Salivary microRNA and Metabolic Profiles in a Mouse Model of Subchronic and Mild Social Defeat Stress
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Food Intake, and Water Intake
2.2. Social Interaction (SI) Test
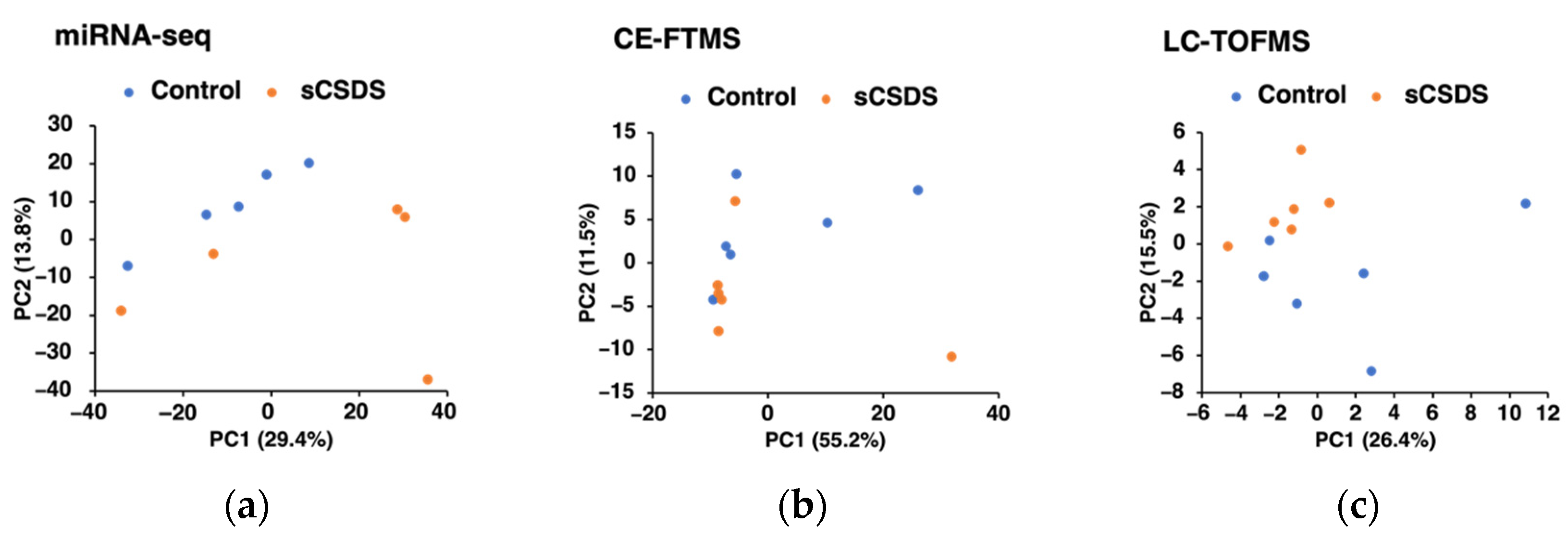
2.3. Saliva microRNA-seq Analysis
2.4. Saliva Metabolome Analyses
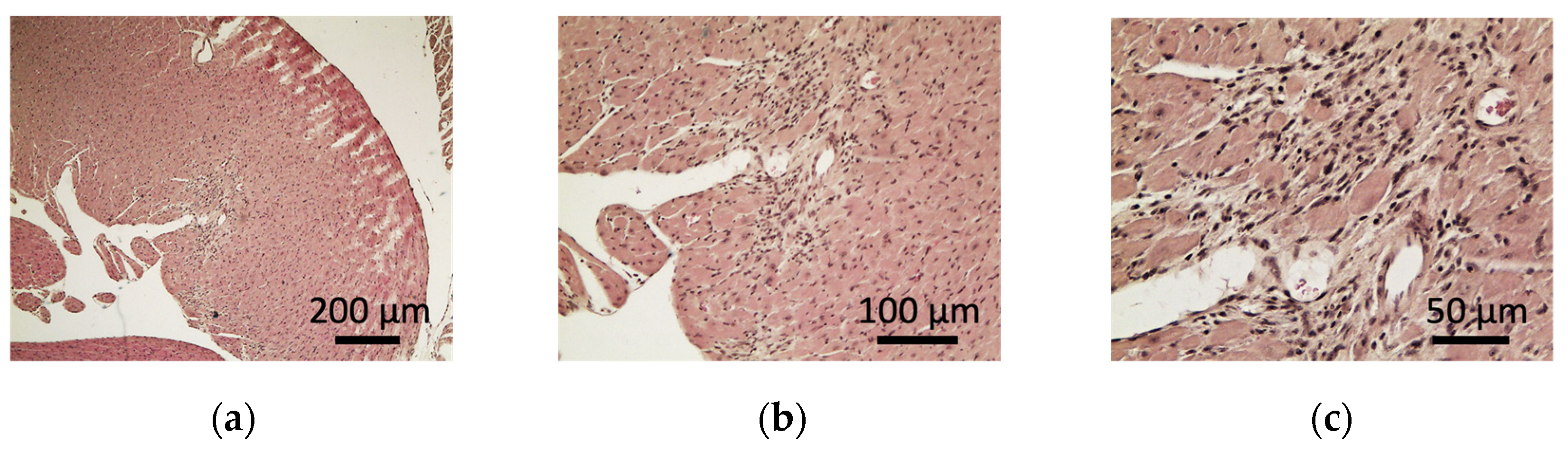
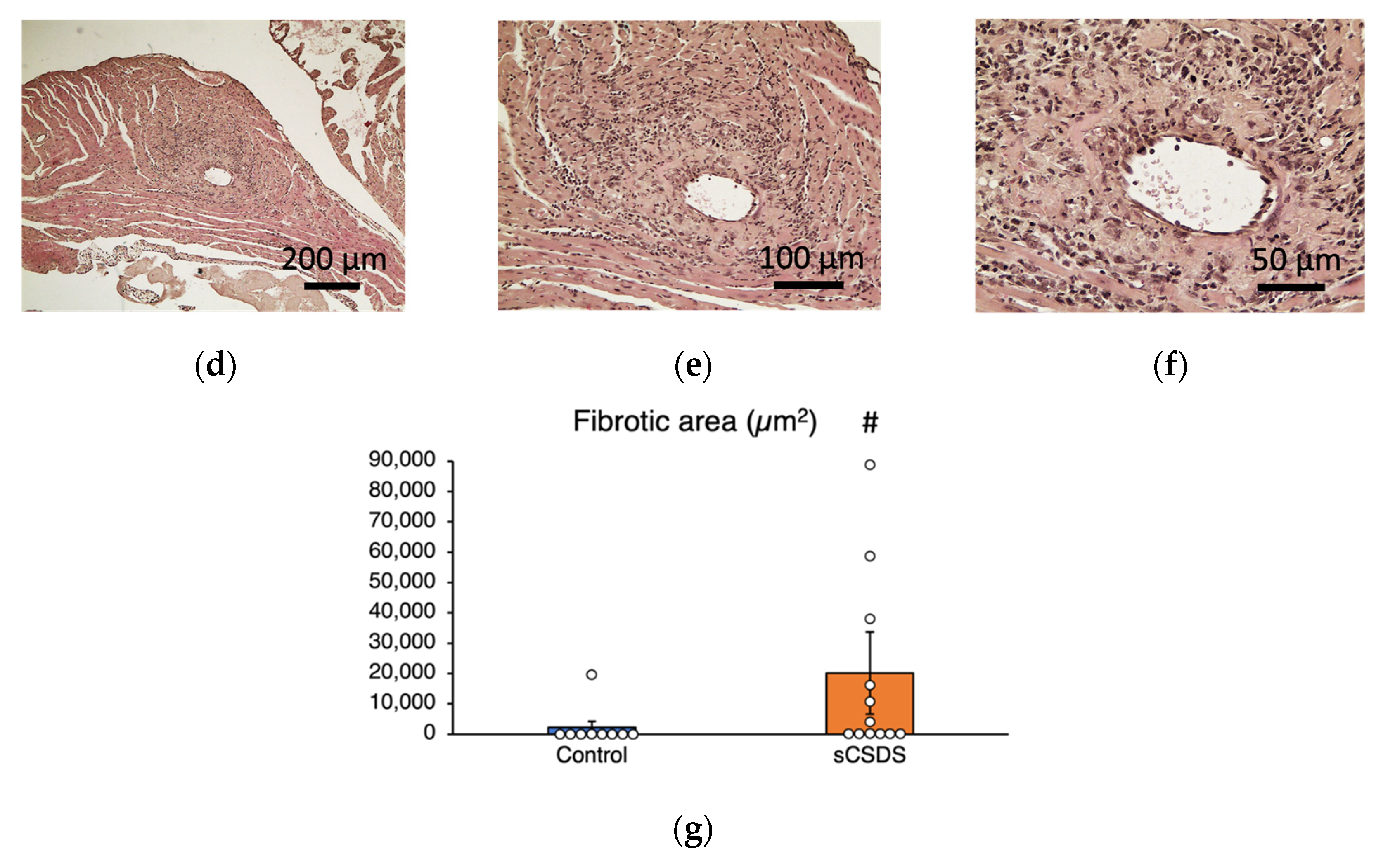
2.5. Pathological Analysis of the Heart of sCSDS Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Subchronic and Mild Social Defeat Stress (sCSDS)
4.3. Social Interaction Test
4.4. Sample Collection
4.5. Saliva microRNA-seq Analysis
4.6. Gene Ontology and Pathway Analyses
4.7. Saliva Metabolome Analysis (CE-FTMS)
4.8. Saliva Metabolome Analysis (LC-TOFMS)
4.9. Additional Study for Detection of Histological Abnormalities
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Depression is the leading cause of disability around the world. J. Am. Med. Assoc. 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Bica, T.; Castelló, R.; Toussaint, L.L.; Montesó-Curto, P. Depression as a risk factor of organic diseases: An international integrative review. J. Nurs. Scholarsh. 2017, 49, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR* D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Bonne, N.J.; Wong, D.T. Salivary biomarker development using genomic, proteomic and metabolomic approaches. Genome Med. 2012, 4, 82. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Vaze, A.; Rao, S. Clinical diagnosis of depression in primary care: A meta-analysis. Lancet 2009, 374, 609–619. [Google Scholar] [CrossRef]
- Maffioletti, E.; Cattaneo, A.; Rosso, G.; Maina, G.; Maj, C.; Gennarelli, M.; Tardito, D.; Bocchio-Chiavetto, L. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J. Affect. Disord. 2016, 200, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.P.; Fiori, L.M.; Cruceanu, C.; Lin, R.; Labonte, B.; Cates, H.M.; Heller, E.A.; Vialou, V.; Ku, S.M.; Gerald, C.; et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat. Commun. 2017, 8, 15497. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Xu, M.; Gao, Z.H.; Wang, Y.Q.; Yue, Z.; Zhang, Y.X.; Li, X.X.; Zhang, C.; Xie, S.Y.; Wang, P.Y. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS ONE 2013, 8, e63648. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Dunbar, M.; Shelton, R.C.; Dwivedi, Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology 2017, 42, 864–875. [Google Scholar] [CrossRef]
- Belzeaux, R.; Bergon, A.; Jeanjean, V.; Loriod, B.; Formisano-Tréziny, C.; Verrier, L.; Loundou, A.; Baumstarck-Barrau, K.; Boyer, L.; Gall, V.; et al. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl. Psychiatry 2012, 2, e185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.M.; Sun, X.Y.; Guo, W.; Zhong, A.F.; Niu, W.; Zhao, L.; Dai, Y.H.; Guo, Z.M.; Zhang, L.Y.; Lu, J. Differential expression of microRNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients. J. Psychiatr. Res. 2014, 59, 45–52. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, X.; Jiang, K.; Peng, D.; Hong, W.; Fang, Y.; Qian, Y.; Yu, S.; Li, H. Alterations of microRNA-124 expression in peripheral blood mononuclear cells in pre- and post-treatment patients with major depressive disorder. J. Psychiatr. Res. 2016, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.P.; Fiori, L.M.; Gross, J.A.; Labonte, B.; Yerko, V.; Mechawar, N.; Turecki, G. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int. J. Neuropsychopharmacol. 2014, 17, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Torres-Berrío, A.; Lopez, J.P.; Bagot, R.C.; Nouel, D.; Dal Bo, G.; Cuesta, S.; Zhu, L.; Manitt, C.; Eng, C.; Cooper, H.M.; et al. DCC confers susceptibility to depression-like behaviors in humans and mice and is regulated by miR-218. Biol. Psychiatry 2017, 81, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, Y.; Roy, B.; Dwivedi, Y. Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects. Neuropsychopharmacology 2021, 46, 900–910. [Google Scholar] [CrossRef]
- Kawamura, N.; Shinoda, K.; Sato, H.; Sasaki, K.; Suzuki, M.; Yamaki, K.; Fujimori, T.; Yamamoto, H.; Osei-Hyiaman, D.; Ohashi, Y. Plasma metabolome analysis of patients with major depressive disorder. Psychiatry Clin. Neurosci. 2018, 72, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Nishi, D.; Ishikawa, H.; Kawakami, N. Prevalence of mental disorders and mental health service use in Japan. Psychiatry Clin. Neurosci. 2019, 73, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Setoyame, D.; Yoshino, A.; Takamura, M.; Okada, G.; Iwata, M.; Tsunetomi, K.; Ohgidani, M.; Kuwano, N.; Yoshimoto, J.; Okamoto, Y.; et al. Personality classification enhances blood metabolome analysis and biotyping for major depressive disorders: Two-species investigation. J. Affect. Disord. 2021, 279, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kubota, Y.; Tanaka, Y.; Iio, W.; Moriya, N.; Toyoda, A. Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia-like features in mice. Behav. Brain Res. 2014, 270, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Toyoda, A. A mouse model of subchronic and mild social defeat stress for understanding stress-induced behavioral and physiological deficits. J. Vis. Exp. 2015, 105, 52973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Lu, W.; Sun, Y.; Feng, J.; Wang, J. mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress. J. Psychiatr. Res. 2019, 118, 44–65. [Google Scholar] [CrossRef]
- Wiegand, C.; Heusser, P.; Klinger, C.; Cysarz, D.; Büssing, A.; Ostermann, T.; Savelsbergh, A. Stress-associated changes in salivary microRNAs can be detected in response to the Trier Social Stress Test: An exploratory study. Sci. Rep. 2018, 8, 7112. [Google Scholar] [CrossRef] [Green Version]
- Van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J.; Olson, E.N. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Carvalho, V.; Carvalho, V.O.; Bocchi, E.A. The emerging role of miR-208a in the heart. DNA Cell Biol. 2013, 32, 8–12. [Google Scholar] [CrossRef]
- Wang, J.; Song, C.; Cao, X.; Li, H.; Cai, H.; Ma, Y.; Huang, Y.; Lan, X.; Lei, C.; Ma, Y.; et al. MiR-208b regulates cell cycle and promotes skeletal muscle cell proliferation by targeting CDKN1A. J. Cell. Physiol. 2019, 234, 3720–3729. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Chen, F.; Zhang, L.; Chen, X.; Yang, C.; Han, Z. Circulating miR-208b: A potentially sensitive and reliable biomarker for the diagnosis and prognosis of acute myocardial infarction. Clin. Lab. 2017, 63, 101–109. [Google Scholar] [CrossRef]
- Agiannitopoulos, K.; Pavlopoulou, P.; Tsamis, K.; Bampali, K.; Samara, P.; Nasioulas, G.; Mertzanos, G.; Babalis, D.; Lamnissou, K. Expression of miR-208b and miR-499 in Greek patients with acute myocardial infarction. Vivo 2018, 32, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Strike, P.C.; Steptoe, A. Psychosocial factors in the development of coronary artery disease. Prog. Cardiovasc. Dis. 2004, 46, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Koolhaas, J.; De Boer, S.; Musso, E.; Stilli, D.; Buwalda, B.; Meerlo, P. Social stress, autonomic neural activation, and cardiac activity in rats. Neurosci. Biobehav. Rev. 1999, 23, 915–923. [Google Scholar] [CrossRef]
- Sgoifo, A.; Carnevali, L.; Grippo, A.J. The socially stressed heart. Insights from studies in rodents. Neurosci. Biobehav. Rev. 2014, 39, 51–60. [Google Scholar] [CrossRef]
- Costoli, T.; Bartolomucci, A.; Graiani, G.; Stilli, D.; Laviola, G.; Sgoifo, A. Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2133–H2140. [Google Scholar] [CrossRef] [Green Version]
- Carrer, M.; Liu, N.; Grueter, C.E.; Williams, A.H.; Frisard, M.I.; Hulver, M.W.; Bassel-Duby, R.; Olson, E.N. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc. Natl. Acad. Sci. USA 2012, 109, 15330–15335. [Google Scholar] [CrossRef] [Green Version]
- Kato, T. Neurobiological basis of bipolar disorder: Mitochondrial dysfunction hypothesis and beyond. Schizophr. Res. 2017, 187, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Mao, C.; Quattrochi, B.; Friedline, R.H.; Zhu, L.J.; Jung, D.Y.; Kim, J.K.; Lewis, B.; Wang, Y.X. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat. Commun. 2014, 5, 4725. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, N.; Hioki, H.; Kaneko, T.; Nakamura, K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014, 20, 346–358. [Google Scholar] [CrossRef]
- Patterson, Z.R.; Khazall, R.; Mackay, H.; Anisman, H.; Abizaid, A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology 2013, 154, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liao, H.; Sun, H.; Zhang, Y.; Cao, Z. MicroRNA-3064-3p regulates the differentiation of cementoblasts through targeting DKK1. J. Periodont. Res. 2018, 53, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yan, X.J.; Lei, F.; Wang, M.L.; He, L.L.; Luo, Y.Y.; Gao, H.W.; Feng, Y.L.; Yang, S.L.; Li, J.; et al. Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: Pivotal sites of oxidative phosphorylation. Mol. Brain 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanarik, M.; Alttoa, A.; Matrov, D.; Kõiv, K.; Sharp, T.; Panksepp, J.; Harro, J. Brain responses to chronic social defeat stress: Effects on regional oxidative metabolism as a function of a hedonic trait, and gene expression in susceptible and resilient rats. Eur. Neuropsychopharmacol. 2011, 21, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.; Gui, S.; Zhou, C.; Chen, J.; Yang, C.; Hu, Z.; Wang, H.; Zhong, X.; Zeng, L.; et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. NeuroReport 2018, 29, 417–425. [Google Scholar] [CrossRef]
- Chung, Y.E.; Chen, H.; Chou, H.L.; Chen, I.; Lee, M.; Chuang, L.; Liu, Y.; Lu, M.; Chen, C.; Wu, C.; et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef]
- Christodoulou, D.; Kuehne, A.; Estermann, A.; Fuhrer, T.; Lang, P.; Sauer, U. Reserve flux capacity in the pentose phosphate pathway by NADPH binding is conserved across kingdoms. Iscience 2019, 19, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Maurel, O.M.; Torrisi, S.A.; Barbagallo, C.; Purrello, M.; Salomone, S.; Drago, F.; Ragusa, M.; Leggio, G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. Int. J. Mol. Sci. 2021, 22, 5157. [Google Scholar] [CrossRef] [PubMed]
- Solich, J.; Kolasa, M.; Faron-Górecka, A.; Hajto, J.; Piechota, M.; Dziedzicka-Wasylewska, M. MicroRNA Let-7e in the mouse prefrontal cortex differentiates restraint-stress-resilient genotypes from susceptible genotype. Int. J. Mol. Sci. 2021, 22, 9439. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, Y.Y.; Eijssen, L.M.; Mews, P.; Ramakrishnan, A.; Alvarez, K.; Lardner, C.K.; Cates, H.M.; Walker, D.M.; Torres-Berrío, A.; Browne, C.J.; et al. Blood miR-144-3p: A novel diagnostic and therapeutic tool for depression. Mol. Psychiat. 2022; in press. [Google Scholar] [CrossRef]
- Takahashi, A.; Chung, J.R.; Zhang, S.; Zhang, H.; Grossman, Y.; Aleyasin, H.; Flanigan, M.E.; Pfau, M.L.; Menard, C.; Dumitriu, D.; et al. Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 2017, 7, 12838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Nagayasu, K.; Nishitani, N.; Kaneko, S.; Koide, T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS ONE 2014, 9, e94657. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slenter, D.N.; Kutmon, M.; Hanspers, K.; Riutta, A.; Windsor, J.; Nunes, N.; Mélius, J.; Cirillo, E.; Coort, S.L.; Digles, D.; et al. WikiPathways: A multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018, 46, D661–D667. [Google Scholar] [CrossRef]
- Kutmon, M.; van Iersel, M.P.; Bohler, A.; Kelder, T.; Nunes, N.; Pico, A.R.; Evelo, C.T. PathVisio 3: An extendable pathway analysis toolbox. PLOS Comput. Biol. 2015, 11, e1004085. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Sagawa, H.; Suzuki, M.; Yamamoto, H.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomics platform with capillary electrophoresis coupled with high-resolution mass spectrometry for plasma analysis. Anal. Chem. 2019, 91, 1295–1301. [Google Scholar] [CrossRef]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
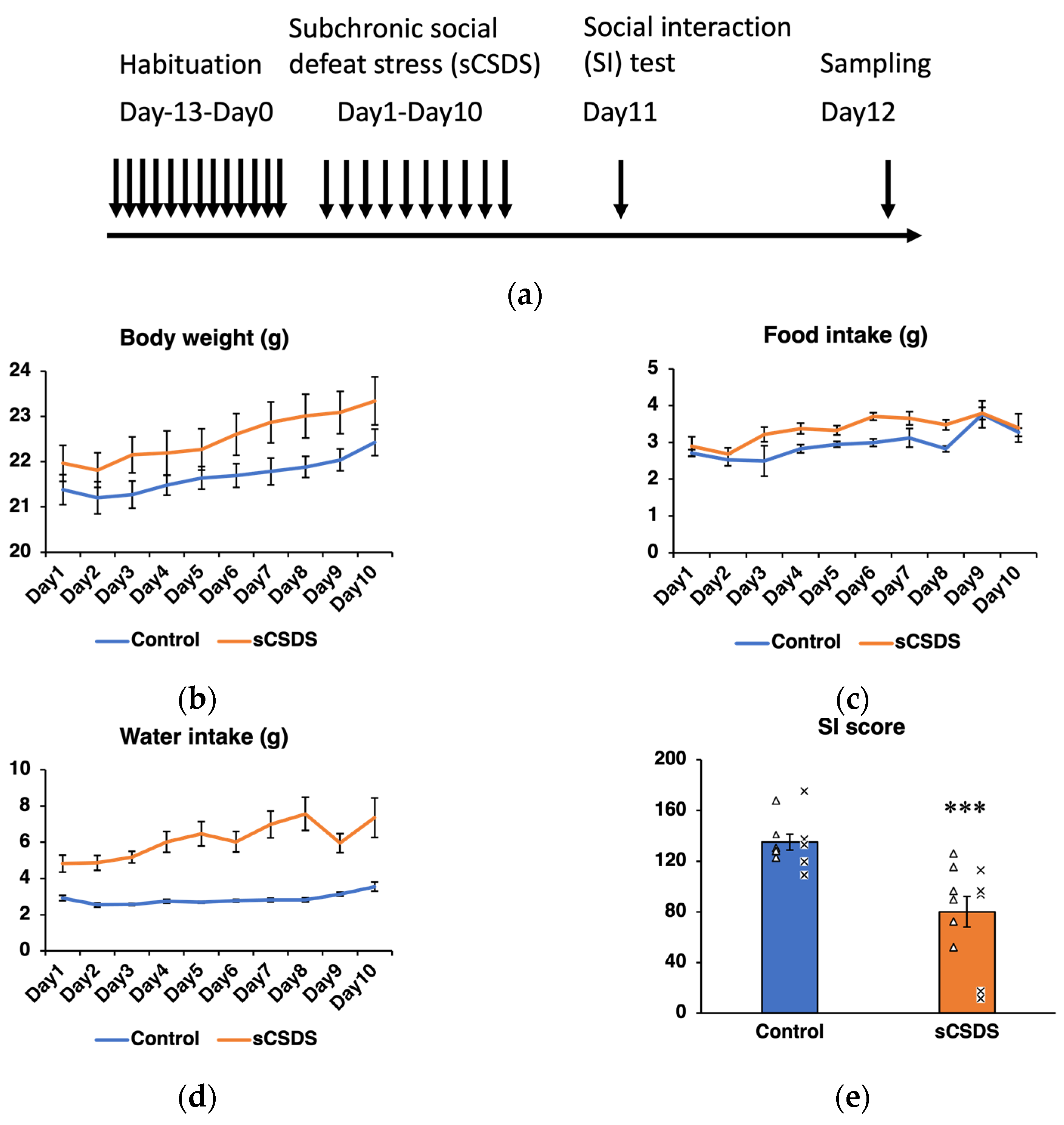
| Stress | Time | Stress × Time | |
|---|---|---|---|
| Body weight | F1,180 = 3.28 p = 0.08 | F9,180 = 18.42 p < 0.001 | F9,180 = 0.97 p > 0.1 |
| Food intake | F1,180 = 21.30 p < 0.001 | F9,180 = 5.85 p < 0.001 | F9,180 = 0.89 p > 0.1 |
| Water intake | F1,180 = 59.05 p < 0.001 | F9,180 = 5.00 p < 0.001 | F9,180 = 2.89 p < 0.01 |
| miRNA/piRNA | Fold Change | p-Value | q-Value |
|---|---|---|---|
| mmu-miR-6985-3p | 7.66 | 5.36 × 10−5 | 0.0609 |
| mmu-miR-7092-5p | 9.13 | 9.25 × 10−5 | 0.0609 |
| mmu-miR-208b-3p | 10.77 | 9.34 × 10−5 | 0.0609 |
| mmu-miR-378a-5p | 12.21 | 0.000147 | 0.0685 |
| mmu-miR-6944-3p | 6.2 | 0.000175 | 0.0685 |
| mmu_piR_000159 | 5.94 | 0.000217 | 0.0707 |
| mmu-miR-3106-3p | −9.12 | 0.000332 | 0.0926 |
| mmu-miR-3064-3p | 7.33 | 0.000402 | 0.098 |
| Pathway | Criterion for Z Score | Permuted p-Value |
|---|---|---|
| Oxidative phosphorylation | 2.7 | 0.008 |
| ApoE and miR-146 in inflammation and atherosclerosis | 2.55 | 0.014 |
| Small ligand GPCRs | 3.34 | 0.016 |
| BMP signaling pathway in eyelid development | 3.12 | 0.021 |
| Pentose phosphate pathway | 2.77 | 0.023 |
| Robo4 and VEGF signaling pathways crosstalk | 3.04 | 0.033 |
| Monoamine GPCRs | 2.15 | 0.033 |
| Mouse | Pathological Phenotype | Mouse | Pathological Phenotype |
|---|---|---|---|
| Control#1 | - | sCSDS#1 | Fibrotic tissue accumulation |
| Control#2 | - | sCSDS#2 | Fibrotic tissue accumulation |
| Control#3 | Fibrotic tissue accumulation | sCSDS#3 | Fibrotic tissue accumulation Inflammatory cell infiltration |
| Control#4 | - | sCSDS#4 | Fibrotic tissue accumulation |
| Control#5 | Fibrotic tissue accumulation | sCSDS#5 | Fibrotic tissue accumulation |
| Control#6 | - | sCSDS#6 | - |
| Control#7 | - | sCSDS#7 | Fibrotic tissue accumulation |
| Control#8 | - | sCSDS#8 | Fibrotic tissue accumulation |
| Control#9 | - | sCSDS#9 | Inflammatory cell infiltration |
| Control#10 | - | sCSDS#10 | - |
| sCSDS#11 | - | ||
| sCSDS#12 | - | ||
| sCSDS#13 | Fibrotic tissue accumulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, Y.; Yajima, Y.; Kawakami, K.; Nakamura, S.-i.; Tsukahara, T.; Oishi, K.; Toyoda, A. Salivary microRNA and Metabolic Profiles in a Mouse Model of Subchronic and Mild Social Defeat Stress. Int. J. Mol. Sci. 2022, 23, 14479. https://doi.org/10.3390/ijms232214479
Yoshida Y, Yajima Y, Kawakami K, Nakamura S-i, Tsukahara T, Oishi K, Toyoda A. Salivary microRNA and Metabolic Profiles in a Mouse Model of Subchronic and Mild Social Defeat Stress. International Journal of Molecular Sciences. 2022; 23(22):14479. https://doi.org/10.3390/ijms232214479
Chicago/Turabian StyleYoshida, Yuta, Yuhei Yajima, Kina Kawakami, Shin-ichi Nakamura, Takamitsu Tsukahara, Katsutaka Oishi, and Atsushi Toyoda. 2022. "Salivary microRNA and Metabolic Profiles in a Mouse Model of Subchronic and Mild Social Defeat Stress" International Journal of Molecular Sciences 23, no. 22: 14479. https://doi.org/10.3390/ijms232214479





