Dapsone Lowers Neutrophil to Lymphocyte Ratio and Mortality in COVID-19 Patients Admitted to the ICU
Abstract
:1. Introduction
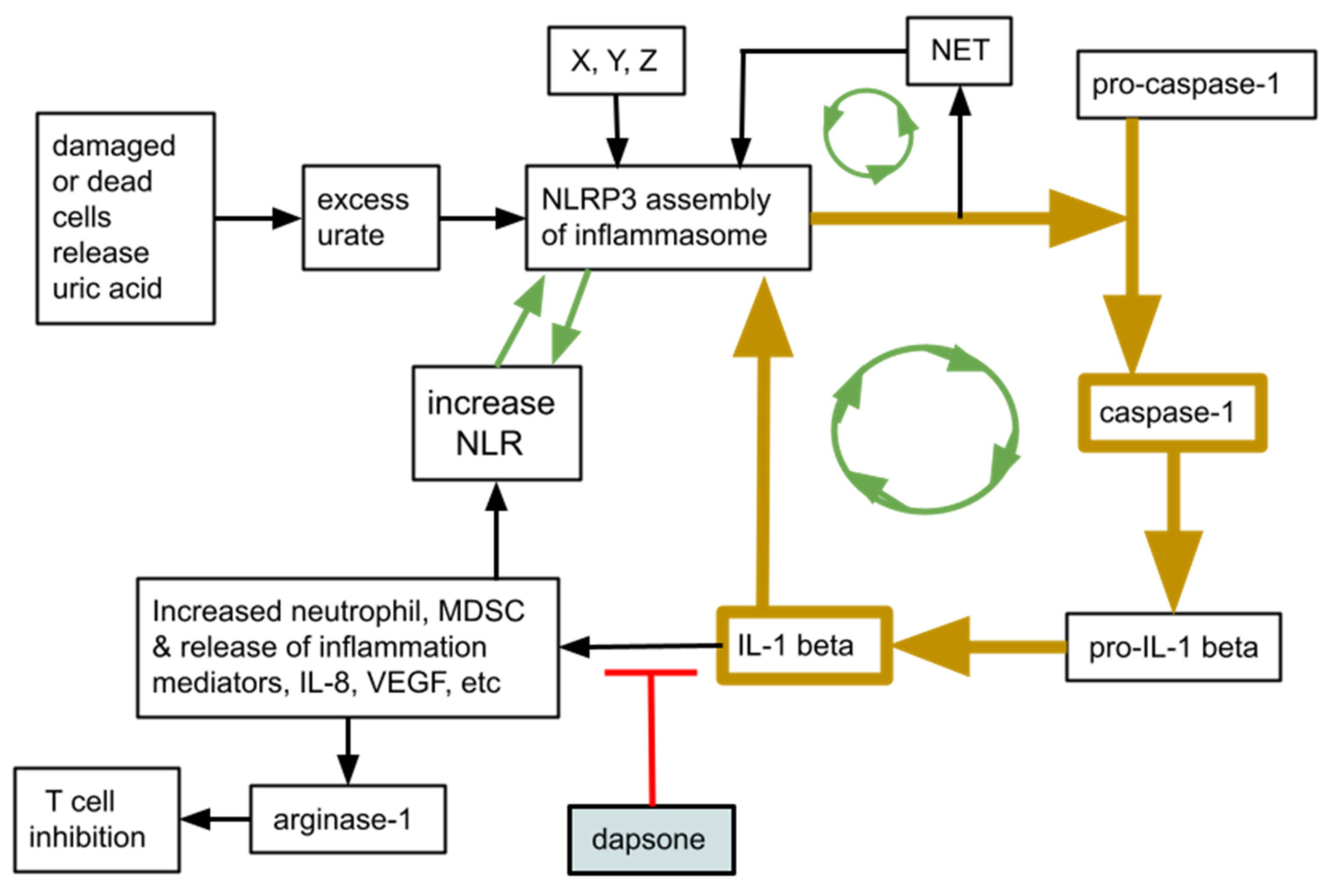
2. Rationale
3. Patient Population
4. Results—What Chart Review Revealed
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chotirmall, S.H.; Leither, L.M.; Çoruh, B.; Chan, L.L.Y.; Joudi, A.M.; Brown, S.M.; Singer, B.D.; Seam, N. Update in COVID-19 2020. Am. J. Respir. Crit. Care Med. 2021, 203, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.J.; Subramaniam, A.; Reddy, M.P.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Berking, C.; Biedermann, T.; Buhl, T.; Erpenbeck, L.; Eyerich, K.; Eyerich, S.; Ghoreschi, K.; Goebeler, M.; Ludwig, R.J.; et al. COVID-19 and immunological regulations—From basic and translational aspects to clinical implications. JDDG J. der Dtsch. Dermatol. Ges. 2020, 18, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Dapsone as treatment adjunct in ARDS. Exp. Lung Res. 2020, 46, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwar, B.A.; Khattak, A.; Balentine, J.; Lee, J.H.; Kast, R.E. Benefits of Using Dapsone in Patients Hospitalized with COVID-19. Vaccines 2022, 10, 195. [Google Scholar] [CrossRef]
- Hafkamp, F.M.; Mol, S.; Waqué, I.; De Jong, E.C. Dexamethasone, but Not Vitamin D or A, Dampens the Inflammatory Neutrophil Response to Protect At-risk COVID-19 Patients. Immune Netw. 2022, 22, e36. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, É.D.A.; Galvão, J.G.F.M.; Silva, J.S.D.F.D.; de Sales-Neto, J.M.; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021, 90, 107233. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
- Seren, S.; Derian, L.; Keleş, I.; Guillon, A.; Lesner, A.; Gonzalez, L.; Baranek, T.; Si-Tahar, M.; Marchand-Adam, S.; Jenne, D.E.; et al. Proteinase release from activated neutrophils in mechanically ventilated patients with non-COVID-19 and COVID-19 pneumonia. Eur. Respir. J. 2021, 57, 2003755. [Google Scholar] [CrossRef]
- Mehta, M. Cimetidine and dapsone-mediated methaemoglobinaemia. Anaesthesia 2007, 62, 1188. [Google Scholar] [CrossRef]
- Coleman, M.D. Improvement of patient tolerance to dapsone: Current and future developments. Dermatol. Online J. 2007, 13, 18. [Google Scholar] [CrossRef]
- Rhodes, L.; Tingle, M.; Park, B.; Chu, P.; Verbov, J.; Friedmann, P. Cimetidine improves the therapeutic/toxic ratio of dapsone in patients on chronic dapsone therapy. Br. J. Dermatol. 1995, 132, 257–262. [Google Scholar] [CrossRef]
- Wolf, R.; Matz, H.; Orion, E.; Tuzun, B.; Tuzun, Y. Dapsone. Dermatol Online J. 2002, 8, 2. [Google Scholar] [CrossRef]
- Ghaoui, N.; Hanna, E.; Abbas, O.; Kibbi, A.; Kurban, M. Update on the use of dapsone in dermatology. Int. J. Dermatol. 2020, 59, 787–795. [Google Scholar] [CrossRef]
- Kurien, G.; Jamil, R.T.; Preuss, C.V. Dapsone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wozel, V.E.G. Innovative Use of Dapsone. Dermatol. Clin. 2010, 28, 599–610. [Google Scholar] [CrossRef]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2013, 306, 103–124. [Google Scholar] [CrossRef] [Green Version]
- Kaltenmeier, C.; Yazdani, H.O.; Handu, S.; Popp, B.; Geller, D.; Tohme, S. The Role of Neutrophils as a Driver in Hepatic Ischemia-Reperfusion Injury and Cancer Growth. Front. Immunol. 2022, 13, 887565. [Google Scholar] [CrossRef]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef]
- Kast, R. Research Supporting a Pilot Study of Metronomic Dapsone during Glioblastoma Chemoirradiation. Med. Sci. 2021, 9, 12. [Google Scholar] [CrossRef]
- Wu, M.; Hu, W.; Wang, G.; Yao, Y.; Yu, X.-F. Nicotinamide N-Methyltransferase Is a Prognostic Biomarker and Correlated With Immune Infiltrates in Gastric Cancer. Front. Genet. 2020, 11, 580299. [Google Scholar] [CrossRef]
- McKenna, E.; Wubben, R.; Isaza-Correa, J.M.; Melo, A.M.; Mhaonaigh, A.U.; Conlon, N.; O’Donnell, J.S.; Cheallaigh, C.N.; Hurley, T.; Stevenson, N.J.; et al. Neutrophils in COVID-19: Not Innocent Bystanders. Front. Immunol. 2022, 13, 864387. [Google Scholar] [CrossRef]
- Silberberg, E.; Filep, J.G.; Ariel, A. Weathering the Storm: Harnessing the Resolution of Inflammation to Limit COVID-19 Pathogenesis. Front. Immunol. 2022, 13, 863449. [Google Scholar] [CrossRef] [PubMed]
- Codd, A.S.; Hanna, S.J.; Compeer, E.B.; Richter, F.C.; Pring, E.J.; Gea-Mallorquí, E.; Borsa, M.; Moon, O.R.; Scourfield, D.O.; The Oxford-Cardiff COVID-19 Literature Consortium; et al. Neutrophilia, lymphopenia and myeloid dysfunction: A living review of the quantitative changes to innate and adaptive immune cells which define COVID-19 pathology. Oxf. Open Immunol. 2021, 2, iqab016. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Looney, M.R. Is neutrophilic inflammation treatable in COVID-19? Lancet Respir. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Farouk, A.; Salman, S. Dapsone and doxycycline could be potential treatment modalities for COVID-19. Med. Hypotheses 2020, 140, 109768. [Google Scholar] [CrossRef]
- Ventura-Santana, E.; Ninan, J.R.; Snyder, C.M.; Okeke, E.B. Neutrophil Extracellular Traps, Sepsis and COVID-19—A Tripod Stand. Front. Immunol. 2022, 13, 902206. [Google Scholar] [CrossRef]
- Münzer, P.; Negro, R.; Fukui, S.; di Meglio, L.; Aymonnier, K.; Chu, L.; Cherpokova, D.; Gutch, S.; Sorvillo, N.; Shi, L.; et al. NLRP3 Inflammasome Assembly in Neutrophils Is Supported by PAD4 and Promotes NETosis Under Sterile Conditions. Front. Immunol. 2021, 12, 683803. [Google Scholar] [CrossRef]
- Burke, R.M.; Dale, B.L.; Dholakia, S. The NLRP3 Inflammasome: Relevance in Solid Organ Transplantation. Int. J. Mol. Sci. 2021, 22, 10721. [Google Scholar] [CrossRef]
- Tall, A.R.; Westerterp, M. Inflammasomes, neutrophil extracellular traps, and cholesterol. J. Lipid Res. 2019, 60, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Kast, R.E. High Neutrophil-to-Lymphocyte Ratio Facilitates Cancer Growth—Currently Marketed Drugs Tadalafil, Isotretinoin, Colchicine, and Omega-3 to Reduce It: The TICO Regimen. Cancers 2022, 14, 4965. [Google Scholar] [CrossRef]
- Prochetto, E.; Borgna, E.; Jiménez-Cortegana, C.; Sánchez-Margalet, V.; Cabrera, G. Myeloid-derived suppressor cells and vaccination against pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 1003781. [Google Scholar] [CrossRef]
- Alsalman, A.; Al-Mterin, M.A.; Elkord, E. Role of T Regulatory Cells and Myeloid-Derived Suppressor Cells in COVID-19. J. Immunol. Res. 2022, 2022, 5545319. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Ostapchuk, Y.O.; Tleulieva, R.; Kali, A.; Abdolla, N.; Krasnoshtanov, V.K.; Perfilyeva, A.V.; Belyaev, N.N. Myeloid-derived suppressor cells in COVID-19: A review. Clin. Immunol. 2022, 238, 109024. [Google Scholar] [CrossRef]
- Falck-Jones, S.; Österberg, B.; Smed-Sörensen, A. Respiratory and systemic monocytes, dendritic cells, and myeloid-derived suppressor cells in COVID-19: Implications for disease severity. J. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Beliakova-Bethell, N.; Maruthai, K.; Xu, R.; Salvador, L.C.M.; Garg, A. Monocytic-Myeloid Derived Suppressor Cells Suppress T-Cell Responses in Recovered SARS CoV2-Infected Individuals. Front. Immunol. 2022, 13, 894543. [Google Scholar] [CrossRef]
- Bline, K.; Andrews, A.; Moore-Clingenpeel, M.; Mertz, S.; Ye, F.; Best, V.; Sayegh, R.; Tomatis-Souverbielle, C.; Quintero, A.M.; Maynard, Z.; et al. Myeloid-Derived Suppressor Cells and Clinical Outcomes in Children With COVID-19. Front. Pediatr. 2022, 10, 893045. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Théroude, C.; Antonakos, N.; Regina, J.; Le Roy, D.; Bart, P.; Chiche, J.; Perreau, M.; Pantaleo, G.; Calandra, T.; et al. COVID-19 rapidly increases MDSCs and prolongs innate immune dysfunctions. Eur. J. Immunol. 2022, 52, 1676–1679. [Google Scholar] [CrossRef]
- Geyfman, M.; Debabov, D.; Poloso, N.; Alvandi, N. Mechanistic insight into the activity of a sulfone compound dapsone on Propionibacterium (Newly Reclassified as Cutibacterium) Acnes-mediated cytokine production. Exp. Dermatol. 2018, 28, 190–197. [Google Scholar] [CrossRef]
- Zinellu, A.; A Mangoni, A. A systematic review and meta-analysis of the association between the neutrophil, lymphocyte, and platelet count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio and COVID-19 progression and mortality. Expert Rev. Clin. Immunol. 2022, 18, 1187–1202. [Google Scholar] [CrossRef]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef]
- Marques, P.; Fernandez-Presa, L.; Carretero, A.; Gómez-Cabrera, M.-C.; Viña, J.; Signes-Costa, J.; Sanz, M.-J. The radiographic assessment of lung edema score of lung edema severity correlates with inflammatory parameters in patients with coronavirus disease 2019—Potential new admission biomarkers to predict coronavirus disease 2019 worsening. Front. Med. 2022, 9, 871714. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, Z.; Bayissa, A.B.; Diriba, T.; Chernet, N.; Tsegaye, S.; Tsega, M. Neutrophil-to-Lymphocyte Ratio and Cut-off Values as Predictor of Severity and Mortality in COVID-19 Patients in Millennium COVID-19 Care Center, Addis Ababa, Ethiopia. Int. J. Gen. Med. 2022, 15, 6739–6755. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.S.; Akram, M.; Yasmin, F.; Najeeb, H.; Naeem, U.; Gaddam, M.; Jafri, M.S.; Tahir, M.J.; Yasin, I.; Mahmood, H.; et al. Comparative analysis of neutrophil to lymphocyte ratio and derived neutrophil to lymphocyte ratio with respect to outcomes of in-hospital coronavirus disease 2019 patients: A retrospective study. Front. Med. 2022, 9, 951556. [Google Scholar] [CrossRef]
- Karaaslan, T.; Karaaslan, E. Predictive Value of Systemic Immune-inflammation Index in Determining Mortality in COVID-19 Patients. J. Crit. Care Med. 2022, 8, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ziuzia-Januszewska, L.; Januszewski, M.; Sosnowska-Nowak, J.; Janiszewski, M.; Dobrzyński, P.; Jakimiuk, A.A.; Jakimiuk, A.J. COVID-19 Severity and Mortality in Two Pandemic Waves in Poland and Predictors of Poor Outcomes of SARS-CoV-2 Infection in Hospitalized Young Adults. Viruses 2022, 14, 1700. [Google Scholar] [CrossRef]
- Parthasarathi, A.; Padukudru, S.; Arunachal, S.; Basavaraj, C.K.; Krishna, M.T.; Ganguly, K.; Upadhyay, S.; Anand, M.P. The Role of Neutrophil-to-Lymphocyte Ratio in Risk Stratification and Prognostication of COVID-19: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1233. [Google Scholar] [CrossRef]
- Piovani, D.; Tsantes, A.G.; Bonovas, S. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients with COVID-19. J. Clin. Med. 2022, 11, 4688. [Google Scholar] [CrossRef]
- Keri, V.C.; Jorwal, P.; Verma, R.; Ranjan, P.; Upadhyay, A.D.; Aggarwal, A.; Sarda, R.; Sharma, K.; Sahni, S.; Rajanna, C. Novel Scoring Systems to Predict the Need for Oxygenation and ICU Care, and Mortality in Hospitalized COVID-19 Patients: A Risk Stratification Tool. Cureus 2022, 14, e27459. [Google Scholar] [CrossRef]
- Higaki, A.; Okayama, H.; Homma, Y.; Sano, T.; Kitai, T.; Yonetsu, T.; Torii, S.; Kohsaka, S.; Kuroda, S.; Node, K.; et al. Predictive value of neutrophil-to-lymphocyte ratio for the fatality of COVID-19 patients complicated with cardiovascular diseases and/or risk factors. Sci. Rep. 2022, 12, 13606. [Google Scholar] [CrossRef]
- Rasarathnam, A.; Haynes-Smith, T.; Wassif, W.S.; Dodd, M.S. Haematological and biochemical pathology markers for a predictive model for ITU admission and death from COVID-19: A retrospective study. eJHaem 2022, 3, 660–668. [Google Scholar] [CrossRef]
- Ortega-Rojas, S.; Salazar-Talla, L.; Romero-Cerdán, A.; Soto-Becerra, P.; Díaz-Vélez, C.; Urrunaga-Pastor, D.; Maguiña, J.L. The Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio as Predictors of Mortality in Older Adults Hospitalized with COVID-19 in Peru. Dis. Markers 2022, 2022, 2497202. [Google Scholar] [CrossRef]
- Dutta, D.; Liu, J.; Xiong, H. NLRP3 inflammasome activation and SARS-CoV-2-mediated hyperinflammation, cytokine storm and neurological syndromes. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 138–160. [Google Scholar]
- Islamuddin, M.; Mustfa, S.A.; Ullah, S.N.M.N.; Omer, U.; Kato, K.; Parveen, S. Innate Immune Response and Inflammasome Activation during SARS-CoV-2 Infection. Inflammation 2022, 45, 1849–1863. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Kashir, J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med. Hypotheses 2020, 143, 109906. [Google Scholar] [CrossRef]
- Carstens, L.B.; D’Amico, R.C.; de Moura, K.F.; de Castro, E.M.; Centenaro, F.; Barbosa, G.S.; da Silva, G.V.C.; Brenny, I.; D’Agostini, J.C.H.; Hlatchuk, E.C.; et al. Lung Inflammasome Activation in SARS-CoV-2 Post-Mortem Biopsies. Int. J. Mol. Sci. 2022, 23, 13033. [Google Scholar] [CrossRef]
- Jafari, R.M.; Shayesteh, S.; Ala, M.; Yousefi-Manesh, H.; Rashidian, A.; Hashemian, S.M.; Sorouri, M.; Dehpour, A.R. Dapsone Ameliorates Colitis through TLR4/NF-kB Pathway in TNBS Induced Colitis Model in Rat. Arch. Med. Res. 2021, 52, 595–602. [Google Scholar] [CrossRef]
- Rashidian, A.; Rashki, A.; Abdollahi, A.; Haddadi, N.-S.; Chamanara, M.; Mumtaz, F.; Dehpour, A.R. Dapsone reduced acetic acid-induced inflammatory response in rat colon tissue through inhibition of NF-kB signaling pathway. Immunopharmacol. Immunotoxicol. 2019, 41, 607–613. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Joo, H.-G. Dapsone modulates lipopolysaccharide-activated bone marrow cells by inducing cell death and down-regulating tumor necrosis factor-α production. J. Vet. Sci. 2018, 19, 744–749. [Google Scholar] [CrossRef]
- Abe, M.; Shimizu, A.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O. A possible inhibitory action of diaminodiphenyl sulfone on tumour necrosis factor-α production from activated mononuclear cells on cutaneous lupus erythematosus. Clin. Exp. Dermatol. 2008, 33, 759–763. [Google Scholar] [CrossRef]
| 1 | COVID death rate with dapsone | 9/29 = 31% |
| 2 | COVID death rate no dapsone | 18/30 = 60% |
| 3 | entry NLR, all 29 pts before receiving dapsone | av 10.2, median 8.8 |
| 4 | entry NLR, all 30 pts no dapsone | av 12.7, median 8.1 |
| 5 | discharge NLR alive, with dapsone, 20/29, | av 8.6, median 5.9 |
| 6 | discharge NLR dead, with dapsone, 9/29 | av 38, median 20 |
| 7 | discharge NLR alive, no dapsone, 12/30 | av 19.8, median 9.6 |
| 8 | discharge NLR dead, no dapsone, 18/30 | av 32, median 30 |
| 9 | entry NLR, died, with dapsone, 9/29 = 31% | av 13.3, median 10.9 |
| 10 | entry NLR, survive, with dapsone, 20/29 = 69% | av 8.8, median 8.0 |
| 11 | entry NLR, died, no dapsone, 18/30 = 60% | av 10.2, median 7.6 |
| 12 | entry NLR, survive, no dapsone, 12/30 = 40% | av 16.4, median 10.1 |
| 13 | NLR increased, # pts, with dapsone | 15/29 = 52% |
| 14 | NLR decreased, # pts, with dapsone | 14/29 = 48% |
| 15 | NLR increased # pts, no dapsone | 21/30 = 70% |
| 16 | NLR decreased # pts, no dapsone | 9/30 = 30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwar, B.; Khattak, A.; Kast, R.E. Dapsone Lowers Neutrophil to Lymphocyte Ratio and Mortality in COVID-19 Patients Admitted to the ICU. Int. J. Mol. Sci. 2022, 23, 15563. https://doi.org/10.3390/ijms232415563
Kanwar B, Khattak A, Kast RE. Dapsone Lowers Neutrophil to Lymphocyte Ratio and Mortality in COVID-19 Patients Admitted to the ICU. International Journal of Molecular Sciences. 2022; 23(24):15563. https://doi.org/10.3390/ijms232415563
Chicago/Turabian StyleKanwar, Badar, Asif Khattak, and Richard E. Kast. 2022. "Dapsone Lowers Neutrophil to Lymphocyte Ratio and Mortality in COVID-19 Patients Admitted to the ICU" International Journal of Molecular Sciences 23, no. 24: 15563. https://doi.org/10.3390/ijms232415563





