Effect of Antibiotic Amphotericin B Combinations with Selected 1,3,4-Thiadiazole Derivatives on RPTECs in an In Vitro Model
Abstract
:1. Introduction
2. Results
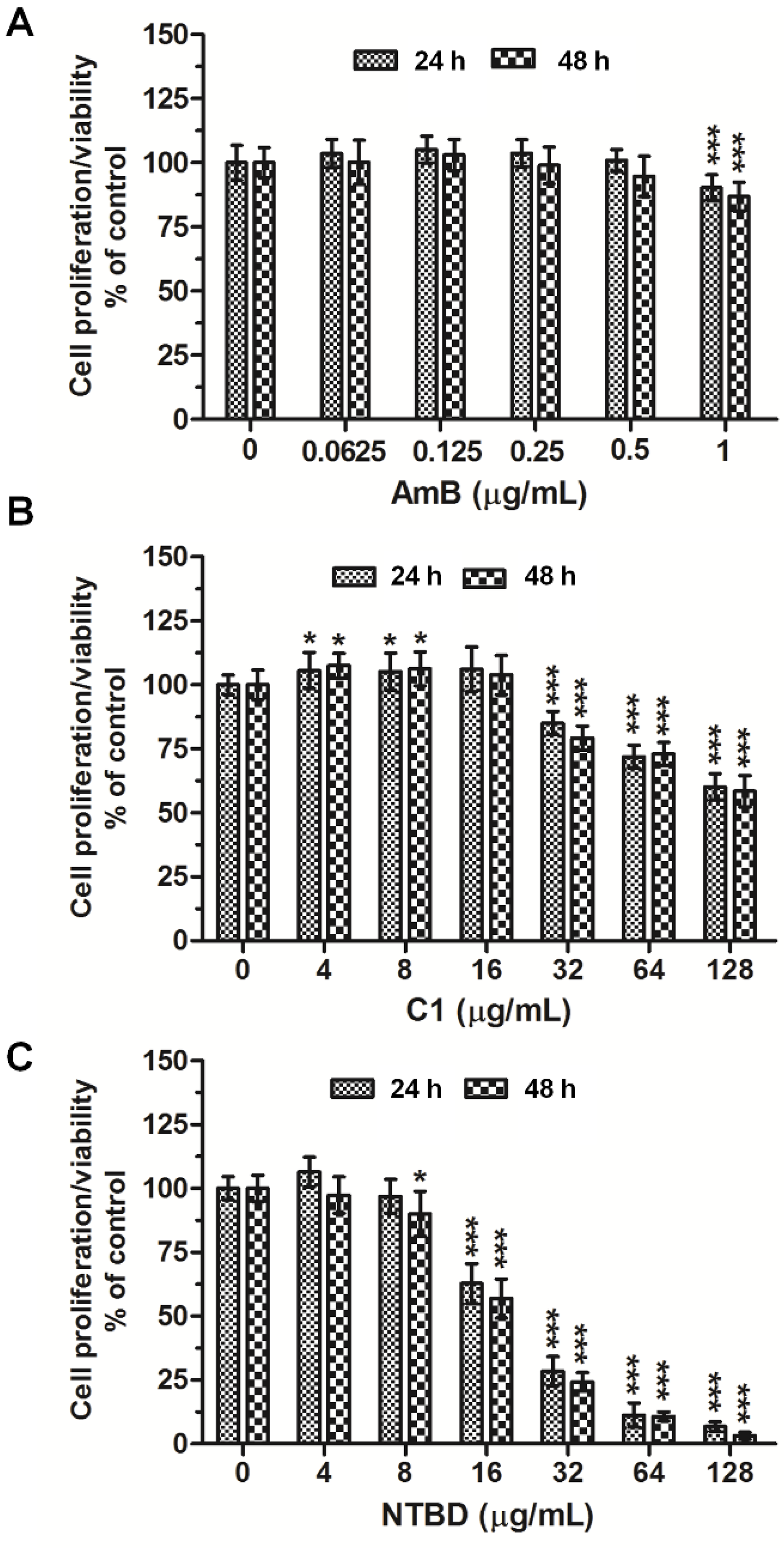
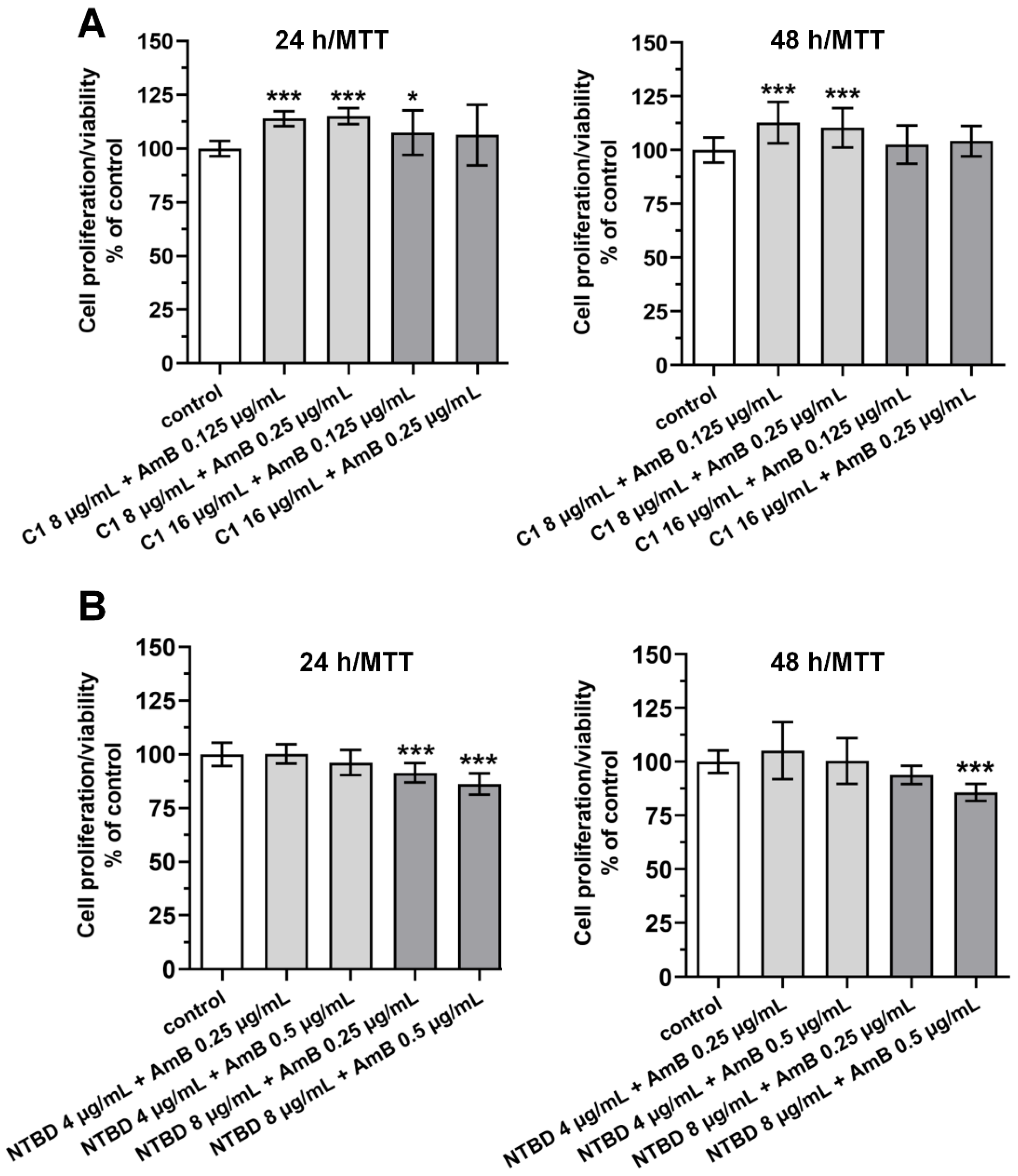
2.1. Mitochondrial Dehydrogenase Activity
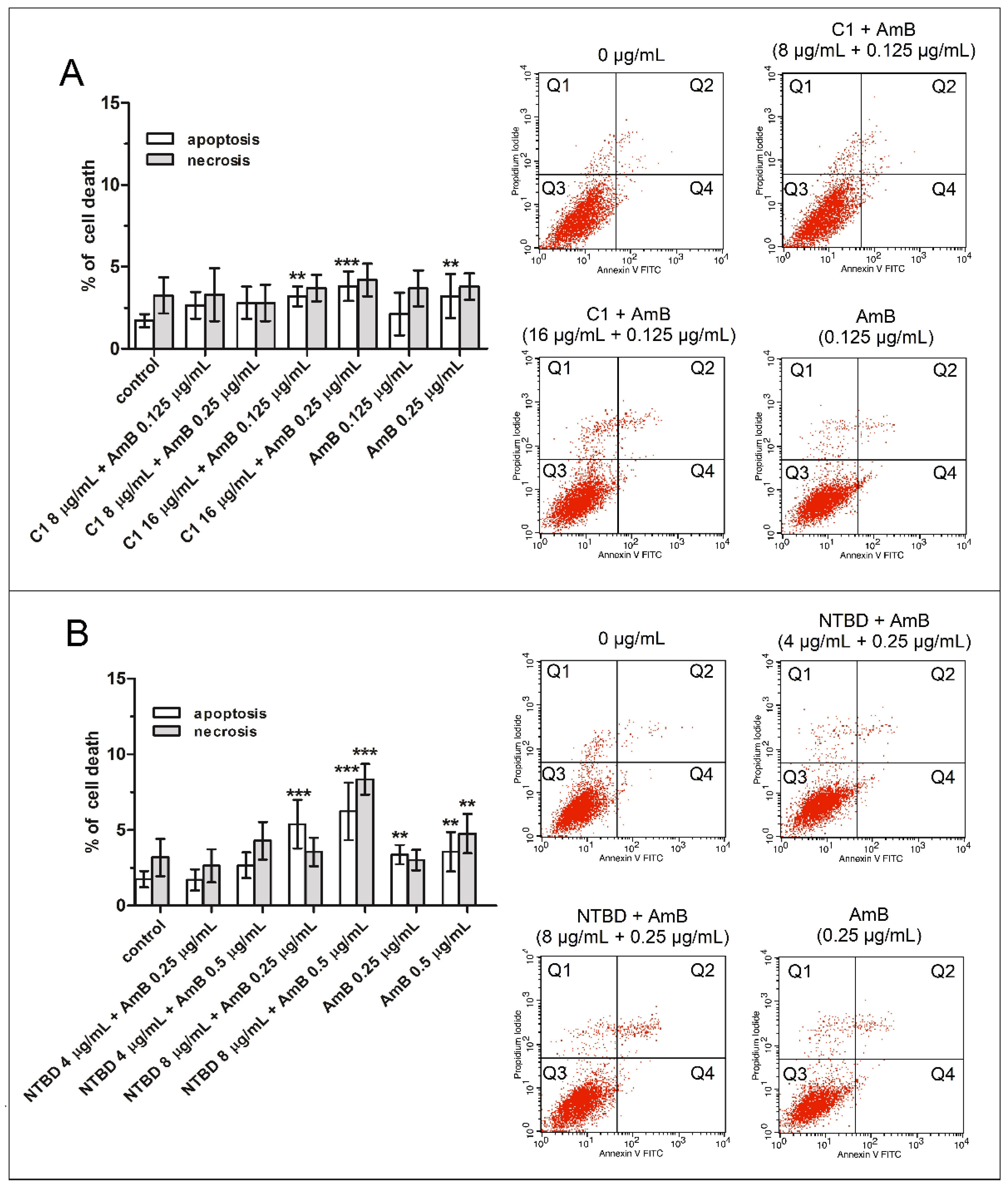
2.2. Impact of Thiadiazole Derivatives and Their Combinations with AmB on Necrotic and Apoptotic Cell Death in RPTEC Culture
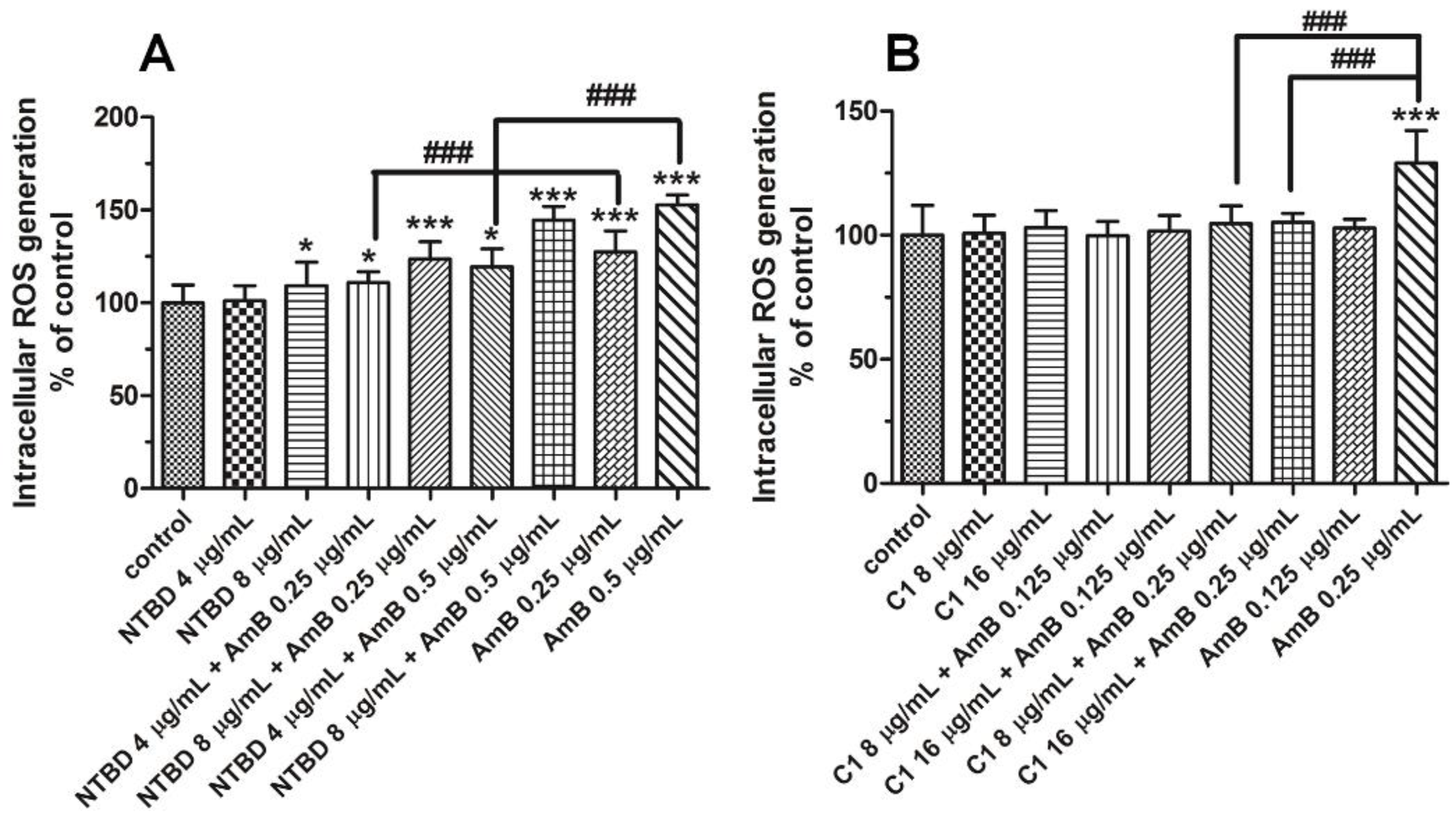
2.3. Impact of Thiadiazole Derivatives and Their Combinations with AmB on Intracelluar ROS Production
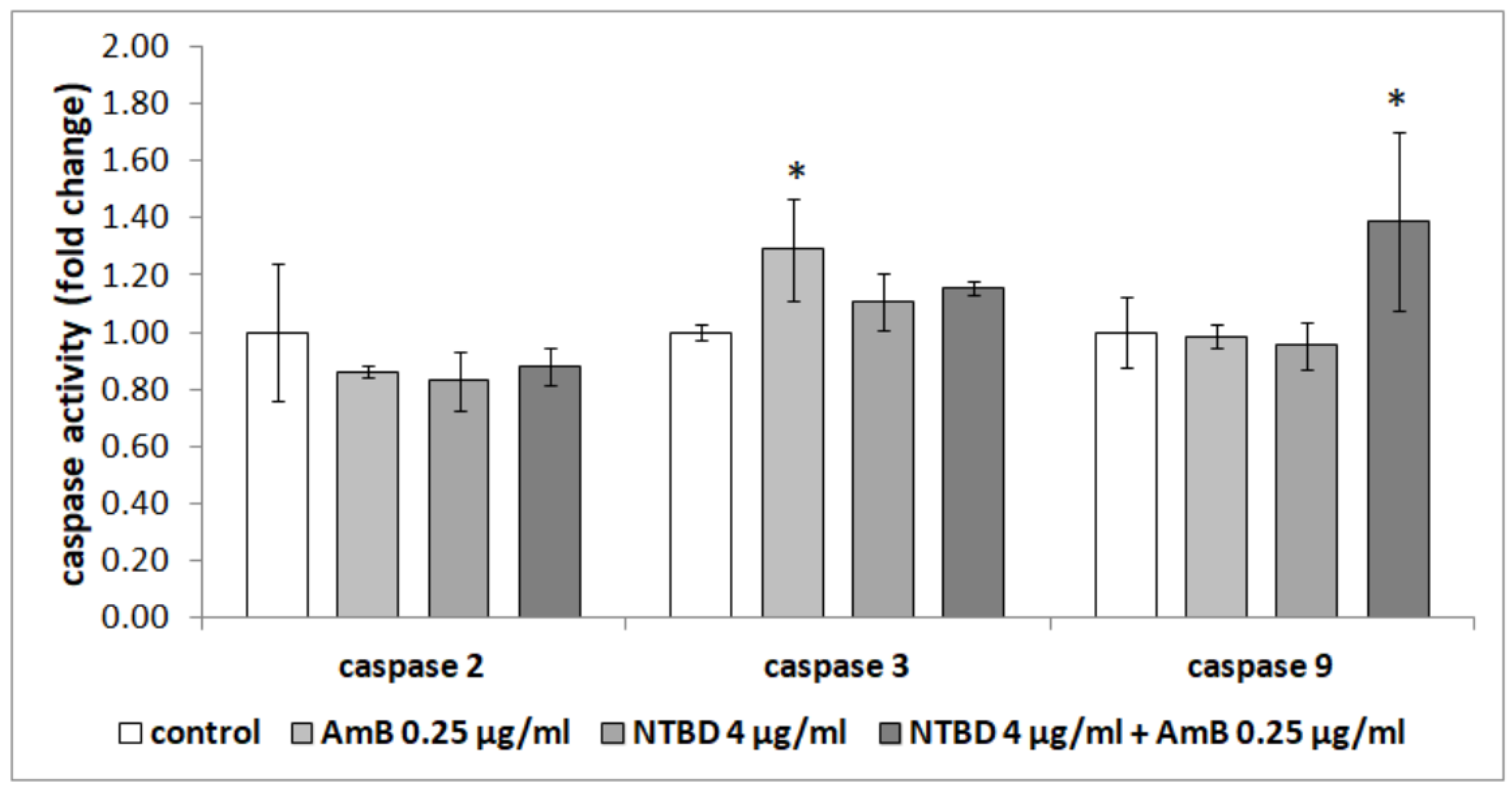
2.4. Effect of Thiadiazole Derivatives and Their Combinations with AmB on the Activity of Caspases in RPTEC Culture
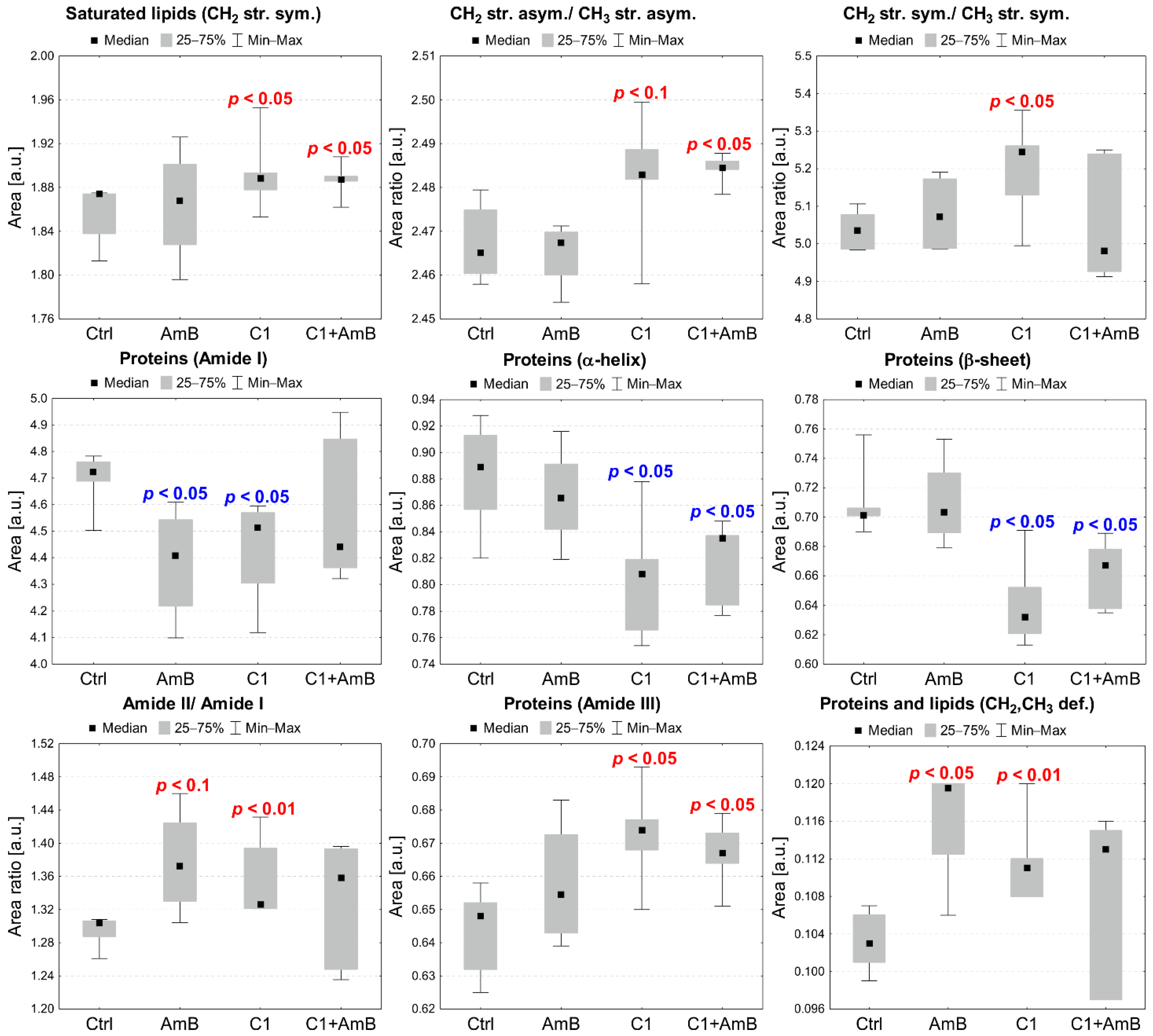
2.5. Semi-Quantitative ATR-FTIR Analysis of the C1+AmB and NTBD+AmB In Vitro Effect in the RPTEC Model
3. Discussion
4. Materials and Methods
4.1. Human Renal Proximal Tubule Cell Culture
4.2. Antifungal Substances
4.3. Determination of Mitochondrial Dehydrogenase Activity (MTT Assay)
4.4. Flow Cytometry
4.5. Determination of ROS
4.6. Caspase Activity Assay
4.7. Sample Preparation for ATR-FTIR Semi-Quantitative Analysis
4.8. ATR-FTIR Spectroscopy and Semi-Quantitative Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased Antimicrobial Resistance during the COVID-19 Pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudzik, B.; Bonio, K.; Dabrowski, W.; Pietrzak, D.; Niewiadomy, A.; Olender, A.; Malodobry, K.; Gagoś, M. Synergistic Antifungal Interactions of Amphotericin B with 4-(5-Methyl-1,3,4-Thiadiazole-2-Yl) Benzene-1,3-Diol. Sci. Rep. 2019, 9, 12945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudzik, B.; Bonio, K.; Dabrowski, W.; Pietrzak, D.; Niewiadomy, A.; Olender, A.; Pawlikowska-Pawlęga, B.; Gagoś, M. Antifungal Effects of a 1,3,4-Thiadiazole Derivative Determined by Cytochemical and Vibrational Spectroscopic Studies. PLoS ONE 2019, 14, e0222775. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. 2021, 1, e210. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L. Cytotoxicity Testing: Measuring Viable Cells, Dead Cells, and Detecting Mechanism of Cell Death. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 103–114. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Angius, F.; Floris, A. Liposomes and MTT Cell Viability Assay: An Incompatible Affair. Toxicol. Vitr. 2015, 29, 314–319. [Google Scholar] [CrossRef]
- Janik-Olchawa, N.; Drozdz, A.; Ryszawy, D.; Pudełek, M.; Planeta, K.; Setkowicz, Z.; Śniegocki, M.; Żądło, A.; Ostachowicz, B.; Chwiej, J. Comparison of Ultrasmall IONPs and Fe Salts Biocompatibility and Activity in Multi-Cellular in Vitro Models. Sci. Rep. 2020, 10, 15447. [Google Scholar] [CrossRef]
- Janik-Olchawa, N.; Drozdz, A.; Ryszawy, D.; Pudelek, M.; Planeta, K.; Setkowicz, Z.; Sniegocki, M.; Wytrwal-Sarna, M.; Gajewska, M.; Chwiej, J. The Influence of IONPs Core Size on Their Biocompatibility and Activity in in Vitro Cellular Models. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2018; Volume 1709, pp. 107–127. ISBN 9781493974771. [Google Scholar]
- Death, C.; Cytometry, F.; Chen, D.; Eyupoglu, I.Y.; Savaskan, N. Cell Viability Assays. In Methods in Molecular Biology; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; Volume 1601, pp. 71–77. ISBN 978-1-4939-6959-3. [Google Scholar]
- Darzynkiewicz, Z.; Bedner, E.; Smolewski, P. Flow Cytometry in Analysis of Cell Cycle and Apoptosis. Semin. Hematol. 2001, 38, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Sławińska-Brych, A.; Król, S.K.; Dmoszyńska-Graniczka, M.; Zdzisińska, B.; Stepulak, A.; Gagoś, M. Xanthohumol Inhibits Cell Cycle Progression and Proliferation of Larynx Cancer Cells in Vitro. Chem. Biol. Interact. 2015, 240, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Sławińska-Brych, A.; Zdzisińska, B.; Dmoszyńska-Graniczka, M.; Jeleniewicz, W.; Kurzepa, J.; Gagoś, M.; Stepulak, A. Xanthohumol Inhibits the Extracellular Signal Regulated Kinase (ERK) Signalling Pathway and Suppresses Cell Growth of Lung Adenocarcinoma Cells. Toxicology 2016, 357–358, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Sivasailam, A.; Maliakkal, R.T.; Pillai, P.R.; Surabhi, S.V.; Prasad, T.; Santhoshkumar, T.R. Quantitative Analysis of Apoptosis and Necrosis in Live Cells Using Flow Cytometry. Methods Mol. Biol. 2022, 2543, 57–69. [Google Scholar]
- Bertho, Á.L.; Santiago, M.A.; Coutinho, S.G. Flow Cytometry in the Study of Cell Death. Mem. Inst. Oswaldo Cruz 2000, 95, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Araya, L.E.; Soni, I.V.; Hardy, J.A.; Julien, O. Deorphanizing Caspase-3 and Caspase-9 Substrates in and Out of Apoptosis with Deep Substrate Profiling. ACS Chem. Biol. 2021, 16, 2280–2296. [Google Scholar] [CrossRef]
- Kopeina, G.S.; Zhivotovsky, B. Caspase-2 as a Master Regulator of Genomic Stability. Trends Cell Biol. 2021, 31, 712–720. [Google Scholar] [CrossRef]
- An, H.-K.; Chung, K.M.; Park, H.; Hong, J.; Gim, J.-E.; Choi, H.; Lee, Y.W.; Choi, J.; Mun, J.Y.; Yu, S.-W. CASP9 (Caspase 9) Is Essential for Autophagosome Maturation through Regulation of Mitochondrial Homeostasis. Autophagy 2020, 16, 1598–1617. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Tiernan, H.; Byrne, B.; Kazarian, S.G. ATR-FTIR Spectroscopy and Spectroscopic Imaging for the Analysis of Biopharmaceuticals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118636. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. Applications of ATR-FTIR Spectroscopic Imaging to Biomedical Samples. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 858–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR Spectroscopic Imaging: Recent Advances and Applications to Biological Systems. Analyst 2013, 138, 1940. [Google Scholar] [CrossRef] [PubMed]
- Glassford, S.E.; Byrne, B.; Kazarian, S.G. Recent Applications of ATR FTIR Spectroscopy and Imaging to Proteins. Biochim. Biophys. Acta-Proteins Proteom. 2013, 1834, 2849–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew Chan, K.L.; Kazarian, S.G. Attenuated Total Reflection Fourier-Transform Infrared (ATR-FTIR) Imaging of Tissues and Live Cells. Chem. Soc. Rev. 2016, 45, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; WILEY: New York, NY, USA, 2004; ISBN 9780470011140. [Google Scholar]
- Petibois, C.; Déléris, G. Analysis and Monitoring of Oxidative Stress in Exercise and Training by FTIR Spectrometry. Int. J. Sports Physiol. Perform. 2008, 3, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petibois, C.; Déléris, G. Oxidative Stress Effects on Erythrocytes Determined by FT-IR Spectrometry. Analyst 2004, 129, 912–916. [Google Scholar] [CrossRef]
- Gaudenzi, S.; Pozzi, D.; Toro, P.; Silvestri, I.; Morrone, S.; Congiu Castellano, A. Cell Apoptosis Specific Marker Found by Fourier Transform Infrared Spectroscopy. Spectroscopy 2004, 18, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR Spectroscopy of Whole Cells for the Monitoring of Yeast Apoptosis Mediated by P53 Over-Expression and Its Suppression by Nigella Sativa Extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef]
- Drozdz, A.; Matusiak, K.; Setkowicz, Z.; Ciarach, M.; Janeczko, K.; Sandt, C.; Borondics, F.; Horak, D.; Babic, M.; Chwiej, J. FTIR Microspectroscopy Revealed Biochemical Changes in Liver and Kidneys as a Result of Exposure to Low Dose of Iron Oxide Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118355. [Google Scholar] [CrossRef]
- Chwiej, J.; Skoczen, A.; Janeczko, K.; Kutorasinska, J.; Matusiak, K.; Figiel, H.; Dumas, P.; Sandt, C.; Setkowicz, Z. The Biochemical Changes in Hippocampal Formation Occurring in Normal and Seizure Experiencing Rats as a Result of a Ketogenic Diet. Analyst 2015, 140, 2190–2204. [Google Scholar] [CrossRef] [PubMed]
- Skoczen, A.; Setkowicz, Z.; Janeczko, K.; Sandt, C.; Borondics, F.; Chwiej, J. The Influence of High Fat Diets with Different Ketogenic Ratios on the Hippocampal Accumulation of Creatine–FTIR Microspectroscopy Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Li, C.; Shi, J.; Yue, J.; Wang, X. FTIR Microspectroscopic Study of Biomacromolecular Changes in As2O3 Induced MGC803 Cells Apoptosis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120220. [Google Scholar] [CrossRef]
- Zong, L.; Li, C.; Zhong, Y.; Shi, J.; Yuan, Z.; Wang, X. FTIR Microspectroscopic Investigation of Lactobacillus Paracasei Apoptosis Induced by Cisplatin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119542. [Google Scholar] [CrossRef] [PubMed]
- Kokot, I.; Mazurek, S.; Piwowar, A.; Szostak, R.; Jędryka, M.; Kratz, E.M. ATR-IR Spectroscopy Application to Diagnostic Screening of Advanced Endometriosis. Oxid. Med. Cell Longev. 2022, 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Yin, J.; Ma, J.; Zhou, X.; Chang, C. Identification of Hepatocellular Carcinoma and Paracancerous Tissue Based on the Peak Area in FTIR Microspectroscopy. Anal. Methods 2022, 14, 3115–3124. [Google Scholar] [CrossRef]
- Kawon, K.; Setkowicz, Z.; Drozdz, A.; Janeczko, K.; Chwiej, J. The Methods of Vibrational Microspectroscopy Reveals Long-Term Biochemical Anomalies within the Region of Mechanical Injury within the Rat Brain. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 263, 120214. [Google Scholar] [CrossRef]
- Shirali, A.; Pazhayattil, G.S. Drug-Induced Impairment of Renal Function. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 457. [Google Scholar] [CrossRef] [Green Version]
- Heidari-Soreshjani, S.; Asadi-Samani, M.; Yang, Q.; Saeedi-Boroujeni, A. Phytotherapy of Nephrotoxicity-Induced by Cancer Drugs: An Updated Review. J. Nephropathol. 2017, 6, 254–263. [Google Scholar] [CrossRef]
- Mihevc, M.; Petreski, T.; Maver, U.; Bevc, S. Renal Proximal Tubular Epithelial Cells: Review of Isolation, Characterization, and Culturing Techniques. Mol. Biol. Rep. 2020, 47, 9865–9882. [Google Scholar] [CrossRef] [PubMed]
- Dreissig, I.; Machill, S.; Salzer, R.; Krafft, C. Quantification of Brain Lipids by FTIR Spectroscopy and Partial Least Squares Regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Lasch, P.; Baldauf, E.; Beekes, M.; Naumann, D. Detection of Pathological Molecular Alterations in Scrapie-Infected Hamster Brain by Fourier Transform Infrared (FT-IR) Spectroscopy. Biochim. Biophys. Acta-Mol. Basis Dis. 2000, 1501, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-Based Infrared and X-Ray Imaging Shows Focalized Accumulation of Cu and Zn Co-Localized with β-Amyloid Deposits in Alzheimer’s Disease. J. Struct. Biol. 2006, 155, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, A.; Wang, Q.; Kneipp, J.; Lasch, P.; Beekes, M.; Miller, L.; Naumann, D. FTIR-Microspectroscopy of Prion-Infected Nervous Tissue. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 948–959. [Google Scholar] [CrossRef] [Green Version]
- Szczerbowska-Boruchowska, M.; Dumas, P.; Kastyak, M.Z.; Chwiej, J.; Lankosz, M.; Adamek, D.; Krygowska-Wajs, A. Biomolecular Investigation of Human Substantia Nigra in Parkinson’s Disease by Synchrotron Radiation Fourier Transform Infrared Microspectroscopy. Arch. Biochem. Biophys. 2007, 459, 241–248. [Google Scholar] [CrossRef]
- Rugiel, M.M.; Setkowicz, Z.K.; Drozdz, A.K.; Janeczko, K.J.; Kutorasińska, J.; Chwiej, J.G. The Use of Fourier Transform Infrared Microspectroscopy for the Determination of Biochemical Anomalies of the Hippocampal Formation Characteristic for the Kindling Model of Seizures. ACS Chem. Neurosci. 2021, 12, 4564–4579. [Google Scholar] [CrossRef]
- Petibois, C.; Déléris, G. Evidence That Erythrocytes Are Highly Susceptible to Exercise Oxidative Stress: FT-IR Spectrometric Studies at the Molecular Level. Cell Biol. Int. 2005, 29, 709–716. [Google Scholar] [CrossRef]
- Petibois, C.; Déléris, G. Chemical Mapping of Tumor Progression by FT-IR Imaging: Towards Molecular Histopathology. Trends Biotechnol. 2006, 24, 455–462. [Google Scholar] [CrossRef]
- Sawaya, B.P.; Briggs, J.P.; Schnermann, J. Amphotericin B Nephrotoxicity: The Adverse Consequences of Altered Membrane Properties. J. Am. Soc. Nephrol. 1995, 6, 154–164. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Fanos, V.; Cataldi, L. Amphotericin B-Induced Nephrotoxicity: A Review. J. Chemother. 2000, 12, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Brajtburg, J.; Powderly, W.G.; Kobayashi, G.S.; Medoff, G. Amphotericin B: Current Understanding of Mechanisms of Action. Antimicrob. Agents Chemother. 1990, 34, 183–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabra, R.; Branch, R.A. Amphotericin B Nephrotoxicity. Drug Saf. 1990, 5, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Folk, A.; Balta, C.; Herman, H.; Ivan, A.; Boldura, O.M.; Paiusan, L.; Ardelean, A.; Hermenean, A. Flucytosine and Amphotericin B Coadministration Induces Dose-Related Renal Injury. Dose-Response 2017, 15, 155932581770346. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.W.; Su, L.; Yu, D.T.; Chertow, G.M.; Seger, D.L.; Gomes, D.R.J.; Platt, R. Correlates of Acute Renal Failure in Patients Receiving Parenteral Amphotericin B. Kidney Int. 2001, 60, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Goldman, R.D.; Koren, G. Amphotericin B Nephrotoxicity in Children. J. Pediatr. Hematol. Oncol. 2004, 26, 421–426. [Google Scholar] [CrossRef]
- Perazella, M.A. Pharmacology behind Common Drug Nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef] [Green Version]
- Mizokami, F.; Mizuno, T. Acute Kidney Injury Induced by Antimicrobial Agents in the Elderly: Awareness and Mitigation Strategies. Drugs Aging 2015, 32, 1–12. [Google Scholar] [CrossRef]
- GOLA, J.; SKUBIS, A.; SIKORA, B.; KRUSZNIEWSKA-RAJS, C.; ADAMSKA, J.; MAZUREK, U.; STRZAŁKA-MROZIK, B.; CZERNEL, G.; GAGOŚ, M. Expression Profiles of Genes Related to Melatonin and Oxidative Stress in Human Renal Proximal Tubule Cells Treated with Antibiotic Amphotericin B and Its Modified Forms. TURKISH J. Biol. 2015, 39, 856–864. [Google Scholar] [CrossRef]
- Magalhães, E.P.; Silva, B.P.; Aires, N.L.; Ribeiro, L.R.; Ali, A.; Cavalcanti, M.M.; Nunes, J.V.S.; Sampaio, T.L.; de Menezes, R.R.P.P.B.; Martins, A.M.C. (−)-α-Bisabolol as a Protective Agent against Epithelial Renal Cytotoxicity Induced by Amphotericin B. Life Sci. 2022, 291, 120271. [Google Scholar] [CrossRef] [PubMed]
- Varlam, D.E.; Siddiq, M.M.; Parton, L.A.; Rüssmann, H. Apoptosis Contributes to Amphotericin B- Induced Nephrotoxicity. Antimicrob. Agents Chemother. 2001, 45, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowska, S.; Khylyuk, D.; Bielawska, A.; Szymanowska, A.; Gornowicz, A.; Bielawski, K.; Noworól, J.; Mandziuk, S.; Wujec, M. New 1,3,4-Thiadiazole Derivatives with Anticancer Activity. Molecules 2022, 27, 1814. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Paneth, A.; Wujec, M. Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review. Molecules 2020, 25, 4309. [Google Scholar] [CrossRef] [PubMed]
- Kluczyk, D.; Matwijczuk, A.; Górecki, A.; Karpińska, M.M.; Szymanek, M.; Niewiadomy, A.; Gagoś, M. Molecular Organization of Dipalmitoylphosphatidylcholine Bilayers Containing Bioactive Compounds 4-(5-Heptyl-1,3,4-Thiadiazol-2-Yl) Benzene-1,3-Diol and 4-(5-Methyl-1,3,4-Thiadiazol-2-Yl) Benzene-1,3-Diols. J. Phys. Chem. B 2016, 120, 12047–12063. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Górecki, A.; Kamiński, D.; Myśliwa-Kurdziel, B.; Fiedor, L.; Niewiadomy, A.; Karwasz, G.P.; Gagoś, M. Influence of Solvent Polarizability on the Keto-Enol Equilibrium in 4-[5-(Naphthalen-1-Ylmethyl)-1,3,4-Thiadiazol-2-Yl]Benzene-1,3-Diol. J. Fluoresc. 2015, 25, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, M. Thiadiazole Derivatives as Anticancer Agents. Pharmacol. Rep. 2020, 72, 1079–1100. [Google Scholar] [CrossRef] [PubMed]
- Bimoussa, A.; Oubella, A.; Bamou, F.Z.; Khdar, Z.A.; Fawzi, M.; Laamari, Y.; Itto, M.Y.A.; Morjani, H.; Auhmani, A.; Ait Itto, M.Y.; et al. New 1,3,4-Thiadiazoles Derivatives: Synthesis, Antiproliferative Activity, Molecular Docking and Molecular Dynamics. Future Med. Chem. 2022, 14, 881–897. [Google Scholar] [CrossRef]
- Altıntop, M.; Ciftci, H.; Radwan, M.; Sever, B.; Kaplancıklı, Z.; Ali, T.; Koga, R.; Fujita, M.; Otsuka, M.; Özdemir, A. Design, Synthesis, and Biological Evaluation of Novel 1,3,4-Thiadiazole Derivatives as Potential Antitumor Agents against Chronic Myelogenous Leukemia: Striking Effect of Nitrothiazole Moiety. Molecules 2017, 23, 59. [Google Scholar] [CrossRef] [Green Version]
- Matysiak, J.; Skrzypek, A.; Niewiadomy, A. Synthesis and Antifungal Activity of Novel 5-Substituted 4-(1,3,4-Thiadiazol-2-Yl)Benzene-1,3-Diols. Heteroat. Chem. 2010, 21, 533–540. [Google Scholar] [CrossRef]
- Lin, L.; Liu, A.; Peng, Z.; Lin, H.-J.; Li, P.-K.; Li, C.; Lin, J. STAT3 Is Necessary for Proliferation and Survival in Colon Cancer–Initiating Cells. Cancer Res. 2011, 71, 7226–7237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, M.; Li, Y.-C.; Wahl, G.M. MDM2, MDMX and P53 in Oncogenesis and Cancer Therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell Death in the Kidney. Int. J. Mol. Sci. 2019, 20, 3598. [Google Scholar] [CrossRef] [Green Version]
- Udawatte, N.S.; Kang, S.W.; Wang, Y.; Arumugam, T.V.; Seneviratne, C.J. Predictive Nephrotoxicity Profiling of a Novel Antifungal Small Molecule in Comparison to Amphotericin B and Voriconazole. Front. Pharmacol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.T.; Palasiewicz, K.; Gadiyar, V.; Lahey, K.; Calianese, D.; Birge, R.B.; Ucker, D.S. Phosphatidylserine Externalization by Apoptotic Cells Is Dispensable for Specific Recognition Leading to Innate Apoptotic Immune Responses. J. Biol. Chem. 2022, 298, 102034. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Fu, Z.; Lu, H.; Zhu, Z.; Yan, L.; Jiang, Y.; Cao, Y. Combination of Baicalein and Amphotericin B Accelerates Candida Albicans Apoptosis. Biol. Pharm. Bull. 2011, 34, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Woo, E.-R.; Lee, D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida Albicans. IUBMB Life 2019, 71, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, S.M.; Aly, A.A.; Bräse, S.; Nieger, M.; Abdelhafez, S.M.N.; Abdelzaher, W.Y.; Abdelhafez, E.-S.M.N. Synthesis, Characterization, and In Vivo Study of Some Novel 3,4,5-Trimethoxybenzylidene-Hydrazinecarbothioamides and Thiadiazoles as Anti-Apoptotic Caspase-3 Inhibitors. Molecules 2022, 27, 2266. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The Roles of Cellular Reactive Oxygen Species, Oxidative Stress and Antioxidants in Pregnancy Outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Djukic, M.; Fesatidou, M.; Xenikakis, I.; Geronikaki, A.; Angelova, V.T.; Savic, V.; Pasic, M.; Krilovic, B.; Djukic, D.; Gobeljic, B.; et al. In Vitro Antioxidant Activity of Thiazolidinone Derivatives of 1,3-Thiazole and 1,3,4-Thiadiazole. Chem. Biol. Interact. 2018, 286, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-Induced Oxidative Damage and Killing of Candida Albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Kiser, P.F.; Feinberg, B.A. Are Reduction Potentials of Antifungal Agents Relevant to Activity? Pharm. Res. 1990, 7, 283–288. [Google Scholar] [CrossRef] [PubMed]
- LAMP-FREUND, M.T.; FERREIRA, V.F.N.; SCHREIER, S. Mechanism of Inactivation of the Polyene Antibiotic Amphotericin B. Evidence for Radical Formation in the Process of Autooxidation. J. Antibiot. (Tokyo) 1985, 38, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Muğlu, H.; Akın, M.; Çavuş, M.S.; Yakan, H.; Şaki, N.; Güzel, E. Exploring of Antioxidant and Antibacterial Properties of Novel 1,3,4-Thiadiazole Derivatives: Facile Synthesis, Structural Elucidation and DFT Approach to Antioxidant Characteristics. Comput. Biol. Chem. 2022, 96, 107618. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It Only Takes One to Do Many Jobs: Amphotericin B as Antifungal and Immunomodulatory Drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef] [Green Version]
- Surowka, A.D.; Pilling, M.; Henderson, A.; Boutin, H.; Christie, L.; Szczerbowska-Boruchowska, M.; Gardner, P. FTIR Imaging of the Molecular Burden around Aβ Deposits in an Early-Stage 3-Tg-APP-PSP1-TAU Mouse Model of Alzheimer’s Disease. Analyst 2017, 142, 156–168. [Google Scholar] [CrossRef] [PubMed]




| Band [cm−1] or Bands Ratio | Origin | Characteristics |
|---|---|---|
| 3008 | =CH str. | Level of unsaturated lipids |
| 2958 | CH3 str. asym. | Level of saturated lipids |
| 2921 | CH2 str. asym. | |
| 2873 | CH3 str. sym. | |
| 2851 | CH2 str. sym. | |
| 1740 | C=O str. | Level of lipids, phospholipids, esters |
| 1687 | C=O str., NH bend. (Amide I) | Level of proteins (antiparallel β-sheet and β-turn) |
| 1654 | Level of proteins (α-helix and Amide I maximum) | |
| 1635 | Level of proteins (β-sheet) | |
| 1541 | NH bend., CN str. (Amide II) | Level of proteins |
| 1360–1480 | CH2, CH3 def. | Level of proteins and lipids |
| 1335 | CN def. (Amide III) | Level of proteins |
| 1310 | ||
| 3008/2958 | =CH str./CH3 str. asym. | Changes in lipid unsaturation level |
| 2921/2958 | CH2 str. asym./CH3 str. asym. | Changes in lipid chain length, branching, and/or saturation level |
| 2851/2873 | CH2 str. sym./CH3 str. sym. | |
| 1635/1654 | β-sheet/α-helix | Changes in relative level of β-sheet and α-helix secondary structures |
| 1541/1654 | Amide II/Amide I | Changes in secondary structure of proteins |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dróżdż, A.; Sławińska-Brych, A.; Kubera, D.; Kimsa-Dudek, M.; Gola, J.M.; Adamska, J.; Kruszniewska-Rajs, C.; Matwijczuk, A.; Karcz, D.; Dąbrowski, W.; et al. Effect of Antibiotic Amphotericin B Combinations with Selected 1,3,4-Thiadiazole Derivatives on RPTECs in an In Vitro Model. Int. J. Mol. Sci. 2022, 23, 15260. https://doi.org/10.3390/ijms232315260
Dróżdż A, Sławińska-Brych A, Kubera D, Kimsa-Dudek M, Gola JM, Adamska J, Kruszniewska-Rajs C, Matwijczuk A, Karcz D, Dąbrowski W, et al. Effect of Antibiotic Amphotericin B Combinations with Selected 1,3,4-Thiadiazole Derivatives on RPTECs in an In Vitro Model. International Journal of Molecular Sciences. 2022; 23(23):15260. https://doi.org/10.3390/ijms232315260
Chicago/Turabian StyleDróżdż, Agnieszka, Adrianna Sławińska-Brych, Dominika Kubera, Magdalena Kimsa-Dudek, Joanna Magdalena Gola, Jolanta Adamska, Celina Kruszniewska-Rajs, Arkadiusz Matwijczuk, Dariusz Karcz, Wojciech Dąbrowski, and et al. 2022. "Effect of Antibiotic Amphotericin B Combinations with Selected 1,3,4-Thiadiazole Derivatives on RPTECs in an In Vitro Model" International Journal of Molecular Sciences 23, no. 23: 15260. https://doi.org/10.3390/ijms232315260






