Effect of a Vegan Diet on Alzheimer’s Disease
Abstract
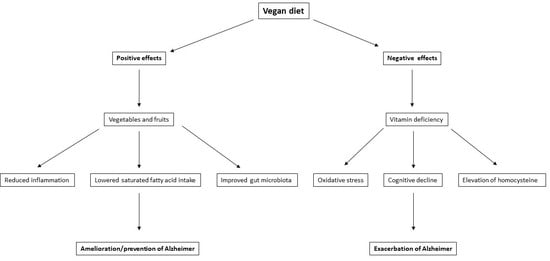
:1. Introduction
2. Vegan Diet and Brain Function
3. Possible Beneficial Effects of a Vegan Diet on the Brain and the Risk of AD
3.1. Fruits and Vegetables
3.2. Reduction in Inflammation
3.3. Modifiable Risk Factors for AD
3.4. GI Tract
3.5. TMAO Reduction
3.6. Mental Health
4. Possible Detrimental Effects of a Vegan Diet on the Brain and the Risk of AD
4.1. Vitamin B12 Deficiency
4.2. Vitamin D Deficiency
4.3. Omega-3 Polyunsaturated Fatty Acids Deficiency
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Share of Vegans in European Countries. 2021. Available online: https://www.statista.com/forecasts/1256518/share-of-vegans-in-european-countries/ (accessed on 31 August 2022).
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and Nutritional Aspects of Sustainable Diet Strategies and Their Association with Environmental Impacts: A Global Modelling Analysis with Country-Level Detail. Lancet Planet. Health 2018, 2, e451–e461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reasons for Being Vegan in Europe. 2019. Available online: https://www.statista.com/statistics/1263270/survey-reasons-for-being-vegan-in-europe/ (accessed on 31 August 2022).
- Miki, A.J.; Livingston, K.A.; Karlsen, M.C.; Folta, S.C.; McKeown, N.M. Using Evidence Mapping to Examine Motivations for Following Plant-Based Diets. Curr. Dev. Nutr. 2020, 4, nzaa013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Katzman, R.; Salmon, D.; Jin, H.; Cai, G.; Wang, Z.; Qu, G.; Grant, I.; Yu, E.; Levy, P.; et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: Impact of age, gender, and education. Ann. Neurol. 1990, 27, 428–437. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Evans, D.A.; Hebert, L.; Langa, K.M.; Heeringa, S.G.; Plassman, B.L.; Kukull, W.A. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer’s Dement. 2011, 7, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Green, R.C.; Cupples, L.A.; Kurz, A.; Auerbach, S.; Go, R.; Sadovnick, D.; Duara, R.; Kukull, W.A.; Chui, H.; Edeki, T.; et al. Depression as a Risk Factor for Alzheimer Disease. Arch. Neurol. 2003, 60, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Ownby, R.L.; Crocco, E.; Acevedo, A.; John, V.; Loewenstein, D. Depression and Risk for Alzheimer Disease. Arch. Gen. Psychiatry 2006, 63, 530. [Google Scholar] [CrossRef] [Green Version]
- Cantón-Habas, V.; Rich-Ruiz, M.; Romero-Saldaña, M.; Carrera-González, M.D.P. Depression as a Risk Factor For Dementia and Alzheimer’s Disease. Biomedicines 2020, 8, 457. [Google Scholar] [CrossRef]
- Lennon, M.J.; Makkar, S.R.; Crawford, J.D.; Sachdev, P.S. Midlife Hypertension and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 71, 307–316. [Google Scholar] [CrossRef]
- Rajan, K.B.; Barnes, L.L.; Wilson, R.S.; Weuve, J.; McAninch, E.A.; Evans, D.A. Blood Pressure and Risk of Incident Alzheimer’s Disease Dementia by Antihypertensive Medications and APOE Ε4 Allele. Ann. Neurol. 2018, 83, 935–944. [Google Scholar] [CrossRef]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Iivonen, S.; Mannermaa, A.; Tuomilehto, J.; Nissinen, A.; et al. Apolipoprotein E Epsilon4 Allele, Elevated Midlife Total Cholesterol Level, and High Midlife Systolic Blood Pressure Are Independent Risk Factors for Late-Life Alzheimer Disease. Ann. Intern. Med. 2002, 137, 149–155. [Google Scholar] [CrossRef]
- Huang, C.C.; Chung, C.M.; Leu, H.B.; Lin, L.Y.; Chiu, C.C.; Hsu, C.Y.; Chiang, C.H.; Huang, P.H.; Chen, T.J.; Lin, S.J.; et al. Diabetes Mellitus and the Risk of Alzheimer’s Disease: A Nationwide Population-Based Study. PLoS ONE 2014, 9, e87095. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Hassing, L.B.; Dahl, A.K.; Thorvaldsson, V.; Berg, S.; Gatz, M.; Pedersen, N.L.; Johansson, B. Overweight in Midlife and Risk of Dementia: A 40-Year Follow-Up Study. Int. J. Obes. 2009, 33, 893–898. [Google Scholar] [CrossRef] [Green Version]
- Razay, G.; Vreugdenhil, A. Obesity in Middle Age and Future Risk of Dementia: Midlife Obesity Increases Risk of Future Dementia. BMJ 2005, 331, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and Vascular Risk Factors at Midlife and the Risk of Dementia and Alzheimer Disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [Green Version]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2008, 39, 3–11. [Google Scholar] [CrossRef]
- Beckett, M.W.; Ardern, C.I.; Rotondi, M.A. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer’s disease in older adults. BMC Geriatr. 2015, 15, 9. [Google Scholar] [CrossRef]
- Stern, Y.; Gurland, B.; Tatemichi, T.K.; Tang, M.X.; Wilder, D.; Mayeux, R. Influence of Education and Occupation on the Incidence of Alzheimer’s Disease. JAMA. 1994, 271, 1004–1010. [Google Scholar] [CrossRef]
- Samadi, M.; Moradi, S.; Moradinazar, M.; Mostafai, R.; Pasdar, Y. Dietary pattern in relation to the risk of Alzheimer’s disease: A systematic review. Neurol. Sci. 2019, 40, 2031–2043. [Google Scholar] [CrossRef]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional Patterns Associated with the Maintenance of Neurocognitive Functions and the Risk of Dementia and Alzheimer’s Disease: A Focus on Human Studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Van Den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; Van De Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Slows Cognitive Decline with Aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO European Office for the Prevention and Control of Noncommunicable Diseases. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence; WHO Regional Office for Europe: Copenhagen, Denmark, 2021; pp. 1–11. [Google Scholar]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. Vegan Diet. Position of the German Nutrition Society (DGE). Ernaehrungs Umsch. Int. 2016, 63, 92–102. [Google Scholar] [CrossRef]
- Pye, A.; Bash, K.; Joiner, A.; Beenstock, J. Good for the Planet and Good for Our Health: The Evidence for Whole-Food Plant-Based Diets. BJPsych Int. 2022, 19, 90–92. [Google Scholar] [CrossRef]
- Termannsen, A.D.; Clemmensen, K.K.B.; Thomsen, J.M.; Nørgaard, O.; Díaz, L.J.; Torekov, S.S.; Quist, J.S.; Færch, K. Effects of Vegan Diets on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2022, 23, e13462. [Google Scholar] [CrossRef]
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a Vegan Diet for Health Benefits and Risks—An Umbrella Review of Meta-Analyses of Observational and Clinical Studies. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and Adequacy of the Vegan Diet. A Systematic Review of the Evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Cao, L.; Shi, M.; Liu, H.; Zhao, Y.; Xia, Y. Fruit and Vegetable Consumption and Cognitive Disorders in Older Adults: A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 871061. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Walach, H. Fruit, Vegetables and Prevention of Cognitive Decline or Dementia: A Systematic Review of Cohort Studies. J. Nutr. Health Aging 2012, 16, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Fieldhouse, J.L.P.; Doorduijn, A.S.; de Leeuw, F.A.; Verhaar, B.J.H.; Koene, T.; Wesselman, L.M.P.; de van der Schueren, M.; Visser, M.; van de Rest, O.; Scheltens, P.; et al. A Suboptimal Diet Is Associated with Poorer Cognition: The NUDAD Project. Nutrients 2020, 12, 703. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate Immunity in Alzheimer’s Disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Su, C.; Zhao, K.; Xia, H.; Xu, Y. Peripheral Inflammatory Biomarkers in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Psychogeriatrics 2019, 19, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, L.; Tsvetanov, K.A.; Jones, P.S.; Bevan-Jones, W.R.; Arnold, R.; Borchert, R.J.; Mak, E.; Su, L.; O’Brien, J.T.; Rowe, J.B. Neuroinflammation and Functional Connectivity in Alzheimer’s Disease: Interactive Influences on Cognitive Performance. J. Neurosci. 2019, 39, 7218–7226. [Google Scholar] [CrossRef] [Green Version]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Menzel, J.; Biemann, R.; Longree, A.; Isermann, B.; Mai, K.; Schulze, M.B.; Abraham, K.; Weikert, C. Associations of a Vegan Diet with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šebeková, K.; Krajčovičová-Kudláčková, M.; Schinzel, R.; Faist, V.; Klvanová, J.; Heidland, A. Plasma Levels of Advanced Glycation End Products in Healthy, Long-Term Vegetarians and Subjects on a Western Mixed Diet. Eur. J. Nutr. 2001, 40, 275–281. [Google Scholar] [CrossRef]
- Franco-De-Moraes, A.C.; De Almeida-Pititto, B.; Da Rocha Fernandes, G.; Gomes, E.P.; Da Costa Pereira, A.; Ferreira, S.R.G. Worse Inflammatory Profile in Omnivores than in Vegetarians Associates with the Gut Microbiota Composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutliffe, J.T.; Wilson, L.D.; de Heer, H.D.; Foster, R.L.; Carnot, M.J. C-Reactive Protein Response to a Vegan Lifestyle Intervention. Complement. Ther. Med. 2015, 23, 32–37. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [Green Version]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic Review and Meta-Analysis of the Associations of Vegan and Vegetarian Diets with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef]
- Chen, H.; Du, Y.; Liu, S.; Ge, B.; Ji, Y.; Huang, G. Association between Serum Cholesterol Levels and Alzheimer’s Disease in China: A Case-Control Study. Int. J. Food Sci. Nutr. 2019, 70, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Walker, R.; Bobb, J.F.; Sin, M.K.; Gray, S.L.; Bowen, J.D.; McCormick, W.; McCurry, S.M.; Crane, P.K.; Larson, E.B. Serum Cholesterol and Incident Alzheimer’s Disease: Findings from the Adult Changes in Thought Study. J. Am. Geriatr. Soc. 2018, 66, 2344–2352. [Google Scholar] [CrossRef]
- Rantanen, K.K.; Strandberg, A.Y.; Pitkälä, K.; Tilvis, R.; Salomaa, V.; Strandberg, T.E. Cholesterol in Midlife Increases the Risk of Alzheimer’s Disease during an up to 43-Year Follow-Up. Eur. Geriatr. Med. 2014, 5, 390–393. [Google Scholar] [CrossRef]
- Helzner, E.P.; Luchsinger, J.A.; Scarmeas, N.; Cosentino, S.; Brickman, A.M.; Glymour, M.M.; Stern, Y. Contribution of Vascular Risk Factors to the Progression in Alzheimer Disease. Arch. Neurol. 2009, 66, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.N.; Davey, G.K.; Key, T.J. Hypertension and Blood Pressure among Meat Eaters, Fish Eaters, Vegetarians and Vegans in EPIC–Oxford. Public Health Nutr. 2002, 5, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollakova, D.; Andreadi, A.; Pacifici, F.; Della-Morte, D.; Lauro, D.; Tubili, C. The Impact of Vegan Diet in the Prevention and Treatment of Type 2 Diabetes: A Systematic Review. Nutrients 2021, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Ylönen, K.; Saloranta, C.; Kronberg-Kippilä, C.; Groop, L.; Aro, A.; Virtanen, S.M. Associations of Dietary Fiber with Glucose Metabolism in Nondiabetic Relatives of Subjects with Type 2 Diabetes: The Botnia Dietary Study. Diabetes Care 2003, 26, 1979–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef] [PubMed]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in Neuroinflammation and Synaptic Dysfunction: A Focus on Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.; Sherzai, A.; Adeghate, J.; Sherzai, D. Gut Dysbiosis, Insulin Resistance and Alzheimer’s Disease: Review of a Novel Approach to Neurodegeneration. Front. Biosci. (Schol. Ed.) 2021, 13, 17–29. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, Gut Microbiota, and Alzheimer’s Disease. J. Neuroinflamm. 2019, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawczynski, C.; Weidauer, T.; Richert, C.; Schlattmann, P.; Dawczynski, K.; Kiehntopf, M. Nutrient Intake and Nutrition Status in Vegetarians and Vegans in Comparison to Omnivores—The Nutritional Evaluation (NuEva) Study. Front. Nutr. 2022, 9, 819106. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.; Yi, C.H.; Liu, T.T.; Lei, W.Y.; Hung, J.S.; Lin, C.L.; Lin, S.Z.; Chen, C.L. Impact of Vegan Diets on Gut Microbiota: An Update on the Clinical Implications. Tzu-Chi Med. J. 2018, 30, 200–203. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohnert, E.; Kreutz, C.; Binder, N.; Hannibal, L.; Gorkiewicz, G.; Müller, A.; Storz, M.A.; Huber, R.; Lederer, A.K. Changes in Gut Microbiota after a Four-Week Intervention with Vegan vs. Meat-Rich Diets in Healthy Participants: A Randomized Controlled Trial. Microorganisms 2021, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Veneruso, I.; Gong, C.; Cecarini, V.; Bonfili, L.; Eleuteri, A.M. Gut Microbiome and Mycobiome Alterations in an In Vivo Model of Alzheimer’s Disease. Genes 2022, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients With Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, M.; Budinska, E.; Kuzma, M.; Pelantova, H.; Hradecky, J.; Heczkova, M.; Daskova, N.; Bratova, M.; Modos, I.; Videnska, P.; et al. Vegan Diet Is Associated With Favorable Effects on the Metabolic Performance of Intestinal Microbiota: A Cross-Sectional Multi-Omics Study. Front. Nutr. 2022, 8, 783302. [Google Scholar] [CrossRef]
- Losno, E.A.; Sieferle, K.; Armando Perez-Cueto, F.J.; Ritz, C.; Losno, C.; Sieferle, E.A.; Perez-Cueto, K.; Ritz, F.J.A. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host–Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef]
- Trefflich, I.; Dietrich, S.; Braune, A.; Abraham, K.; Weikert, C. Short-and Branched-Chain Fatty Acids as Fecal Markers for Microbiota Activity in Vegans and Omnivores. Nutrients 2021, 13, 1808. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative Metabolomics in Vegans and Omnivores Reveal Constraints on Diet-Dependent Gut Microbiota Metabolite Production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.; Jacobi, M.; Rusch, K.; Andreas, S. Association of Dietary Type with Fecal Microbiota and Short Chain Fatty Acids in Vegans and Omnivores. J. Int. Soc. Microbiota 2016, 1, 1–19. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.S.; Yip, C.M.; Huang, T.H.J.; Chakrabartty, A.; Fraser, P.E. Manipulating the amyloid-β aggregation pathway with chemical chaperones. JBC 1999, 274, 32970–32974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The Gut Microbiota-Derived Metabolite Trimethylamine N-Oxide Is Elevated in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrona Cardoza, P.; Spillane, M.B.; Morales Marroquin, E. Alzheimer’s disease and gut microbiota: Does trimethylamine N-oxide (TMAO) play a role? Nutr. Rev. 2022, 8, 271–281. [Google Scholar] [CrossRef]
- Zarbock, K.R.; Han, J.H.; Singh, A.P.; Thomas, S.P.; Bendlin, B.B.; Denu, J.M.; Yu, J.-P.J.; Rey, F.E.; Ulland, T.K. Trimethylamine N-Oxide Reduces Neurite Density and Plaque Intensity in a Murine Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 585–597. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell. 2018, 17, e12768. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Wang, X.; Fu, S.; Zhang, X.; Wang, R.; Zhang, X. Decreased levels of circulating trimethylamine N-oxide alleviate cognitive and pathological deterioration in transgenic mice: A potential therapeutic approach for Alzheimer’s disease. Aging 2019, 11, 8642–8663. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G. The Influence of Animal- or Plant-Based Diets on Blood and Urine Trimethylamine-N-Oxide (TMAO) Levels in Humans. Curr. Nutr. Rep. 2022, 11, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Argyridou, S.; Davies, M.J.; Biddle, G.J.H.; Bernieh, D.; Suzuki, T.; Dawkins, N.P.; Rowlands, A.V.; Khunti, K.; Smith, A.C.; Yates, T. Evaluation of an 8-Week Vegan Diet on Plasma Trimethylamine-N-Oxide and Postchallenge Glucose in Adults with Dysglycemia or Obesity. J. Nutr. 2021, 151, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Obeid, R.; Awwad, H.M.; Keller, M.; Geisel, J. Trimethylamine-N-oxide and its biological variations in vegetarians. Eur. J. Nutr. 2017, 56, 2599–2609. [Google Scholar] [CrossRef]
- Jain, R.; Larsuphrom, P.; Degremont, A.; Latunde-Dada, G.O.; Philippou, E. Association between Vegetarian and Vegan Diets and Depression: A Systematic Review. Nutr. Bull. 2022, 47, 27–49. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and Veganism Compared with Mental Health and Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Link, L.B.; Hussaini, N.S.; Jacobson, J.S. Change in Quality of Life and Immune Markers after a Stay at a Raw Vegan Institute: A Pilot Study. Complement. Ther. Med. 2008, 16, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M. Mechanistic Insights and Perspectives Involved in Neuroprotective Action of Quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Dixon Clarke, S.E.; Ramsay, R.R. Dietary Inhibitors of Monoamine Oxidase A. J. Neural Transm. 2011, 118, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Chen, J.Y.; Ouyang, D.; Lu, J.H. Quercetin in animal models of Alzheimer’s disease: A systematic review of preclinical studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paula, P.C.; Maria, S.G.A.; Luis, C.H.; Patricia, C.G.G. Preventive effect of quercetin in a triple transgenic Alzheimer’s disease mice model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Li, H.R.; Chen, X.X.; Gao, X.R.; Huang, L.L.; Du, A.Q.; Jiang, C.; Li, H.; Ge, J.F. Quercetin alleviates LPS-induced depression-like behavior in rats via regulating BDNF-related imbalance of copine 6 and TREM1/2 in the hippocampus and PFC. Front. Pharmacol. 2020, 10, 544. [Google Scholar] [CrossRef] [Green Version]
- Taber, L.; Chiu, C.H.; Whelan, J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids 1998, 33, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Mądry, E.; Lisowska, A.; Grebowiec, P.; Walkowiak, J. The Impact of Vegan Diet on B-12 Status in Healthy Omnivores: Five-Year Prospective Study. Acta Sci. Pol. Technol. Aliment. 2012, 2, 209–212. [Google Scholar]
- Schüpbach, R.; Wegmüller, R.; Berguerand, C.; Bui, M.; Herter-Aeberli, I. Micronutrient Status and Intake in Omnivores, Vegetarians and Vegans in Switzerland. Eur. J. Nutr. 2017, 56, 283–293. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gøbel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of Macro- and Micronutrients in Danish Vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef] [Green Version]
- Rathod, R.; Kale, A.; Joshi, S. Novel Insights into the Effect of Vitamin B12 and Omega-3 Fatty Acids on Brain Function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef] [Green Version]
- Scalabrino, G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog. Neurobiol. 2009, 88, 203–220. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Refsum, H.; Johnston, C.; Smith, S.M.; Bradley, K.M.; De Jager, C.; Budge, M.M.; Smith, A.D. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008, 71, 826–832. [Google Scholar] [CrossRef]
- Tangney, C.C.; Aggarwal, N.T.; Li, H.; Wilson, R.S.; Decarli, C.; Evans, D.A.; Morris, M.C. Vitamin B12, cognition, and brain MRI measures: A cross-sectional examination. Neurology 2011, 77, 1773. [Google Scholar] [CrossRef] [Green Version]
- Jarquin Campos, A.; Risch, L.; Nydegger, U.; Wiesner, J.; Vazquez Van Dyck, M.; Renz, H.; Stanga, Z.; Risch, M. Diagnostic Accuracy of Holotranscobalamin, Vitamin B12, Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a Large, Mixed Patient Population. Dis. Markers 2020, 2020, 7468506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, J.; Chang, H.; Liu, X.; Zhu, R. Homocysteine and Folic Acid: Risk Factors for Alzheimer’s Disease—An Updated Meta-Analysis. Front. Aging Neurosci. 2021, 13, 665114. [Google Scholar] [CrossRef] [PubMed]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Ger. Psych. 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [Green Version]
- Douaud, G.; Refsum, H.; De Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [Green Version]
- Marques de Brito, B.; Campos, V. de M.; Neves, F.J.; Ramos, L.R.; Tomita, L.Y. Vitamin B12 Sources in Non-Animal Foods: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 28, 1–15. [Google Scholar] [CrossRef]
- Nakos, M.; Pepelanova, I.; Beutel, S.; Krings, U.; Berger, R.G.; Scheper, T. Isolation and Analysis of Vitamin B12 from Plant Samples. Food Chem. 2017, 216, 301–308. [Google Scholar] [CrossRef]
- Lederer, A.K.; Hannibal, L.; Hettich, M.; Behringer, S.; Spiekerkoetter, U.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Simmet, T.; et al. Vitamin B12 Status Upon Short-Term Intervention with a Vegan Diet—A Randomized Controlled Trial in Healthy Participants. Nutrients 2019, 11, 2815. [Google Scholar] [CrossRef] [Green Version]
- Selinger, E.; Kühn, T.; Procházková, M.; Anděl, M.; Gojda, J. Vitamin B12 Deficiency Is Prevalent Among Czech Vegans Who Do Not Use Vitamin B12 Supplements. Nutrients 2019, 11, 3019. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Eremin, K.O.; Tuohimaa, P. Mechanisms of Neuroprotective Action of Vitamin D3. Biochemistry 2004, 69, 738–741. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Tian, Q.; Cao, W.; Fan, X.; Wu, L.; Song, M.; Meng, Q.; Wang, W.; Wang, Y. Vitamin D and Multiple Health Outcomes: An Umbrella Review of Observational Studies, Randomized Controlled Trials, and Mendelian Randomization Studies. Adv. Nutr. 2022, 13, 1044–1062. [Google Scholar] [CrossRef]
- Kalra, A.; Teixeira, A.L.; Diniz, B.S. Association of Vitamin D Levels with Incident All-Cause Dementia in Longitudinal Observational Studies: A Systematic Review and Meta-Analysis. J. Prev. Alzheimer’s Dis. 2020, 7, 14–20. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Vitamin D Deficiency Is Associated with Increased Risk of Alzheimer’s Disease and Dementia: Evidence from Meta-Analysis. Nutr. J. 2015, 14, 76. [Google Scholar] [CrossRef] [Green Version]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D Deficiency as a Risk Factor for Dementia and Alzheimer’s Disease: An Updated Meta-Analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Annweiler, C.; Llewellyn, D.J.; Beauchet, O. Low Serum Vitamin D Concentrations in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2013, 33, 659–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavraki, E.; Ioannidis, P.; Tripsianis, G.; Gioka, T.; Kolousi, M.; Vadikolias, K. Vitamin D in Mild Cognitive Impairment and Alzheimer’s Disease. A Study in Older Greek Adults. Hippokratia 2020, 24, 120–126. [Google Scholar]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D Status and Risk of Dementia and Alzheimer’s Disease: A Meta-Analysis of Dose-Response†. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef]
- Menzel, J.; Longree, A.; Abraham, K.; Schulze, M.B.; Weikert, C. Dietary and Plasma Phospholipid Profiles in Vegans and Omnivores—Results from the RBVD Study. Nutrients 2022, 14, 2900. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High Compliance with Dietary Recommendations in a Cohort of Meat Eaters, Fish Eaters, Vegetarians, and Vegans: Results from the European Prospective Investigation into Cancer and Nutrition-Oxford Study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [Green Version]
- Lane, K.E.; Wilson, M.; Hellon, T.G.; Davies, I.G. Bioavailability and Conversion of Plant Based Sources of Omega-3 Fatty Acids—A Scoping Review to Update Supplementation Options for Vegetarians and Vegans. Crit. Rev. Food Sci. Nutr. 2022, 62, 4982–4997. [Google Scholar] [CrossRef]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; Pablo, G.S. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Davis, B.C.; Garg, M.L. Omega-3 polyunsaturated fatty acids and vegetarian diets. Med. J. Aust. 2013, 19, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H. Effects of N-3 Polyunsaturated Fatty Acids on Dementia. J. Clin. Med. Res. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Hjorth, E.; Vedin, I.; Eriksdotter, M.; Freund-Levi, Y.; Wahlund, L.O.; Cederholm, T.; Palmblad, J.; Schultzberg, M. Effects of N-3 FA Supplementation on the Release of Proresolving Lipid Mediators by Blood Mononuclear Cells: The OmegAD Study. J. Lipid Res. 2015, 56, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortés-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of Inflammation Is Altered in Alzheimer’s Disease. Alzheimer’s Dement. 2015, 11, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Faxén-Irving, G.; Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Basun, H.; Hjorth, E.; Palmblad, J.; Vedin, I.; Cederholm, T.; Wahlund, L.O. Effects on Transthyretin in Plasma and Cerebrospinal Fluid by DHA-Rich n − 3 Fatty Acid Supplementation in Patients with Alzheimer’s Disease: The OmegAD Study. J. Alzheimer’s Dis. 2013, 36, 1–6. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Kuchenbecker, J.; Grosgen, S.; Burg, V.K.; Hundsdorfer, B.; Rothhaar, T.L.; Friess, P.; De Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic Acid Reduces Amyloid Beta Production via Multiple Pleiotropic Mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.O.; Basun, H.; et al. Omega-3 Fatty Acids Enhance Phagocytosis of Alzheimer’s Disease-Related Amyloid-Β42 by Human Microglia and Decrease Inflammatory Markers. J. Alzheimer’s Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [Green Version]
- Tully, A.M.; Roche, H.M.; Doyle, R.; Fallon, C.; Bruce, I.; Lawlor, B.; Coakley, D.; Gibney, M.J. Low Serum Cholesteryl Ester-Docosahexaenoic Acid Levels in Alzheimer’s Disease: A Case–Control Study. Br. J. Nutr. 2003, 89, 483–489. [Google Scholar] [CrossRef]
- Chu, C.S.; Hung, C.F.; Ponnusamy, V.K.; Chen, K.C.; Chen, N.C. Higher Serum DHA and Slower Cognitive Decline in Patients with Alzheimers Disease: Two-Year Follow-Up. Nutrients 2022, 14, 1159. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Satizabal, C.L.; Tintle, N.; Melo van Lent, D.; Vasan, R.S.; Beiser, A.S.; Seshadri, S.; Harris, W.S. Red Blood Cell DHA Is Inversely Associated with Risk of Incident Alzheimer´s Disease and All-Cause Dementia: Framingham Offspring Study. Nutrients 2022, 14, 2408. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.F.; Wolf, P.A. Plasma Phosphatidylcholine Docosahexaenoic Acid Content and Risk of Dementia and Alzheimer Disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef]
- Kröger, E.; Verreault, R.; Carmichael, P.H.; Lindsay, J.; Julien, P.; Dewailly, É.; Ayotte, P.; Laurin, D. Omega-3 Fatty Acids and Risk of Dementia: The Canadian Study of Health and Aging. Am. J. Clin. Nutr. 2009, 90, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurin, D.; Verreault, R.; Lindsay, J.; Dewailly, É.; Holub, B.J. Omega-3 Fatty Acids and Risk of Cognitive Impairment and Dementia. J. Alzheimer’s Dis. 2003, 5, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.H.R.; Chappell, H.F.; Zulyniak, M.A. Dietary and Supplemental Long-Chain Omega-3 Fatty Acids as Moderators of Cognitive Impairment and Alzheimer’s Disease. Eur. J. Nutr. 2022, 61, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Araya-Quintanilla, F.; Gutiérrez-Espinoza, H.; Sánchez-Montoya, U.; Muñoz-Yañez, M.J.; Baeza-Vergara, A.; Petersen-Yanjarí, M.; Fernández-Lecaros, L. Effectiveness of Omega-3 Fatty Acid Supplementation in Patients with Alzheimer Disease: A Systematic Review and Meta-Analysis. Neurología (Engl. Ed.) 2020, 35, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Su, K.P.; Cheng, T.C.; Liu, H.C.; Chang, C.J.; Dewey, M.E.; Stewart, R.; Huang, S.Y. The Effects of Omega-3 Fatty Acids Monotherapy in Alzheimer’s Disease and Mild Cognitive Impairment: A Preliminary Randomized Double-Blind Placebo-Controlled Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 Fatty Acid Treatment in 174 Patients with Mild to Moderate Alzheimer Disease: OmegAD Study: A Randomized Double-Blind Trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [Green Version]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of Long-Term Omega 3 Polyunsaturated Fatty Acid Supplementation with or without Multidomain Intervention on Cognitive Function in Elderly Adults with Memory Complaints (MAPT): A Randomised, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; de Souto Barreto, P.; Coley, N.; Cantet, C.; Cesari, M.; Andrieu, S.; Vellas, B. Cognitive Changes with Omega-3 Polyunsaturated Fatty Acids in Non-Demented Older Adults with Low Omega-3 Index. J. Nutr. Health Aging 2017, 21, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Beezhold, B.L.; Johnston, C.S.; Daigle, D.R. Vegetarian diets are associated with healthy mood states: A cross-sectional study in Seventh Day Adventist adults. Nutr. J. 2010, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.-N.; Chiu, T.H.; Chang, C.-E.; Lin, M.-N. The Impact of a Plant-based Dietary Pattern on Dementia Risk: A Prospective Cohort Study. Innov. Aging 2019, 3, S734. [Google Scholar] [CrossRef]
- Brasky, T.M.; Darke, A.K.; Song, X.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J. Natl. Cancer Inst. 2013, 105, 1132–1141. [Google Scholar] [CrossRef] [PubMed]


| Non-Modifiable Risk Factors | Non-Modifiable Risk Factors |
|---|---|
| Advanced age [6,7,8] | Depression [9,10,11] |
| Gender [7,8] | Hypertension [12,13,14] |
| Genetic predisposition [26] | Diabetes [15,16,17] |
| Obesity [18,19,20] | |
| Physical inactivity [21,22] | |
| Low education [6,7,23] | |
| Unhealthy diet [24,25] |
| Positive Effects | Negative Effects |
|---|---|
| Reduction in systemic inflammation [49,50,52,76] | Increased risk of vitamin B12 deficiency [35,36,107] |
| Reduced risk of developing obesity [34] and type II diabetes [59] | Increased risk of vitamin D deficiency [70,108,109] |
| Reduction in TMAO levels in plasma and urine [92,93] | Insufficient intake of DHA and EPA [70] |
| Lower caloric intake and saturated fat intake compared to other types of diets [35,70] | |
| Decreased pro-inflammatory bacteria in the gut [77,84] | |
| Increased production of beneficial bacterially produced metabolites [76,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katonova, A.; Sheardova, K.; Amlerova, J.; Angelucci, F.; Hort, J. Effect of a Vegan Diet on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14924. https://doi.org/10.3390/ijms232314924
Katonova A, Sheardova K, Amlerova J, Angelucci F, Hort J. Effect of a Vegan Diet on Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(23):14924. https://doi.org/10.3390/ijms232314924
Chicago/Turabian StyleKatonova, Alzbeta, Katerina Sheardova, Jana Amlerova, Francesco Angelucci, and Jakub Hort. 2022. "Effect of a Vegan Diet on Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 23: 14924. https://doi.org/10.3390/ijms232314924