Remarkable Plasticity of Bone Iron Homeostasis in Hibernating Daurian Ground Squirrels (Spermophilus dauricus) May Be Involved in Bone Maintenance
Abstract
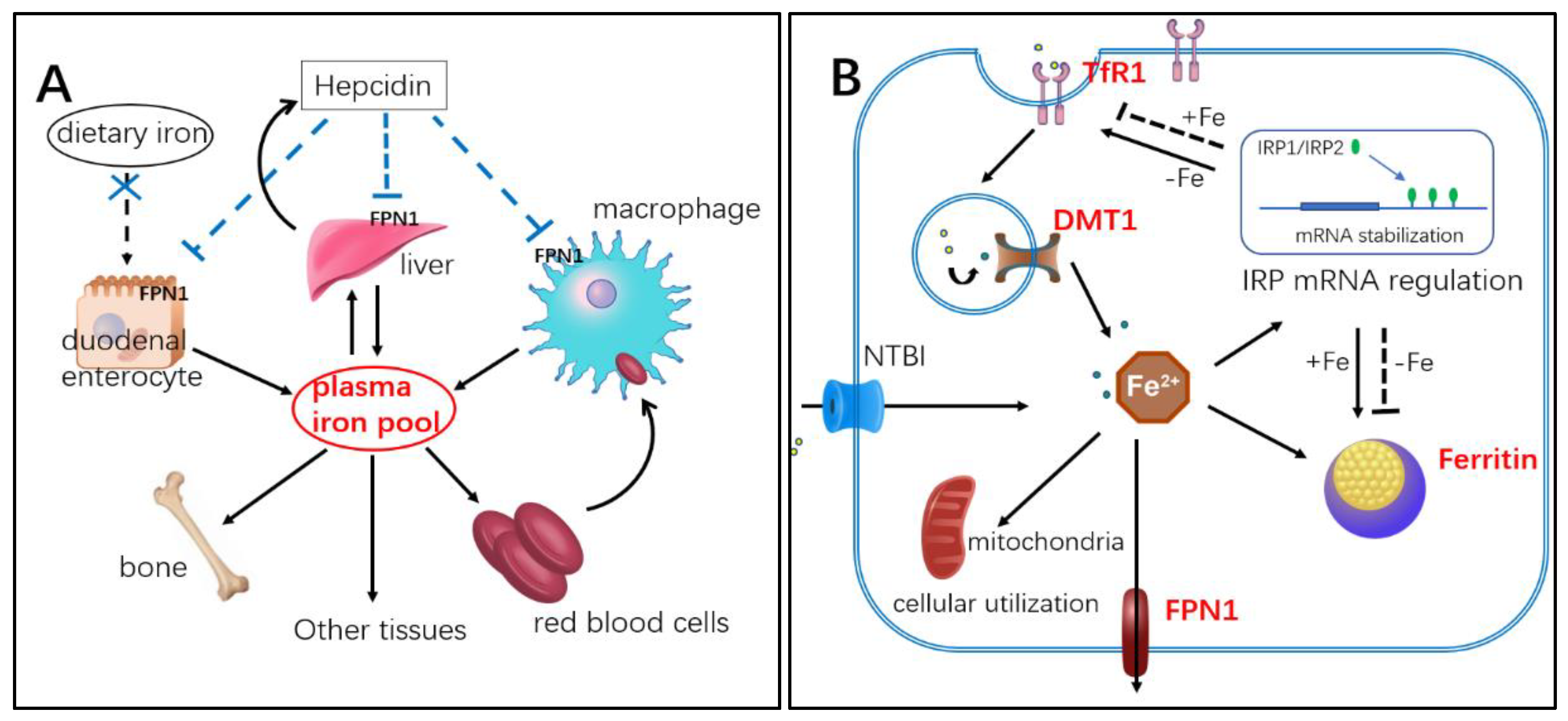
:1. Introduction
2. Results
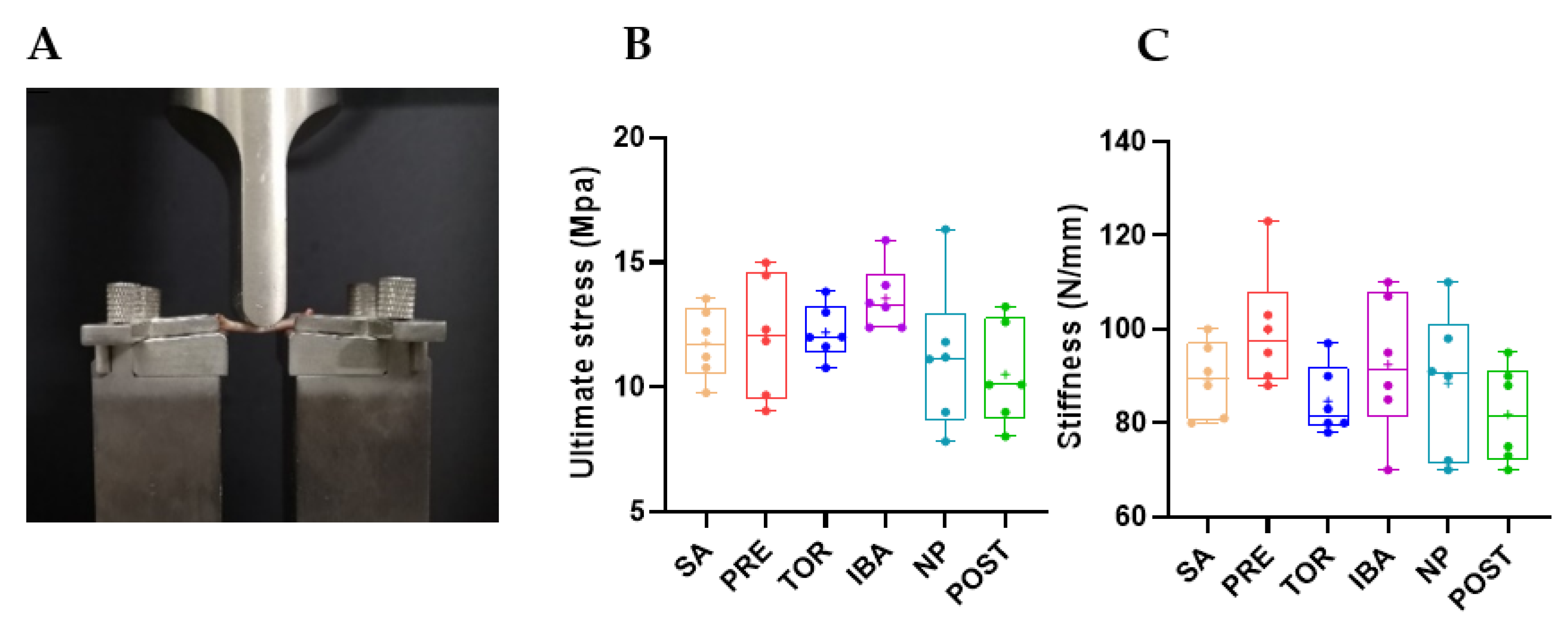
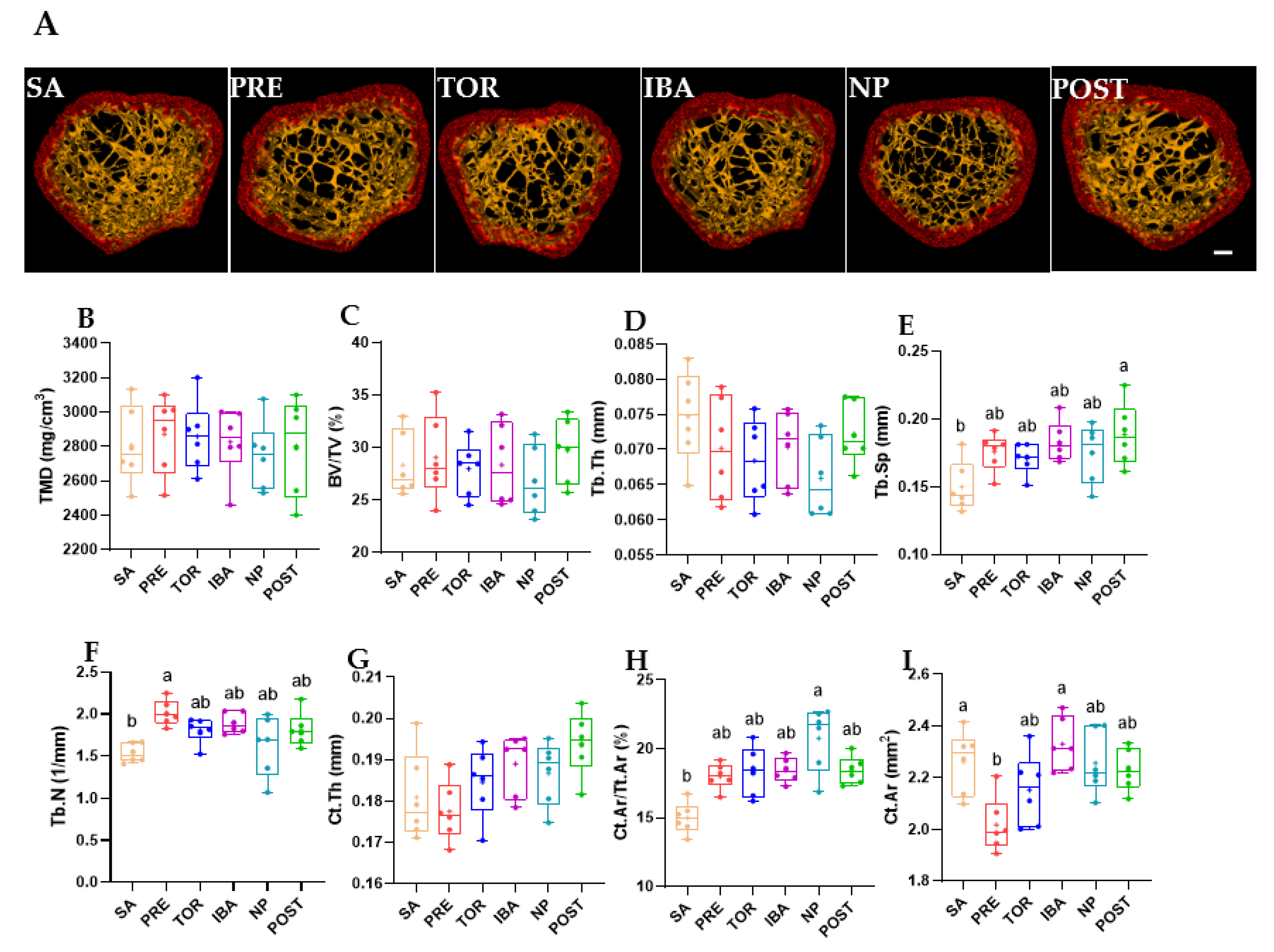
2.1. Bone Mass, Mechanical Properties, and Microstructure
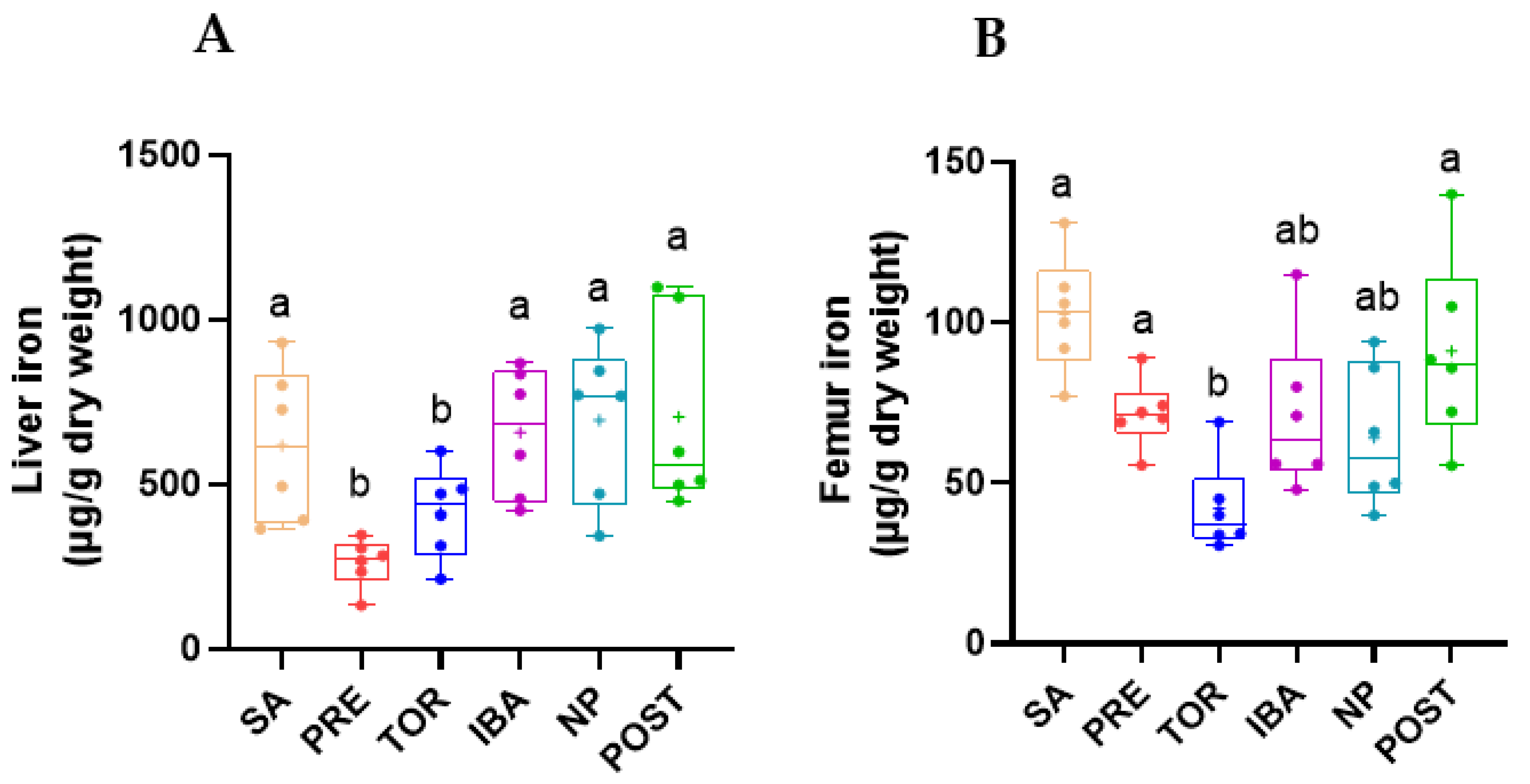
2.2. Total Iron Content in Femur and Liver during Different Periods
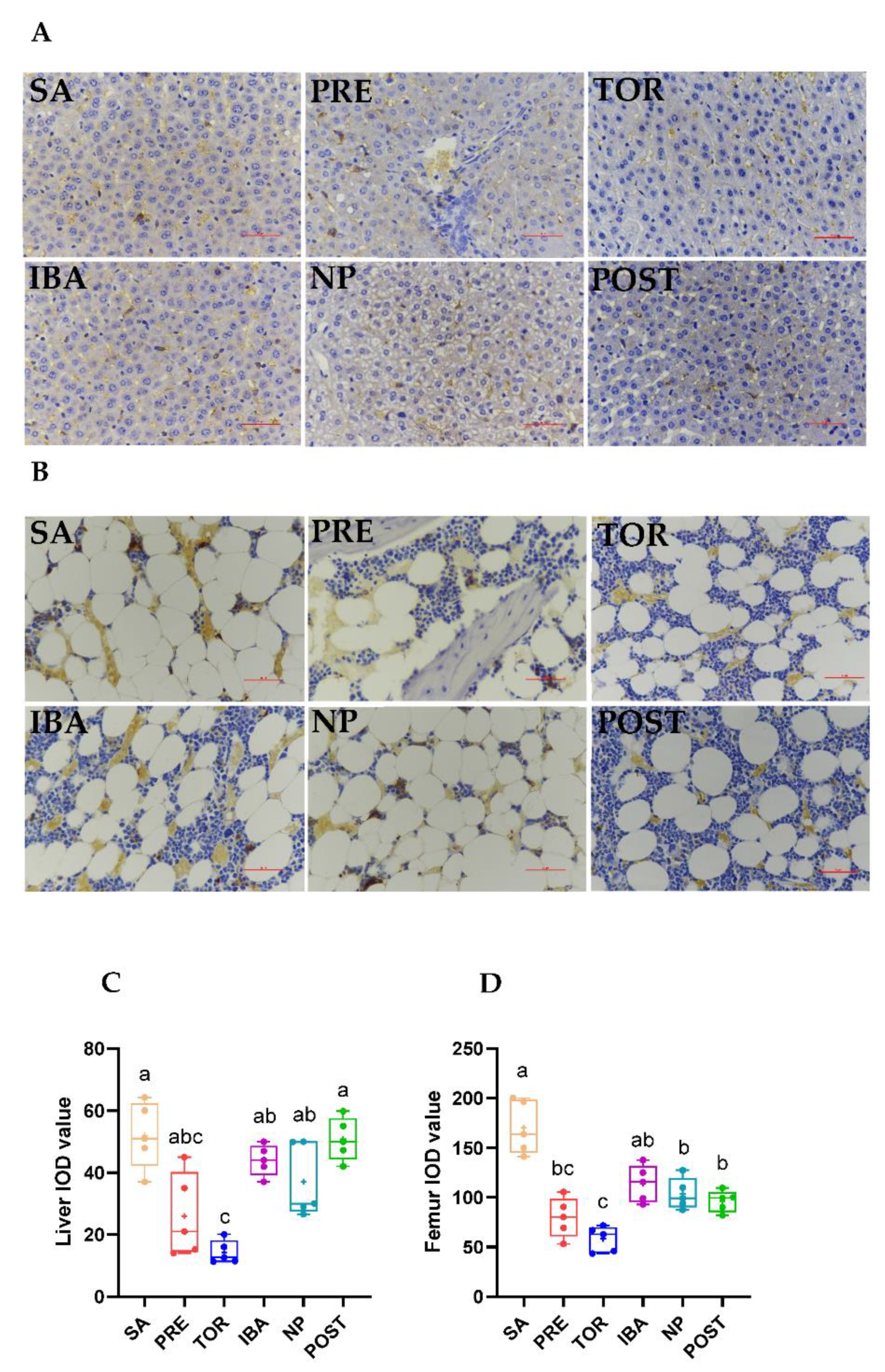
2.3. Iron Distribution in Femur and Liver during Different Periods
2.4. Regulation of Hepcidin in Liver during Different Periods
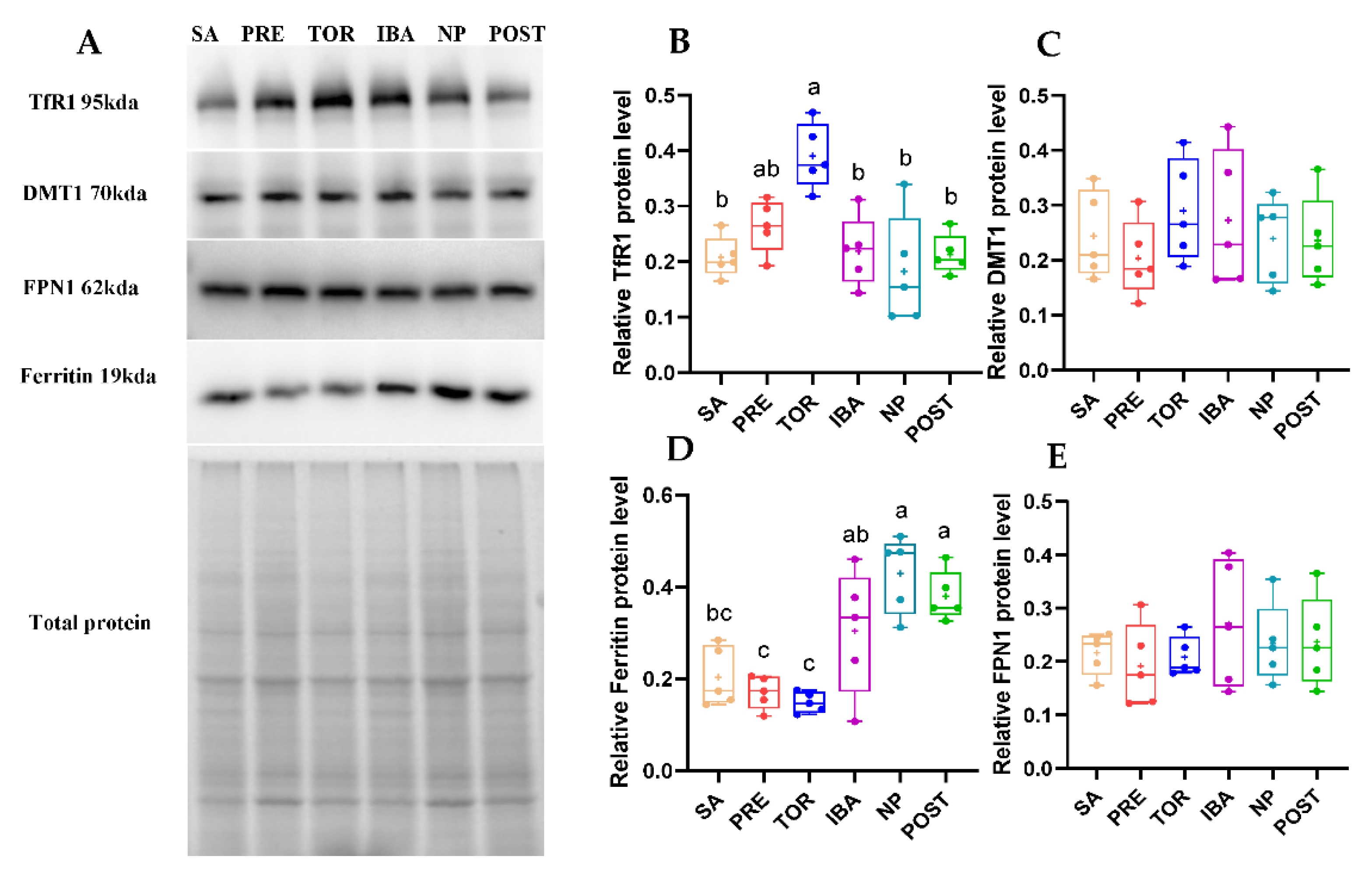
2.5. Iron-Metabolism-Related Protein Expression in Femur during Different Periods
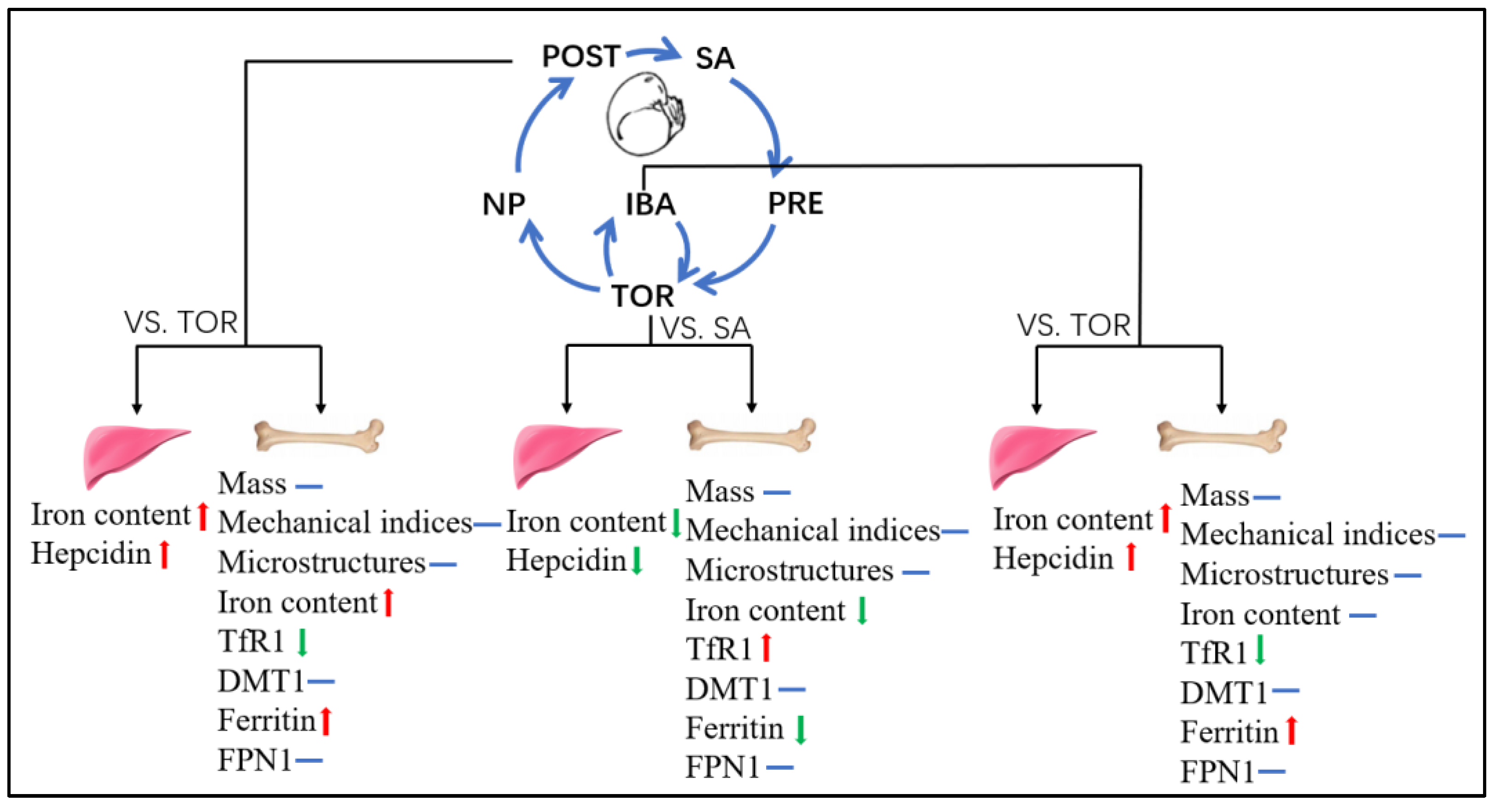
3. Discussion
4. Materials and Methods
4.1. Animals and Groups
4.2. Sample Collection and Preparation
4.3. Micro-Computed Tomography (CT)
4.4. Mechanical Properties
4.5. Iron Measurements
4.6. Histological Analysis
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Crichton, R.R.; Wilmet, S.; Legssyer, R.; Ward, R.J. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 2002, 91, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rockfield, S.; Raffel, J.; Mehta, R.; Rehman, N.; Nanjundan, M. Iron overload and altered iron metabolism in ovarian cancer. Biol. Chem. 2017, 398, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Meroño, T.; Gómez, L.; Sorroche, P.; Boero, L.; Arbelbide, J.; Brites, F. High risk of cardiovascular disease in iron overload patients. Eur. J. Clin. Investig. 2011, 41, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, L.; Tan, Y.; Wang, G.; Lin, X.; Cai, L. Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Curr. Med. Chem. 2009, 16, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Garringer, H.J.; Irimia, J.M.; Li, W.; Goodwin, C.B.; Richine, B.; Acton, A.; Chan, R.J.; Peacock, M.; Muhoberac, B.B.; Ghetti, B.; et al. Effect of Systemic Iron Overload and a Chelation Therapy in a Mouse Model of the Neurodegenerative Disease Hereditary Ferritinopathy. PLoS ONE 2016, 11, e0161341. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, E.D. Iron loading: A risk factor for osteoporosis. Biomet. Int. J. Role Met. Ions Biol. Biochem. Med. 2006, 19, 633–635. [Google Scholar] [CrossRef]
- He, Y.F.; Ma, Y.; Gao, C.; Zhao, G.Y.; Zhang, L.L.; Li, G.F.; Pan, Y.Z.; Li, K.; Xu, Y.J. Iron overload inhibits osteoblast biological activity through oxidative stress. Biol. Trace Elem. Res. 2013, 152, 292–296. [Google Scholar] [CrossRef]
- Jia, P.; Xu, Y.J.; Zhang, Z.L.; Li, K.; Li, B.; Zhang, W.; Yang, H. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 1843–1852. [Google Scholar] [CrossRef]
- Li, G.F.; Pan, Y.Z.; Sirois, P.; Li, K.; Xu, Y.J. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos. Int. 2012, 23, 2403–2408. [Google Scholar] [CrossRef]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Sun, W.; Li, Y.; Ling, S.; Zhao, C.; Zhong, G.; Zhao, D.; Song, J.; Song, H.; Li, J.; et al. The regulation of iron metabolism by hepcidin contributes to unloading-induced bone loss. Bone 2017, 94, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2022, 110, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Dong, D.; Lv, H.; Zhao, B.; Xue, Y.; Shang, P. Iron overload as a high risk factor for microgravity-induced bone loss. Acta Astronaut. 2019, 164, 407–414. [Google Scholar] [CrossRef]
- Yang, J.; Meng, X.; Dong, D.; Xue, Y.; Chen, X.; Wang, S.; Shen, Y.; Zhang, G.; Shang, P. Iron overload involved in the enhancement of unloading-induced bone loss by hypomagnetic field. Bone 2018, 114, 235–245. [Google Scholar] [CrossRef]
- Zwart, S.R.; Morgan, J.L.; Smith, S.M. Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the International Space Station. Am. J. Clin. Nutr. 2013, 98, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Zwart, S.R.; Oliver, S.A.; Fesperman, J.V.; Kala, G.; Krauhs, J.; Ericson, K.; Smith, S.M. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80 (Suppl. 5), A15–A122. [Google Scholar] [CrossRef]
- Morgan, J.L.; Zwart, S.R.; Heer, M.; Ploutz-Snyder, R.; Ericson, K.; Smith, S.M. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. 2012, 113, 1519–1529. [Google Scholar] [CrossRef]
- Wojda, S.J.; McGee-Lawrence, M.E.; Gridley, R.A.; Auger, J.; Black, H.L.; Donahue, S.W. Yellow-bellied marmots (Marmota flaviventris) preserve bone strength and microstructure during hibernation. Bone 2012, 50, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, A.H.; Roteliuk, D.M.; Gookin, S.E.; McGrew, A.K.; Broccardo, C.J.; Condon, K.W.; Prenni, J.E.; Wojda, S.J.; Florant, G.L.; Donahue, S.W. Exploring the Bone Proteome to Help Explain Altered Bone Remodeling and Preservation of Bone Architecture and Strength in Hibernating Marmots. Physiol. Biochem. Zool. 2016, 89, 364–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, S.W.; Vaughan, M.R.; Demers, L.M.; Donahue, H.J. Bone formation is not impaired by hibernation (disuse) in black bears Ursus americanus. J. Exp. Biol. 2003, 206 Pt 23, 4233–4239. [Google Scholar] [CrossRef] [Green Version]
- McGee-Lawrence, M.E.; Stoll, D.M.; Mantila, E.R.; Fahrner, B.K.; Carey, H.V.; Donahue, S.W. Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) show microstructural bone loss during hibernation but preserve bone macrostructural geometry and strength. J. Exp. Biol. 2011, 214 Pt 8, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Ahn, S.H.; Bae, S.J.; Kim, E.H.; Lee, S.H.; Kim, H.K.; Choe, J.W.; Koh, J.M.; Kim, G.S. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: A 3-year retrospective longitudinal study. J. Bone Miner. Res. 2012, 27, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Utz, J.C.; Nelson, S.; O’Toole, B.J.; van Breukelen, F. Bone strength is maintained after 8 months of inactivity in hibernating golden-mantled ground squirrels, Spermophilus lateralis. J. Exp. Biol. 2009, 212, 2746–2752. [Google Scholar] [CrossRef] [Green Version]
- Doherty, A.H.; Frampton, J.D.; Vinyard, C.J. Hibernation does not reduce cortical bone density, area or second moments of inertia in woodchucks (Marmota monax). J. Morphol. 2012, 273, 604–617. [Google Scholar] [CrossRef]
- Turlin, B.; Deugnier, Y. Evaluation and interpretation of iron in the liver. Semin. Diagn. Pathol. 1998, 15, 237–245. [Google Scholar]
- Yin, R.; Zhang, J.; Xu, S.; Kong, Y.; Wang, H.; Gao, Y. Resistance to disuse-induced iron overload in Daurian ground squirrels (Spermophilus dauricus) during extended hibernation inactivity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 257, 110650. [Google Scholar] [CrossRef]
- Græsli, A.R.; Evans, A.L.; Fahlman, Å.; Bertelsen, M.F.; Blanc, S.; Arnemo, J.M. Seasonal variation in haematological and biochemical variables in free-ranging subadult brown bears (Ursus arctos) in Sweden. BMC Vet. Res. 2015, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Prunescu, C.; Serban-Parau, N.; Brock, J.H.; Vaughan, D.M.; Prunescu, P. Liver and kidney structure and iron content in romanian brown bears (Ursus arctos) before and after hibernation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Koechlin-Ramonatxo, C.; Rochdi, S.; Island, M.L.; Orfila, L.; Treffel, L.; Bareille, M.P.; Beck, A.; Gauquelin-Koch, G.; Ropert, M.; et al. Simulated microgravity disturbs iron metabolism and distribution in humans: Lessons from dry immersion, an innovative ground-based human model. FASEB J. 2020, 34, 14920–14929. [Google Scholar] [CrossRef] [PubMed]
- Means, R.T., Jr. Hepcidin and anaemia. Blood Rev. 2004, 18, 219–225. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef]
- Malyszko, J. Hemojuvelin: The hepcidin story continues. Kidney Blood Press. Res. 2009, 32, 71–76. [Google Scholar] [CrossRef]
- Silvestri, L.; Nai, A.; Dulja, A.; Pagani, A. Hepcidin and the BMP-SMAD pathway: An unexpected liaison. Vitam. Horm. 2019, 110, 71–99. [Google Scholar]
- Latour, C.; Besson-Fournier, C.; Gourbeyre, O.; Meynard, D.; Roth, M.P.; Coppin, H. Deletion of BMP6 worsens the phenotype of HJV-deficient mice and attenuates hepcidin levels reached after LPS challenge. Blood 2017, 130, 2339–2343. [Google Scholar] [CrossRef] [Green Version]
- Nay, K.; Martin, D.; Orfila, L.; Saligaut, D.; Martin, B.; Horeau, M.; Cavey, T.; Kenawi, M.; Island, M.L.; Ropert, M.; et al. Intermittent reloading does not prevent reduction in iron availability and hepcidin upregulation caused by hindlimb unloading. Exp. Physiol. 2021, 106, 28–36. [Google Scholar] [CrossRef]
- Cavey, T.; Pierre, N.; Nay, K.; Allain, C.; Ropert, M.; Loréal, O.; Derbré, F. Simulated microgravity decreases circulating iron in rats: Role of inflammation-induced hepcidin upregulation. Exp. Physiol. 2017, 102, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.J. Mechanisms of iron loading and toxicity. Am. J. Hematol. 2007, 82 (Suppl. 12), 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barisani, D.; Conte, D. Transferrin receptor 1 (TfR1) and putative stimulator of Fe transport (SFT) expression in iron deficiency and overload: An overview. Blood Cells Mol. Dis. 2002, 29, 498–505. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorg Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Liang, W.; Ferrara, N. Iron Metabolism in the Tumor Microenvironment: Contributions of Innate Immune Cells. Front. Immunol. 2020, 11, 626812. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, H.; Yin, R.; Xu, S.; Wang, H.; Gao, Y. A temporal study on musculoskeletal morphology and metabolism in hibernating Daurian ground squirrels (Spermophilus dauricus). Bone 2021, 144, 115826. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Ismail, F.; Xu, S.; Wang, Z.; Peng, X.; Yang, C.; Chang, H.; Wang, H.; Gao, Y. Priority Strategy of Intracellular Ca(2+) Homeostasis in Skeletal Muscle Fibers During the Multiple Stresses of Hibernation. Cells 2019, 9, 42. [Google Scholar] [CrossRef]


| Group | Sample Time | Tb of Animal |
|---|---|---|
| SA | Mid-June | >37 °C |
| PRE | Mid-September | >37 °C |
| TOR | After two months hibernation, animals enter a new hibernation bout | ≥5 days with stable Tb of 5–8 °C |
| IBA | Same as TOR | 34–37 °C for less than 12 h |
| NP | March of following year | 5–8 °C for less than 24 h |
| POST | Same as NP | 36–38 °C for more than 3 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Kong, Y.; Yin, R.; Yang, H.; Zhang, J.; Wang, H.; Gao, Y. Remarkable Plasticity of Bone Iron Homeostasis in Hibernating Daurian Ground Squirrels (Spermophilus dauricus) May Be Involved in Bone Maintenance. Int. J. Mol. Sci. 2022, 23, 15858. https://doi.org/10.3390/ijms232415858
He Y, Kong Y, Yin R, Yang H, Zhang J, Wang H, Gao Y. Remarkable Plasticity of Bone Iron Homeostasis in Hibernating Daurian Ground Squirrels (Spermophilus dauricus) May Be Involved in Bone Maintenance. International Journal of Molecular Sciences. 2022; 23(24):15858. https://doi.org/10.3390/ijms232415858
Chicago/Turabian StyleHe, Yue, Yong Kong, Rongrong Yin, Huajian Yang, Jie Zhang, Huiping Wang, and Yunfang Gao. 2022. "Remarkable Plasticity of Bone Iron Homeostasis in Hibernating Daurian Ground Squirrels (Spermophilus dauricus) May Be Involved in Bone Maintenance" International Journal of Molecular Sciences 23, no. 24: 15858. https://doi.org/10.3390/ijms232415858





