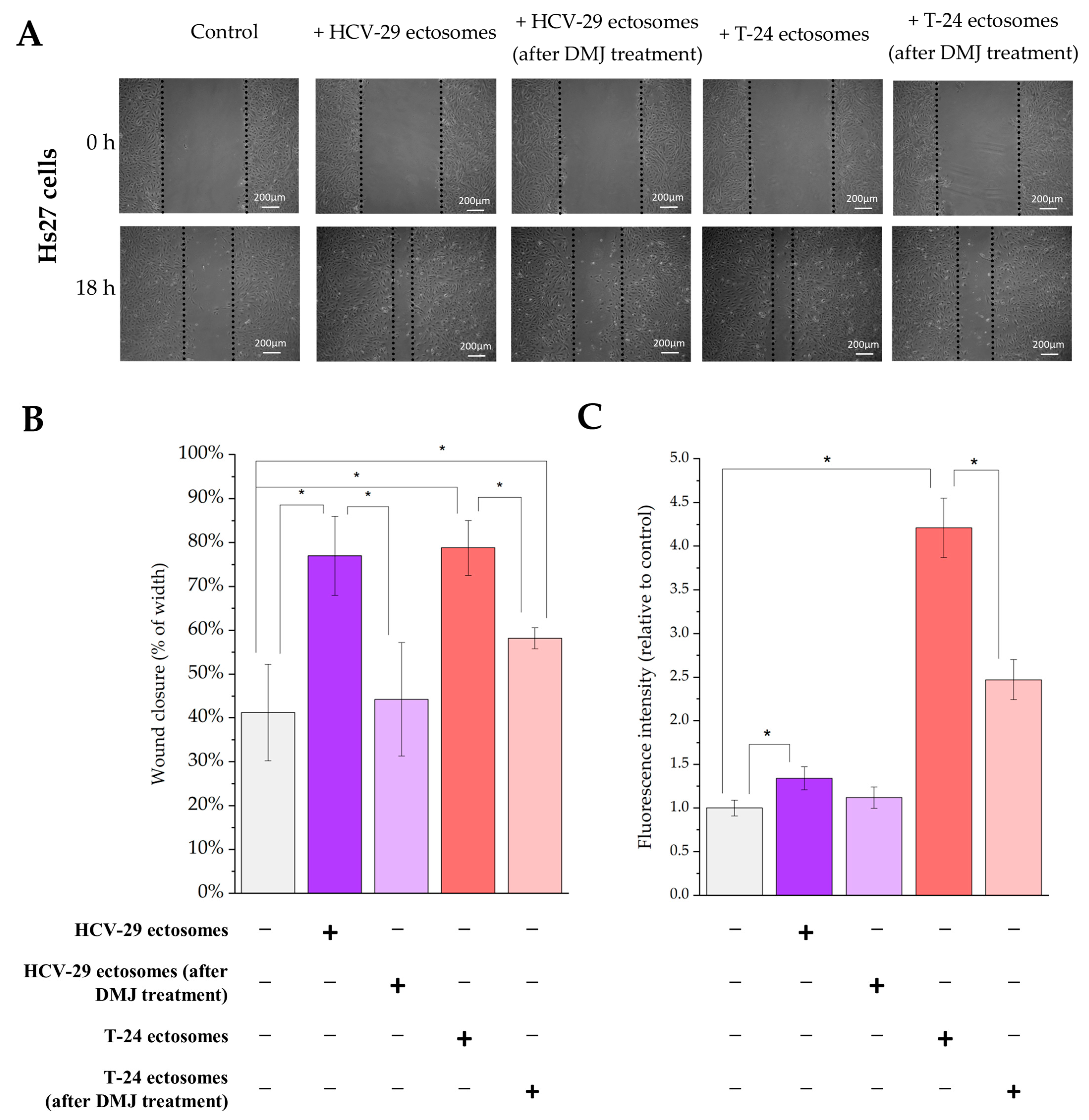
2.4. Functional Effect of HCV-29- and T-24-Derived Ectosomes on Recipient Cells Viability and Migratory Properties
3. Discussion
3.1. Glycan Epitopes in Ectosomal Protein Sorting
3.2. Ectosomal Glycoproteins and Their Functional Role in Cancer
3.3. Ectosomes as Carries of Cancer-Related and Clinically Relevant Glycoepitopes
4. Materials and Methods
4.1. Reagents
4.2. Antibodies
4.3. Cell Lines
4.4. Culture Conditions and Cell Treatment
4.5. Preparation of the Whole Cell Protein Extracts
4.6. Isolation of the Membrane Protein Fraction and Assessment of Its Purity
4.7. Ectosome Isolation and Assessment of the Ectosome Samples Purity
4.8. Comparative Analysis of Cell and Ectosome Glycosylation
4.8.1. Lectin Blotting
4.8.2. Flow Cytometry
4.9. Functional Tests
4.9.1. Alamar Blue Cell Viability Assay
4.9.2. Wound Healing Assay
4.10. Other Methods
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Aleuria aurantia agglutinin |
| BCIP | 5-bromo-4-chloro-3-indolyl-phosphate |
| CAMs | cell adhesion molecules |
| EVs | extracellular vesicles |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GNA | Galanthus nivalis agglutinin |
| MAA | Maackia amurensis agglutinin |
| NTA | Nano Tracking Analysis |
| NBT | 4-nitro blue tetrazolium chloride |
| PHA-E | Phaseolus vulgaris erythroagglutinin |
| PHA-L | Phaseolus vulgaris leukoagglutinin |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SNA | Sambucus nigra agglutinin |
| TACA | tumor-associated carbohydrate antigen |
| TEM | transmission electron microscopy |
References
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [Green Version]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Gao, Y.; Lin, C.; An, Q.; Feng, Y.; Liu, Y.; Luo, H.; Wang, D. The biology and function of extracellular vesicles in cancer development. Front. Cell Dev. Biol. 2021, 9, 777441. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappello, F.; Logozzi, M.; Campanella, C.; Bavisotto, C.C.; Marcilla, A.; Properzi, F.; Fais, S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. 2017, 96, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F.; et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017, 10, 318–329. [Google Scholar] [CrossRef]
- Curtiss, M.; Jones, C.; Babst, M. Efficient cargo sorting by ESCRT-I and the subsequent release of ESCRT-I from multivesicular bodies requires the subunit Mvb12. Mol. Biol. Cell 2007, 18, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; Purushothaman, A.; Ramani, V.C.; Vlodavsky, I.; Sanderson, R.D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013, 288, 10093–10099. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Eng, W.S.; Colquhoun, D.R.; Dinglasan, R.R.; Graham, D.R.; Mahal, L.K. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J. Biol. Chem. 2014, 289, 32526–32537. [Google Scholar] [CrossRef]
- Gomes, J.; Gomes-Alves, P.; Carvalho, S.B.; Peixoto, C.; Alves, P.M.; Altevogt, P.; Costa, J. Extracellular vesicles from ovarian carcinoma cells display specific glycosignatures. Biomolecules 2015, 5, 1741–1761. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.J.; Schnaubelt, M.; Hoti, N.; Hu, Y.; Zhou, Y.; Gooya, M.; Zhang, H. Impact of increased FUT8 expression on the extracellular vesicle proteome in prostate cancer cells. J. Proteome Res. 2020, 19, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wen, T.; Ge, Y.; Liu, J.; Yang, L.; Jiang, Y.; Dong, X.; Liu, H.; Yao, J.; An, G. Disruption of core 1-mediated O-glycosylation oppositely regulates CD44 expression in human colon cancer cells and tumor-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 521, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.-P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, C.A.; Simms, P.E.; Van Huis, A.F.; Foreman, K.E.; Kuo, P.C.; Gupta, G.N. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed. Res. Int. 2014, 2014, 619829. [Google Scholar] [CrossRef] [Green Version]
- Saunderson, S.C.; Dunn, A.C.; Crocker, P.R.; McLellan, A.D. CD169 Mediates the capture of exosomes in spleen and lymph node. Blood 2014, 123, 208–216. [Google Scholar] [CrossRef]
- Hung, M.E.; Leonard, J.N. Stabilization of exosome-targeting peptides via engineered glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.; Pazos, R.; Royo, F.; González, E.; Roura-Ferrer, M.; Martinez, A.; Gamiz, J.; Reichardt, N.C.; Falcón-Pérez, J.M. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci. Rep. 2019, 9, 11920. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Brigstock, D.R. Integrins and heparan sulfate proteoglycans on hepatic stellate cells (HSC) are novel receptors for HSC-derived exosomes. FEBS Lett. 2016, 590, 4263–4274. [Google Scholar] [CrossRef]
- Royo, F.; Cossío, U.; Ruiz de Angulo, A.; Llop, J.; Falcon-Perez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, C.A.; Osorio, H.; Silva, L.; Gomes, C.; David, L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010, 63, 322–329. [Google Scholar] [CrossRef]
- Hoja-Łukowicz, D.; Przybyło, M.; Duda, M.; Pocheć, E.; Bubka, M. On the trail of the glycan codes stored in cancer-related cell adhesion proteins. Biochim. Biophys. Acta 2017, 1861, 3237–3257. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the era of cancer-targeted therapy: Where are we heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.S.; Eng, W.S.; Pilobello, K.T.; Hendricks-Muñoz, K.D.; Mahal, L.K. Identification of a conserved glycan signature for microvesicles. J. Proteome Res. 2011, 10, 4624–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escrevente, C.; Grammel, N.; Kandzia, S.; Zeiser, J.; Tranfield, E.M.; Conradt, H.S.; Costa, J. Sialoglycoproteins and N-glycans from secreted exosomes of ovarian carcinoma cells. PLoS ONE 2013, 8, 78631. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, A.; Kalraiya, R.D. α-2,6 sialylation associated with increased beta 1,6-branched N-oligosaccharides influences cellular adhesion and invasion. J. Biosci. 2013, 38, 867–876. [Google Scholar] [CrossRef]
- Sung, H.; Farley, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Franzen, C.A.; Blackwell, R.H.; Foreman, K.E.; Kuo, P.C.; Flanigan, R.C.; Gupta, G.N. Urinary exosomes: The potential for biomarker utility, intercellular signaling and therapeutics in urological malignancy. J. Urol. 2016, 195, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zieren, R.C.; Wang, Y.; de Reijke, T.M.; Xue, W.; Pienta, K.J. Recent advances in extracellular vesicle research for urological cancers: From technology to application. BBA 2019, 1871, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Kędracka-Krok, S.; Jankowska, U.; Drożdż, A.; Stępień, E.; Przybyło, M. Proteomic profiling of ectosomes derived from paired urothelial bladder cancer and normal cells reveals the presence of biologically-relevant molecules. Int. J. Mol. Sci. 2021, 22, 6816. [Google Scholar] [CrossRef] [PubMed]
- Lityńska, A.; Przybyło, M.; Pocheć, E.; Laidler, P. Adhesion properties of human bladder cell lines with extracellular matrix components: The role of integrins and glycosylation. Acta. Biochim. Pol. 2002, 49, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Przybyło, M.; Lityńska, A.; Pocheć, E. Different adhesion and migration properties of human HCV29 non-malignant urothelial and T24 bladder cancer cells: Role of glycosylation. Biochimie 2005, 87, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.; Kornfeld, R. The effect of 1-deoxymannojirimycin on rat liver alpha-mannosidases. Biochem. Biophys. Res. Commun. 1984, 125, 324–331. [Google Scholar] [CrossRef]
- Surman, M.; Hoja-Łukowicz, D.; Szwed, S.; Drożdż, A.; Stępień, E.; Przybyło, M. Human melanoma-derived ectosomes are enriched with specific glycan epitopes. Life Sci. 2018, 207, 395–411. [Google Scholar] [CrossRef]
- Surman, M.; Hoja-Łukowicz, D.; Szwed, S.; Kędracka-Krok, S.; Jankowska, U.; Kurtyka, M.; Drożdż, A.; Lityńska, A.; Stępień, E.; Przybyło, M. An insight into the proteome of uveal melanoma-derived ectosomes reveals the presence of potentially useful biomarkers. Int. J. Mol. Sci. 2019, 20, 3789. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.R.; Park, J.-J.; Jin, Y.B.; Cao, Y.J.; Park, M.-J.; Kim, E.J.; Lee, M. Silencing of ST6Gal I enhances colorectal cancer metastasis by down-regulating KAI1 via exosome-mediated exportation and thereby rescues integrin signaling. Carcinogenesis 2016, 37, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Borkham-Kamphorst, E.; Van de Leur, E.; Meurer, S.K.; Buhl, E.M.; Weiskirchen, R. N-glycosylation of lipocalin 2 is not required for secretion or exosome targeting. Front. Pharmacol. 2018, 9, 426. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Chen, C.N.; Chu, C.Y.; Lu, J.; Wang, B.J.; Chen, C.H.; Huang, M.C.; Lin, T.H.; Pan, C.C.; Chen, S.S.; et al. Calreticulin activates β1 integrin via fucosylation by fucosyltransferase 1 in J82 human bladder cancer cells. Biochem. J. 2014, 460, 69–78. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, Q.; Chen, S.; Liu, J.; Li, S. Fut7 promotes the epithelial-mesenchymal transition and immune infiltration in bladder urothelial carcinoma. J. Inflamm. Res. 2021, 14, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xiang, L.; Zengqi, T.; Wei, L.; Ganglong, Y.; Feng, G. Alteration of N-glycans and expression of their related glycogenes in the epithelial-mesenchymal transition of HCV29 bladder epithelial cells. Molecules 2014, 19, 20073–20090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaiteiro, C.; Soares, J.; Relvas-Santos, M.; Peixoto, A.; Ferreira, D.; Paul, P.; Brand?o, A.; Fernandes, E.; Azevedo, R.; Palmeira, C.; et al. Glycoproteogenomics characterizes the CD44 splicing code associated with bladder cancer invasion. Theranostics 2022, 12, 3150–3177. [Google Scholar] [CrossRef] [PubMed]
- Horrevorts, S.K.; Stolk, D.A.; van de Ven, R.; Hulst, M.; van Het Hof, B.; Duinkerken, S.; Heineke, M.H.; Ma, W.; Dusoswa, S.A.; Nieuwland, R.; et al. Glycan-Modified Apoptotic Melanoma-Derived Extracellular Vesicles as Antigen Source for Anti-Tumor Vaccination. Cancers 2019, 11, 1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusoswa, S.A.; Horrevorts, S.K.; Ambrosini, M.; Kalay, H.; Paauw, N.J.; Nieuwland, R.; Pegtel, M.D.; Würdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J.J. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targetingof dendritic cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef] [Green Version]
- Menck, K.; Scharf, C.; Bleckmann, A.; Dyck, L.; Rost, U.; Wenzel, D.; Dhople, V.M.; Siam, L.; Pukrop, T.; Binder, C.; et al. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J. Mol. Cell. Biol. 2015, 7, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, J.; Li, L.; Ianni, A.; Kumari, P.; Liu, S.; Sun, P.; Braun, T.; Tan, X.; Xiang, R.; et al. MGAT3-mediated glycosylation of tetraspanin CD82 at asparagine 157 suppresses ovarian cancer metastasis by inhibiting the integrin signaling pathway. Theranostics 2020, 10, 6467–6482. [Google Scholar] [CrossRef]
- Martins, Á.M.; Ramos, C.C.; Freitas, D.; Reis, C.A. Glycosylation of cancer extracellular vesicles: Capture strategies, functional roles and potential clinical applications. Cells 2021, 10, 109. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, Q.; Zeng, H.; Zhao, Q.; Guo, Z.; Zhong, R.; Xie, M.; Cai, X.; Su, J.; He, Z.; et al. Exosomal CA125 as a promising biomarker for ovarian cancer diagnosis. J. Cancer 2020, 11, 6445–6453. [Google Scholar] [CrossRef]
- Yokose, T.; Kabe, Y.; Matsuda, A.; Kitago, M.; Matsuda, S.; Hirai, M.; Nakagawa, T.; Masugi, Y.; Hishiki, T.; Nakamura, Y.; et al. O-glycan-altered extracellular vesicles: A specific serum marker elevated in pancreatic cancer. Cancers 2020, 12, 2469. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Sebastián, V.; Sesé, J.; Pazo-Cid, R.; Mendoza, G.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Isolation of exosomes from whole blood by a new microfluidic device: Proof of concept application in the diagnosis and monitoring of pancreatic cancer. J. Nanobiotechnol. 2020, 18, 150. [Google Scholar] [CrossRef]
- Sakaue, T.; Koga, H.; Iwamoto, H.; Nakamura, T.; Ikezono, Y.; Abe, M.; Wada, F.; Masuda, A.; Tanaka, T.; Fukahori, M.; et al. Glycosylation of ascites-derived exosomal CD133: A potential prognostic biomarker in patients with advanced pancreatic cancer. Med. Mol. Morphol. 2019, 52, 198–208. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Bleckmann, A.; Wachter, A.; Hennies, B.; Ries, L.; Schulz, M.; Balkenhol, M.; Pukrop, T.; Schatlo, B.; Rost, U.; et al. Characterisation of tumour-derived microvesicles in cancer patients’ blood and correlation with clinical outcome. J. Extracell. Vesicles 2017, 6, 1340745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khirul, I. Extracellular Vesicle Glycosylations as Novel Biomarkers of Urological Cancers: Nanoparticle-Aided Glycovariant Assay to Detect Vesicles for the Early Detection of Cancer. Turun Yliopisto. 2022. ISBN 978-951-29-8912-6. Available online: https://www.utupub.fi/handle/10024/154308 (accessed on 20 October 2022).
- Lv, J.; Wang, Z.; Li, F.; Zhang, Y.; Lu, H. Reverse capture for selectively and sensitively revealing the n-glycome of serum exosomes. Chem. Commun. 2019, 55, 14339–14342. [Google Scholar] [CrossRef]
- Walker, S.A.; Aguilar Díaz De León, J.S.; Busatto, S.; Wurtz, G.A.; Zubair, A.C.; Borges, C.R.; Wolfram, J. Glycan node analysis of plasma-derived extracellular vesicles. Cells 2020, 9, 1946. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Natalia, A.; Tang, C.S.L.; Ang, C.B.T.; Ong, C.-A.J.; Teo, M.C.C.; So, J.B.Y.; Shao, H. Dual-selective magnetic analysis of extracellular vesicle glycans. Matter 2020, 2, 150–166. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Hagisawa, S.; Yoshikawa, K.; Tezuka, F.; Kaku, M.; Ohyama, C. Predictive value of N-acetylglucosaminyltransferase-V for superficial bladder cancer recurrence. J. Urol. 2006, 175, 90–93. [Google Scholar] [CrossRef]
- Hegele, A.; Mecklenburg, V.; Varga, Z.; Olbert, P.; Hofmann, R.; Barth, P. CA19.9 and CEA in transitional cell carcinoma of the bladder: Serological and immunohistochemical findings. Anticancer Res. 2010, 30, 5195–5200. [Google Scholar] [PubMed]
- Numahata, K.; Satoh, M.; Handa, K.; Saito, S.; Ohyama, C.; Ito, A.; Takahashi, T.; Hoshi, S.; Orikasa, S.; Hakomori, S.I. Sialosyl-Le(x) expression defines invasive and metastatic properties of bladder carcinoma. Cancer 2002, 94, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Opanuraks, J.; Boonla, C.; Saelim, C.; Kittikowit, W.; Sumpatanukul, P.; Honglertsakul, C.; Tosukhowong, P. Elevated urinary total sialic acid and increased oxidative stress in patients with bladder cancer. Asian Biomedicine 2010, 4, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Li, D.; Cui, Z.; Zhang, C.; Zhang, Z.; Yan, L. Evaluation of the value of preoperative sialic acid levels in diagnosis and localization of urothelial tumors. J. Cancer 2021, 12, 5066–5075. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Neves, M.; Oliveira, M.I.; Dieguez, L.; Freitas, R.; Azevedo, R.; Gaiteiro, C.; Soares, J.; Ferreira, D.; Peixoto, A.; et al. Sialyl-Tn identifies muscle-invasive bladder cancer basal and luminal subtypes facing decreased survival, being expressed by circulating tumor cells and metastases. Urol. Oncol. 2017, 35, e1–e675. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.D. Human urologic cancer cell lines. Invest. Urol. 1980, 17, 359–363. [Google Scholar]
- Bean, M.A.; Pees, H.; Fogh, J.E.; Grabstald, H.; Oettgen, H.F. Cytotoxicity of lymphocytes from patients with cancer of the urinary bladder: Detection by a 3-H-proline microcytotoxicity test. Int. J. Cancer 1974, 14, 186–197. [Google Scholar] [CrossRef]
- Laidler, P.; Lityńska, A.; Hoja-Łukowicz, D.; Łabedz, M.; Przybyło, M.; Ciołczyk-Wierzbicka, D.; Pocheć, E.; Trebacz, E.; Kremser, E. Characterization of glycosylation and adherent properties of melanoma cell lines. Cancer Immunol. Immunother. 2006, 55, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Kędracka-Krok, S.; Hoja-Łukowicz, D.; Jankowska, U.; Drożdż, A.; Stępień, E.Ł.; Przybyło, M. Mass spectrometry-based proteomic characterization of cutaneous melanoma ectosomes reveals the presence of cancer-related molecules. Int. J. Mol. Sci. 2020, 21, 2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1974, 80, 575–599. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surman, M.; Wilczak, M.; Przybyło, M. Lectin-Based Study Reveals the Presence of Disease-Relevant Glycoepitopes in Bladder Cancer Cells and Ectosomes. Int. J. Mol. Sci. 2022, 23, 14368. https://doi.org/10.3390/ijms232214368
Surman M, Wilczak M, Przybyło M. Lectin-Based Study Reveals the Presence of Disease-Relevant Glycoepitopes in Bladder Cancer Cells and Ectosomes. International Journal of Molecular Sciences. 2022; 23(22):14368. https://doi.org/10.3390/ijms232214368
Chicago/Turabian StyleSurman, Magdalena, Magdalena Wilczak, and Małgorzata Przybyło. 2022. "Lectin-Based Study Reveals the Presence of Disease-Relevant Glycoepitopes in Bladder Cancer Cells and Ectosomes" International Journal of Molecular Sciences 23, no. 22: 14368. https://doi.org/10.3390/ijms232214368




