N-Terminally Lipidated Sialorphin Analogs—Synthesis, Molecular Modeling, In Vitro Effect on Enkephalins Degradation by NEP and Treatment of Intestinal Inflammation in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptides Synthesis and Purification
2.2. Effect of Sialorphin and Its Analogs on Degradation of Met-Enkephalin by NEP
2.3. Stability in the Human Plasma
2.4. Molecular Modeling
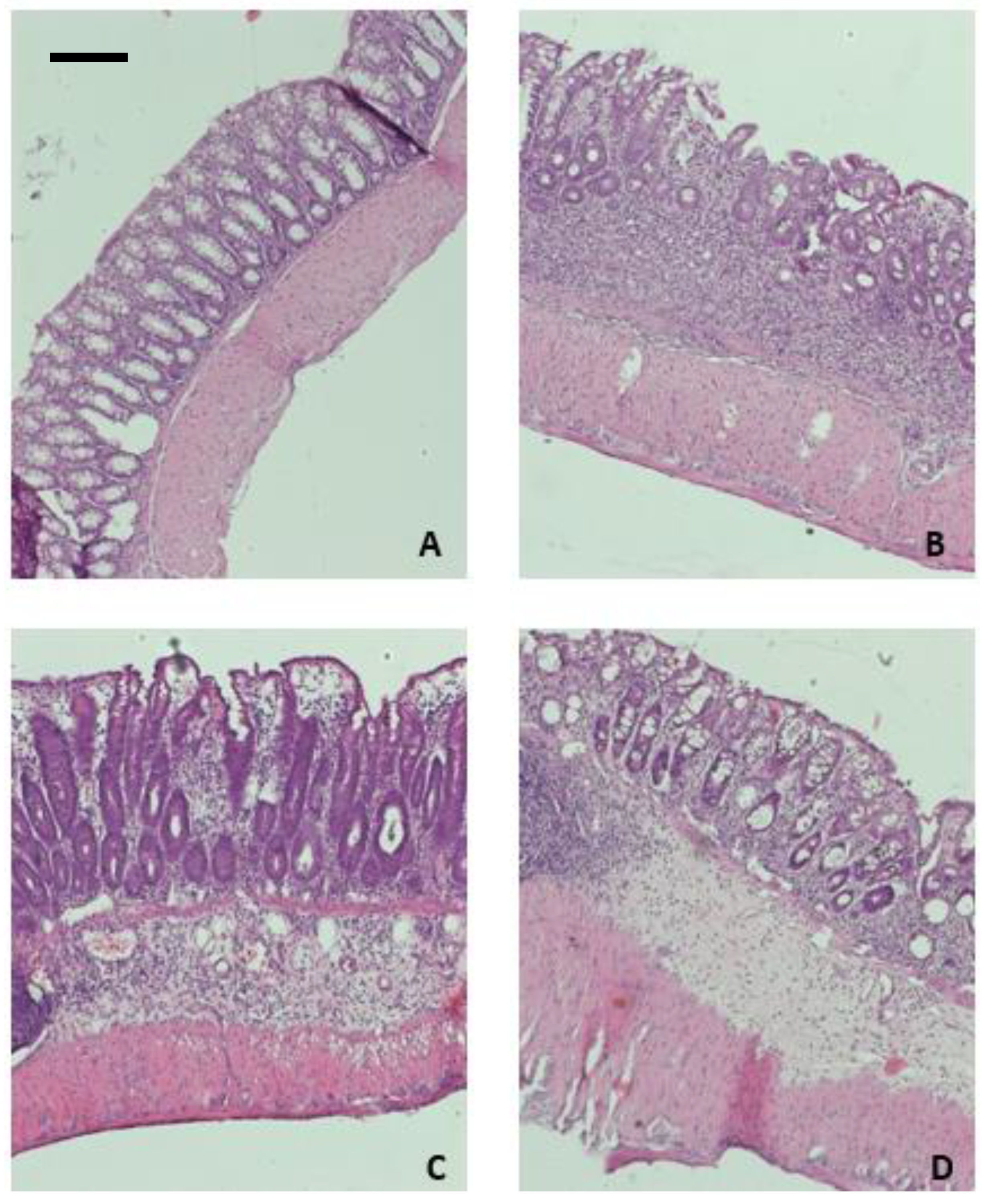
2.5. Peptides VII and VIII Alleviate Inflammation in the DSS-Induced Mouse Model of Colitis
3. Materials and Methods
3.1. Peptides Synthesis and Purification
3.2. Determination of Met-Enkephalin Degradation Rates
3.3. Stability Peptides VII and VIII in Human Plasma
3.4. Molecular Modeling
3.5. Animals
3.5.1. Drugs and Reagents
3.5.2. Induction of Colitis
3.5.3. Pharmacological Treatments
3.5.4. Evaluation of Colonic Damage (Macroscopic and Microscopic Score Evaluation)
3.5.5. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vegh, Z.; Burisch, J.; Pedersen, N.; Kaimakliotis, I.; Duricova, D.; Bortlik, M.; Avnstrøm, S.; Kofod Vinding, K.; Olsen, J.; Nielsen, K.R.; et al. EpiCom-groupet. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: Results of the 2011 ECCO-EpiCom inception cohort. J. Crohns Colitis 2014, 8, 1506–1515. [Google Scholar] [CrossRef] [Green Version]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, M.E.; Mangen, M.J.; Severs, M.; van der Have, M.; Dijkstra, G.; van Bodegraven, A.A.; Fidder, H.H.; De Jong, D.J.; van der Woude, C.J.; Romberg-Camps, M.J.; et al. COIN study group and the Dutch Initiative on Crohn and Colitis. Evolution of Costs of Inflammatory Bowel Disease over Two Years of Follow-Up. PLoS ONE 2016, 21, e0142481. [Google Scholar]
- Mackiewicz, T.; Sowa, A.; Fichna, J. Biomarkers for Early Detection of Colitis-associated Colorectal Cancer—Current Concepts, Future Trends. Curr. Drug Targets 2021, 22, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Fichna, J.; Wiśniewska-Jarosińska, M. Alopecia areata in patients with inflammatory bowel disease: An overview. Folia Med. Cracov. 2016, 56, 5–12. [Google Scholar]
- Mosińska, P.; Zielińska, M.; Fichna, J. Expression and physiology of opioid receptors in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 3–10. [Google Scholar] [CrossRef]
- Jankovic, D.B.; Maric, D. In vivo modulation of the immune system by enkephalins. Int. J. Neurosci. 1990, 51, 167–169. [Google Scholar] [CrossRef]
- Salzet, M.; Tasiemski, A. Involvement of pro-enkephalin-derived peptides in immunity. Dev. Comp. Immunol. 2001, 25, 177–185. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Racz, I.; Michel, K.; Mauer, D.; Zimmer, A.; Klingmüller, D.; Zimmer, A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology 2008, 33, 425–436. [Google Scholar] [CrossRef]
- Owczarek, D.; Cibor, D.; Mach, T.; Cieśla, A.; Pierzchała-Koziec, K.; Sałapa, K.; Kuśnierz-Cabała, B. Met-enkephalins in patients with inflammatory bowel diseases. Adv. Med. Sci. 2011, 56, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Sałaga, M.; Storr, M.A.; Fichna, J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: Current concepts and future perspectives. J. Gastroenterol. 2014, 49, 24–45. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, H.U.; Cremonini, F.; Park, M.I.; Camilleri, M. Opioids and the gut: Pharmacology and current clinical experience. Neurogastroenterol. Motil. 2004, 16, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Galligan, J.J. Function of opioids in the enteric nervous system. Neurogastroenterol. Motil. 2004, 16 (Suppl. 2), 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.M. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav. Immun. 2006, 20, 9–14. [Google Scholar] [CrossRef]
- Oefner, C.; Roques, B.P.; Fournie-Zaluski, M.C.; Dale, G.E. Structural analysis of neprilysin with various specific and potent inhibitors. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 392–396. [Google Scholar] [CrossRef] [Green Version]
- Campbell, D.J. Vasopeptidase Inhibition. A Double-Edged Sword? Hypertension 2003, 41, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Fritz, R.; Mukherjee, A.; Zaghi, S.; Agalliu, I.; Jindal, S.; Tashima, A.K.; Fricker, L.D.; Davies, K.P. Identification and characterization of RSIY-11, a novel seminal peptide derived from semenogelin-1, which acts as a neutral endopeptidase inhibitor modulating sperm motility. J. Assist. Reprod. Genet. 2019, 36, 1891–1900. [Google Scholar] [CrossRef]
- Judge, P.; Haynes, R.; Landray, M.J.; Baigent, C. Neprilysin inhibition in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Nalivaeva, N.N.; Belyaev, N.D.; Zhuravin, I.A.; Turner, A. The Alzheimer’s Amyloid-Degrading Peptidase, Neprilysin: Can We Control It? Int. J. Alzheimers Dis. 2012, 2012, 383796. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.O.W.; Mett, J.; Stahlmann, C.P.; Haupenthal, V.J.; Zimmer, V.C.; Hartmann, T. Neprilysin and Aβ Clearance: Impact of the APP Intracellular Domain in NEP Regulation and Implications in Alzheimer’s Disease. Front Aging Neurosci. 2013, 5, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaga, M.; Mokrowiecka, A.; Jacenik, D.; Cygankiewicz, A.I.; Malecka-Panas, E.; Kordek, R.; Krajewska, W.M.; Sobocinska, M.K.; Kamysz, E.; Fichna, J. Systemic Administration of Sialorphin Attenuates Experimental Colitis in Mice via Interaction With Mu and Kappa Opioid Receptors. J Crohns Colitis 2017, 11, 988–998. [Google Scholar] [CrossRef]
- Kamysz, E.; Sałaga, M.; Sobocińska, M.; Giełdoń, A.; Fichna, J. Anti-inflammatory effect of novel analogs of natural enkephalinase inhibitors in a mouse model of experimental colitis. Future Med. Chem. 2016, 8, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis. A Practical Approach; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Sobocińska, M.; Sałaga, M.; Fichna, J.; Kamysz, E. Anti-Inflammatory Effect of Homo- and Heterodimers of Natural Enkephalinase Inhibitors in Experimental Colitis in Mice. Molecules 2020, 25, 5820. [Google Scholar] [CrossRef]
- Storch, J.; McDermott, L. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 2009, 50, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collini, M.; D’Alfonso, L.; Molinari, H.; Ragona, L.; Catalano, M.; Baldini, G. Competitive binding of fatty acids and the fluorescent probe 1-8-anilinonaphthalene sulfonate to bovine β-lactoglobulin. Protein Sci. 2003, 12, 1596–1603. [Google Scholar] [CrossRef]
- Barlos, K.; Chatzi, O.; Gatos, D.; Stavropoulos, G. 2-Chlorotrityl chloride resin. Studies on anchoring of Fmoc-amino acids and peptide cleavage. Int. J. Pept. Protein Res. 1991, 37, 513–520. [Google Scholar]
- Sobocińska, M.; Giełdoń, A.; Fichna, J.; Kamysz, E. Alanine scan of sialorphin and its hybrids with opiorphin: Synthesis, molecular modelling and effect on enkephalins degradation. Amino Acids 2018, 50, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Sobocińska, M.; Giełdoń, A.; Fichna, J.; Kamysz, E. 1-Substituted sialorphin analogues -synthesis, molecular modelling and in vitro effect on enkephalins degradation by NEP. Amino Acids 2019, 51, 1201–1207. [Google Scholar] [CrossRef] [Green Version]
- Oefner, C.; Pierau, S.; Schulz, H.; Dale, G.E. Structural studies of a bifunctional inhibitor of neprilysin and DPP-IV. Acta Crystallogr. D Biol. Crystallogr. 2007, 63 Pt 9, 975–981. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. An ant colony optimization approach to flexible protein–ligand docking. Swarm Intell. 2007, 1, 115–134. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical Scoring Functions for Advanced Protein−Ligand Docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, D.A.; Case, D.A.; Caldwell, J.W.; Ross, W.S.; Cheatham, T.E., III; DeBolt, S.; Ferguson, D.; Seibel, G.; Kollman, P. AMBER a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to stimulate the structure and energetic properties of molecules. Comput. Phys. Commun. 1995, 91, 1–41. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [Green Version]
- Pikora, M.; Giełdoń, A. RASMOL AB—new functionalities in the program for structure analysis. Acta Biochim Pol. 2015, 62, 629–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaga, M.; Bartoszek, A.; Binienda, A.; Krajewska, J.B.; Fabisiak, A.; Mosińska, P.; Dziedziczak, K.; Niewinna, K.; Talar, M.; Tarasiuk, A.; et al. Activation of Free Fatty Acid Receptor 4 Affects Intestinal Inflammation and Improves Colon Permeability in Mice. Nutrients 2021, 13, 2716. [Google Scholar] [CrossRef]



| Peptide | Sequence | HPLC tR [min] | Molecular Ion | |
|---|---|---|---|---|
| calc. [M]+ | Found [M+H]+ | |||
| I | QHNPR | 5.5 a | 650.3 | 651.4 |
| II | KQHNPR | 5.6 a | 778.7 | 779.7 |
| III | KKQHNPR | 5.5 a | 906.5 | 907.7 |
| IV | KKKQHNPR | 7.9 f | 1034.6 | 1036.0 |
| V | Laur-KKQHNPR | 9.5 e | 1088.8 | 1089.9 |
| VI | Mir-KKQHNPR | 11.5 d | 1116.9 | 1117.9 |
| VII | Palm-KKQHNPR | 9.1 c | 1144.7 | 1146.3 |
| VIII | Stear-KKQHNPR | 7.8 b | 1172.9 | 1174.3 |
| No | Inhibitor | Met-Enkephalin | |
|---|---|---|---|
| 1000 × k [1/min] | t1/2 [min] | ||
| - | without inhibitor | 25.35 ± 1.05 | 27 ± 1 |
| I | QHNPR | 8.84 ± 0.27 *** | 78 ± 2 *** |
| II | KQHNPR | 7.63 ± 0.22 *** | 90 ± 3 *** |
| III | KKQHNPR | 7.17 ± 0.16 *** | 96 ± 2 *** |
| IV | KKKQHNPR | 8.28 ± 0.11 *** | 83 ± 1 *** |
| V | Laur-KKQHNPR | 23.20 ± 0.89 | 30 ± 1 |
| VI | Mir-KKQHNPR | 20.06 ± 1.34 ** | 35 ± 2 |
| VII | Palm-KKQHNPR | 3.46 ± 0.40 *** | 199 ± 2 *** |
| VIII | Stear-KKQHNPR | 0.89 ± 0.10 *** | 724 ± 43 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobocińska, M.; Fichna, J.; Giełdoń, A.; Skowron, P.; Kamysz, E. N-Terminally Lipidated Sialorphin Analogs—Synthesis, Molecular Modeling, In Vitro Effect on Enkephalins Degradation by NEP and Treatment of Intestinal Inflammation in Mice. Int. J. Mol. Sci. 2022, 23, 14450. https://doi.org/10.3390/ijms232214450
Sobocińska M, Fichna J, Giełdoń A, Skowron P, Kamysz E. N-Terminally Lipidated Sialorphin Analogs—Synthesis, Molecular Modeling, In Vitro Effect on Enkephalins Degradation by NEP and Treatment of Intestinal Inflammation in Mice. International Journal of Molecular Sciences. 2022; 23(22):14450. https://doi.org/10.3390/ijms232214450
Chicago/Turabian StyleSobocińska, Małgorzata, Jakub Fichna, Artur Giełdoń, Piotr Skowron, and Elżbieta Kamysz. 2022. "N-Terminally Lipidated Sialorphin Analogs—Synthesis, Molecular Modeling, In Vitro Effect on Enkephalins Degradation by NEP and Treatment of Intestinal Inflammation in Mice" International Journal of Molecular Sciences 23, no. 22: 14450. https://doi.org/10.3390/ijms232214450





