Impact of Saccharomyces cerevisiae on the Field of Single-Molecule Biophysics
Abstract
:1. Introduction: Why Single Molecule Tracking?
2. Know-How of Yeast SMT
2.1. How to Label Yeast for SMT?
2.2. How to Image Yeast for SMT?
2.2.1. Imaging Systems
2.2.2. Detection of Molecules
2.2.3. Tracking of Molecules
2.3. How to Analyze Single Molecule Tracks?
2.3.1. Analysis by Survival Distribution: Bi-Exponential Fit
2.3.2. Analysis by Survival Distribution: Power Law Fit
2.3.3. MSD Analysis
2.3.4. Software for SMT
2.4. Yeast as a Model for SMT: Pros and Cons
3. What Did We Learn from SMT in Yeast and Higher Eukaryotes?
3.1. Kinetics of Protein Complexes
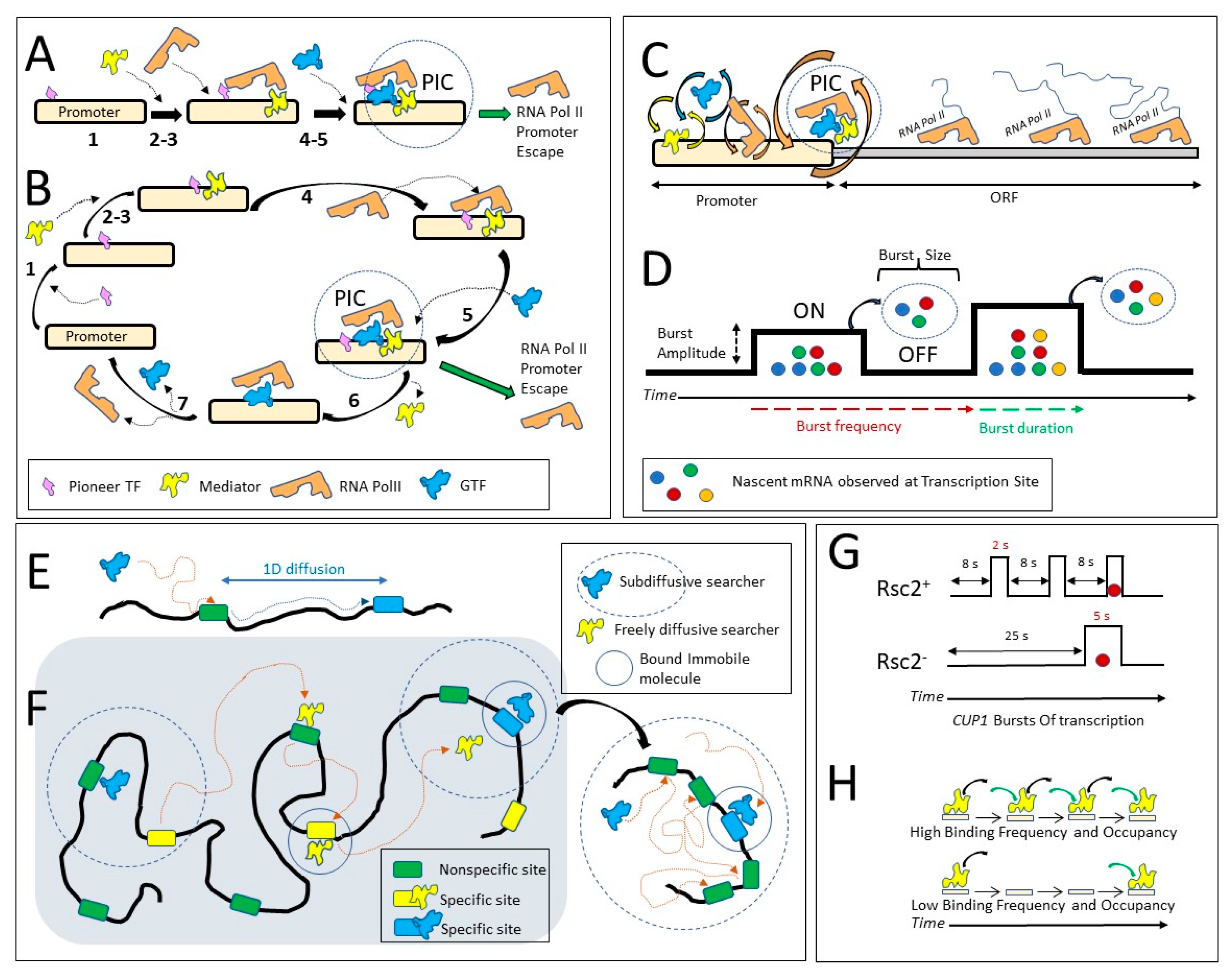
3.1.1. Dynamics of Regulatory Complex Assembly
3.1.2. Dynamics of Processive Complexes
3.2. Fast Kinetics of TFs Translated into Slow Kinetics of Transcription Bursts
3.2.1. Short Binding Events May Lead to Long Bursts of Transcription
3.2.2. Yeast and Mammalian TFs Play Different Roles in Initiation of Transcription
3.3. Search for the Specific Binding Sites
3.3.1. Protein Interactions with DNA during the Search Process
3.3.2. Nuclear Space Sampling during the Search Process in Eukaryotic Nucleus
3.4. Regulation of Promoter Occupancy
3.4.1. Factors Affecting Frequency of Protein Binding to Target Sites
3.4.2. Factors Affecting Protein Residence Time on Target Sites
3.4.3. Long Residence or Frequent Binding?
4. Conclusions: Hopes for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Term | Definition |
| Anisotropic | Having a material that has a different value when measured in different directions, opposite of isotropic (similar in different directions) |
| Anomalous diffusion | A diffusion process with a non-linear relationship between the MSD and time |
| Auto-correlation | The correlation of a signal with a delayed copy of itself as a function of delay; used to identify patterns within a signal |
| Autofluorescence | The fluorescence naturally occurring in a biological structure |
| Bessel-shaped profile | Refers to a wave or beam with an amplitude described by a Bessel function |
| Concavalin A | A lectin originally extracted from the jack-bean (Canavalia ensiformis) |
| Counterstain | A stain contrasting to the principal stain to make clearer the distinction between different compartments within the cell |
| Cross correlation | A measure of the relationship between two signals as a function of the displacement in time of one relative to the other; used to calculate the relative timing of features in the two signals |
| Diffusion | Random motion of molecules within a medium |
| Diffusion coefficient | The area covered by a moving molecule in 1 second |
| Diploids | A cell or organism having two complete sets of chromosomes |
| Dwell time | Refers to a period of time that a molecule remains in a given state |
| Electroporation | A technique in which an electric field is used to make the cell’s membrane more permeable to drugs, chemicals (i.e., dyes), or DNA |
| Elongation rates | The rates by which RNA strands get longer due to the addition of new nucleotides during transcription |
| Ensemble | A group or collective set of data used in an analysis |
| Eukaryotes | Organisms containing nucleus and intracellular organelles |
| Exponential decay | Refers to a decrease of a quantity at a rate proportional to its current value |
| Fluorophore | A fluorescent chemical compound that can re-emit light when excited by a light source (such as lasers) |
| Gaussian filter | A filter whose impulse response is a Gaussian function |
| Gaussian profile | A graph or profile with a characteristic symmetric “bell curve” shape |
| Genome | Refers to the complete set of genes or genetic materials in a cell or organism |
| HaloTag ligand | A synthetic fluorescent molecule of choice with reactive chloroalkane linker forming covalent bonds with HaloTag (a self-labeling protein tag) |
| Heterogeneity | Refers to the quality or state of being diverse in character or content |
| Heterozygous | Referring to a diploid cell or organism having two different alleles of a particular gene in the same genome |
| Histones | Protein components of nucleosome |
| Homozygote | Referring to a diploid cell or organism having two identical alleles of a particular gene in the same genome |
| Housekeeping genes | Genes that are typically always expressed because they are required for the maintenance of basic cellular function |
| In vitro | Latin for “in glass,” refers to studies performed outside the normal biological context |
| In vivo | Latin for “within the living” cell or organism |
| Intranuclear | Refers to being situated or occurring within a nucleus |
| Isotropic diffusion | Presented by a sphere with equal diffusion in all directions in space |
| Kinase | An enzyme that catalyzes the transfer of a phosphate group from ATP to a specific molecule |
| Kinetics | Refers to the study of time-dependent processes |
| Kymograph | A space-time plot that shows the intensity values of a molecule’s path over time, where either the X or Y axis of the 2D space is presented as a maximum intensity projection |
| Lagging strand | A single DNA strand that is discontinuously replicated in the 5-prime to 3-prime direction during DNA replication |
| Leading strand | A single DNA strand that is continuously replicated in the 3-prime to 5-prime direction during DNA replication |
| Liquid-liquid phase separation | A biological phenomenon that refers to the components of similar properties forming droplets or condensates in cells |
| Mediator complex | A general regulator of transcription |
| Metallothionein | Small cysteine-rich protein that binds heavy metal ions and protects against heavy metal stress and toxicity |
| Motif | Refers to a short nucleic acid sequence that has some biological significance |
| Mutation | Change in DNA sequence that may alter gene expression in some way |
| Nascent mRNA | Refers to a newly synthesized messenger RNA molecule |
| Non-specific binding | Refers to the binding of a protein to an unintended site |
| Nuclear bodies | Membrane-less structures found in the cell nuclei of eukaryotic cells and providing specific cellular functions such as splicing, etc. |
| Nucleoplasm | Contents of the cell nucleus |
| Nucleosome | The basic structural unit of DNA packaging, consisting of eight histone proteins, around which a segment of DNA is wrapped |
| Photoactivation | The process of transforming a molecule or substance to an active form by using radiant energy such as laser light |
| Photobleaching | Refers to fading or loss of a fluorescence from a molecule being irradiated |
| Photoinducible fluorophores | Molecules that are normally non-fluorescent, but can be transformed to a fluorescent state by exposure to light with a specific wavelength |
| Photostable dyes | Fluorescent dyes that can withstand long exposure to irradiation before photobleaching |
| Polymerases | Components of replicative complex (replisome): Pol α, Pol ε, and Pol δ |
| Pre-initiation complex | A complex of about 100 proteins that is necessary for initiation of transcription |
| Primer | A short RNA oligonucleotide used by all living organisms in the initiation of DNA synthesis |
| Prokaryotes | Organisms that lack a true nucleus and organelles inside the cell |
| Promoter | A region of DNA where RNA polymerase begins to transcribe a gene |
| Putative binding site | A DNA segment with a sequence corresponding to consensus motif for appropriate binding protein |
| Radius of confinement | An area to which the motion of a molecule is limited |
| Regulatory factors/proteins | Proteins that control the cellular functions, such as transcription |
| Regulatory site | A site to which a regulatory protein binds to perform a regulatory function |
| Replisome | Molecular complex that carries out replication of DNA |
| Reporter | A synthetic DNA sequence inserted into the genome to monitor specific processes, such as to detect formation of the nascent mRNA or the synthesis of specific protein |
| Residual population | A group (for example, of molecules) that were already present when their production was stopped |
| RNA Polymerase II | Molecular complex that is involved in transcribing DNA into mRNA It is one of the three RNA polymerase complexes found in the nucleus of all eukaryotic cells |
| S stage | A phase of the cell cycle in which DNA replication occurs |
| SNAP-tag ligand | A synthetic fluorescent molecule of choice with guanine or chloropyrimidine residue forming covalent bonds with SNAP-tag (a self-labeling protein tag) |
| Stem-loop approach | A technique to observe mRNA in live cells; relies on the insertion into the gene of either MS2 or PP7 phage sequence, RNA of which folds into hairpins; hairpins are bound by appropriate phage coat protein (PCP) labeled with a fluorophore |
| Subdiffusion | Refers to the tendency of particles in a fluid to move slower than expected for Brownian motion; caused by random trapping or frequent binding |
| Temporal resolution | Refers to the discrete time steps at which a measurement is made |
| Transcription | The process of copying a segment of DNA into RNA |
| Transcription factor | A protein that controls the transcription of genetic information from DNA to RNA |
| Transcription site | The location where nascent transcripts are produced |
| Transcriptional output | Refers to the amount of RNA molecules produced during transcription |
| Transcriptional silence | Occurs when the transcription of a gene by RNA Pol II is repressed or turned off |
| Transient binding | Temporary binding that is typically associated with binding of proteins in places other than their specific motif |
| Vacuole | A large vesicle within the cytoplasm of a cell, enclosed by a membrane |
| Xenobiotic compounds | Chemicals or substances from natural or synthetic sources found within an organism that are not naturally produced by the organism |
References
- Métivier, R.; Penot, G.; Hübner, M.R.; Reid, G.; Brand, H.; Koš, M.; Gannon, F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 2003, 115, 751–763. [Google Scholar] [CrossRef] [Green Version]
- Korber, P.; Barbaric, S. The yeast PHO5 promoter: From single locus to systems biology of a paradigm for gene regulation through chromatin. Nucleic Acids Res. 2014, 42, 10888–10902. [Google Scholar] [CrossRef] [Green Version]
- McNally, J.G.; Muller, W.G.; Walker, D.; Wolford, R.; Hager, G.L. The glucocorticoid receptor: Rapid exchange with regulatory sites in living cells. Science 2000, 287, 1262–1265. [Google Scholar] [CrossRef]
- Karpova, T.S.; Kim, M.J.; Spriet, C.; Nalley, K.; Stasevich, T.J.; Kherrouche, Z.; Heliot, L.; McNally, J.G. Concurrent fast and slow cycling of a transcriptional activator at an endogenous promoter. Science 2008, 319, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Karpova, T.S.; Chen, T.Y.; Sprague, B.L.; McNally, J.G. Dynamic interactions of a transcription factor with DNA are accelerated by a chromatin remodeller. EMBO Rep. 2004, 5, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Sprouse, R.O.; Karpova, T.S.; Mueller, F.; Dasgupta, A.; McNally, J.G.; Auble, D.T. Regulation of TATA-binding protein dynamics in living yeast cells. Proc. Natl. Acad. Sci. USA 2008, 105, 13304–13308. [Google Scholar] [CrossRef] [Green Version]
- Mazza, D.; Abernathy, A.; Golob, N.; Morisaki, T.; McNally, J.G. A benchmark for chromatin binding measurements in live cells. Nucleic Acids Res. 2012, 40, e119. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, J.C.M.; Suter, D.M.; Roy, R.; Zhao, Z.W.; Chapman, A.R.; Basu, S.; Maniatis, T.; Xie, X.S. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 2013, 10, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Morisaki, T.; Müller, W.G.; Golob, N.; Mazza, D.; McNally, J.G. Single-molecule analysis of transcription factor binding at transcription sites in live cells. Nat. Commun. 2014, 5, 4456. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Legant, W.R.; Chen, B.C.; Li, L.; Grimm, J.B.; Lavis, L.D.; Betzig, E.; Tjian, R. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. Elife 2014, 3, e04236. [Google Scholar] [CrossRef]
- Ball, D.A.; Mehta, G.D.; Salomon-Kent, R.; Mazza, D.; Morisaki, T.; Mueller, F.; McNally, J.G.; Karpova, T.S. Single molecule tracking of Ace1p in Saccharomyces cerevisiae defines a characteristic residence time for non-specific interactions of transcription factors with chromatin. Nucleic Acids Res. 2016, 44, e160. [Google Scholar] [CrossRef]
- Mehta, G.D.; Ball, D.A.; Eriksson, P.R.; Chereji, R.V.; Clark, D.J.; McNally, J.G.; Karpova, T.S. Single-Molecule Analysis Reveals Linked Cycles of RSC Chromatin Remodeling and Ace1p Transcription Factor Binding in Yeast. Mol. Cell 2018, 72, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Donovan, B.T.; Huynh, A.; Ball, D.A.; Patel, H.P.; Poirier, M.G.; Larson, D.R.; Ferguson, M.L.; Lenstra, T.L. Live-cell imaging reveals the interplay between transcription factors, nucleosomes, and bursting. EMBO J. 2019, 38, e100809. [Google Scholar] [CrossRef]
- Kapadia, N.; El-Hajj, Z.W.; Zheng, H.; Beattie, T.R.; Yu, A.; Reyes-Lamothe, R. Processive Activity of Replicative DNA Polymerases in the Replisome of Live Eukaryotic Cells. Mol. Cell 2020, 80, 114–126. [Google Scholar] [CrossRef]
- Ranjan, A.; Nguyen, V.Q.; Liu, S.; Wisniewski, J.; Kim, J.M.; Tang, X.; Mizuguchi, G.; Elalaoui, E.; Nickels, T.J.; Jou, V.; et al. Live-cell single particle imaging reveals the role of RNA polymerase II in histone H2A.Z eviction. Elife 2020, 9, e55667. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Ranjan, A.; Liu, S.; Tang, X.; Ling, Y.H.; Wisniewski, J.; Mizuguchi, G.; Li, K.Y.; Jou, V.; Zheng, Q.; et al. Spatiotemporal coordination of transcription preinitiation complex assembly in live cells. Mol. Cell 2021, 81, 3560–3575. [Google Scholar] [CrossRef]
- Kim, J.M.; Visanpattanasin, P.; Jou, V.; Liu, S.; Tang, X.; Zheng, Q.; Li, K.Y.; Snedeker, J.; Lavis, L.D.; Lionnet, T.; et al. Single-molecule imaging of chromatin remodelers reveals role of ATPase in promoting fast kinetics of target search and dissociation from chromatin. Elife 2021, 10, e69387. [Google Scholar] [CrossRef]
- England, C.G.; Luo, H.; Cai, W. HaloTag Technology: A Versatile Platform for Biomedical Applications. Bioconjugate Chem. 2015, 26, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Kolberg, K.; Puettmann, C.; Pardo, A.; Fitting, J.; Barth, S. SNAP-Tag Technology: A General Introduction. Curr. Pharm. Des. 2013, 19, 5406–5413. [Google Scholar] [CrossRef]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef]
- Tokunaga, M.; Imamoto, N.; Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 2008, 5, 159–161. [Google Scholar] [CrossRef]
- Presman, D.M.; Ball, D.A.; Paakinaho, V.; Grimm, J.B.; Lavis, L.D.; Karpova, T.S.; Hager, G.L. Quantifying transcription factor binding dynamics at the single-molecule level in live cells. Methods 2017, 123, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Kapadia, N.; El-Hajj, Z.W.; Reyes-Lamothe, R. Bound2Learn: A machine learning approach for classification of DNA-bound proteins from single-molecule tracking experiments. Nucleic Acids Res. 2021, 49, e79. [Google Scholar] [CrossRef]
- Chen, B.C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A., III; Liu, Z.; et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Lanzanò, L.; Gratton, E. Orbital single particle tracking on a commercial confocal microscope using piezoelectric stage feedback. Methods Appl. Fluoresc. 2014, 2, e024010. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Johnson, C.; Welsher, K. Real-Time 3D Single Particle Tracking: Towards Active Feedback Single Molecule Spectroscopy in Live Cells. Molecules 2019, 24, 2826. [Google Scholar] [CrossRef] [Green Version]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2017, 355, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, T.; Iwanski, M.K.; Schentarra, E.M.; Heidebrecht, C.; Schmidt, L.; Heck, J.; Weihs, T.; Schnorrenberg, S.; Hoess, P.; Liu, S.; et al. Direct observation of motor protein stepping in living cells using MINFLUX. bioRxiv 2022, 1–20. [Google Scholar] [CrossRef]
- Li, J.; Dong, A.; Saydaminova, K.; Chang, H.; Wang, G.; Ochiai, H.; Yamamoto, T.; Pertsinidis, A. Single-Molecule Nanoscopy Elucidates RNA Polymerase II Transcription at Single Genes in Live Cells. Cell 2019, 178, 491–506. [Google Scholar] [CrossRef]
- Gustavsson, A.K.; Petrov, P.N.; Lee, M.Y.; Shechtman, Y.; Moerner, W.E. 3D single-molecule super-resolution microscopy with a tilted light sheet. Nat. Commun. 2018, 9, 123. [Google Scholar] [CrossRef]
- Opatovski, N.; Shalev Ezra, Y.; Weiss, L.E.; Ferdman, B.; Orange-Kedem, R.; Shechtman, Y. Multiplexed PSF Engineering for Three-Dimensional Multicolor Particle Tracking. Nano Lett. 2021, 21, 5888–5895. [Google Scholar] [CrossRef]
- Wollman, A.J.; Hedlund, E.G.; Shashkova, S.; Leake, M.C. Mapping the 3D genome through high speed single-molecule tracking of functional transcription factors in single living cells. bioRxiv 2019, 1–15. [Google Scholar] [CrossRef]
- Waddle, J.A.; Karpova, T.S.; Waterston, R.H.; Cooper, J.A. Movement of cortical actin patches in yeast. J. Cell Biol. 1996, 132, 861–870. [Google Scholar] [CrossRef]
- Reisser, M.; Hettich, J.; Kuhn, T.; Popp, A.P.; Große-Berkenbusch, A.; Gebhardt, J.C.M. Inferring quantity and qualities of superimposed reaction rates from single molecule survival time distributions. Sci. Rep. 2020, 10, 1758. [Google Scholar] [CrossRef] [Green Version]
- Lerner, J.; Gómez-García, P.A.; McCarthy, R.L.; Liu, Z.; Lakadamyali, M.; Zaret, K.S. Two-parameter single-molecule analysis for measurement of chromatin mobility. STAR Protoc. 2020, 1, e100223. [Google Scholar] [CrossRef]
- Garcia, D.A.; Johnson, T.A.; Presman, D.M.; Fettweis, G.; Wagh, K.; Rinaldi, L.; Stavreva, D.A.; Paakinaho, V.; Jensen, R.A.; Mandrup, S.; et al. An intrinsically disordered region-mediated confinement state contributes to the dynamics and function of transcription factors. Mol. Cell 2021, 81, 1484–1498. [Google Scholar] [CrossRef]
- Mirny, L.; Slutsky, M.; Wunderlich, Z.; Tafvizi, A.; Leith, J.; Kosmrlj, A. How a protein searches for its site on DNA: The mechanism of facilitated diffusion. J. Phys. A Math. Theor. 2009, 42, e434013. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.A.; Fettweis, G.; Presman, D.M.; Paakinaho, V.; Jarzynski, C.; Upadhyaya, A.; Hager, G.L. Power-law behavior of transcription factor dynamics at the single-molecule level implies a continuum affinity model. Nucleic Acids Res. 2021, 49, 6605–6620. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Li, L.; Chen, B.C.; Revyakin, A.; Hajj, B.; Legant, W.; Dahan, M.; Lionnet, T.; Betzig, E.; et al. Single-Molecule Dynamics of Enhanceosome Assembly in Embryonic Stem Cells. Cell 2014, 156, 1274–1285. [Google Scholar] [CrossRef] [Green Version]
- Woringer, M.; Darzacq, X. Protein motion in the nucleus: From anomalous diffusion to weak interactions. Biochem. Soc. Trans. 2018, 46, 945–956. [Google Scholar] [CrossRef]
- Wieser, S.; Schutz, G.J. Tracking single molecules in the live cell plasma membrane-Do’s and Don’t’s. Methods 2008, 46, 131–140. [Google Scholar] [CrossRef]
- Mazza, D.; Ganguly, S.; McNally, J.G. Monitoring dynamic binding of chromatin proteins in vivo by single-molecule tracking. Methods Mol. Biol. 2013, 1042, 117–137. [Google Scholar]
- Jaqaman, K.; Loerke, D.; Mettlen, M.; Kuwata, H.; Grinstein, S.; Schmid, S.L.; Danuser, G. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 2008, 5, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Tinevez, J.Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Vallotton, P.; Olivier, S. Tri-track: Free software for large-scale particle tracking. Microsc. Microanal. 2013, 19, 451–460. [Google Scholar] [CrossRef]
- Sergé, A.; Bertaux, N.; Rigneault, H.; Marguet, D. Dynamic multiple-target tracing to probe spatiotemporal cartography of cell membranes. Nat. Methods 2008, 5, 687–694. [Google Scholar] [CrossRef]
- Chenouard, N.; Smal, I.; De Chaumont, F.; Maška, M.; Sbalzarini, I.F.; Gong, Y.; Cardinale, J.; Carthel, C.; Coraluppi, S.; Winter, M.; et al. Objective comparison of particle tracking methods. Nat. Methods 2014, 11, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yoo, S.; Tang, X.; Sung, Y.; Wu, C. Sojourner: Statistical Analysis of Single Molecule Trajectories. R Package Version 1.3.0. Available online: https://github.com/sheng-liy/sojourner (accessed on 10 December 2022).
- Hansen, A.S.; Woringer, M.; Grimm, J.B.; Lavis, L.D.; Tjian, R.; Darzacq, X. Robust model-based analysis of single-particle tracking experiments with Spot-On. Elife 2018, 7, e33125. [Google Scholar] [CrossRef]
- Persson, F.; Lindén, M.; Unoson, C.; Elf, J. Extracting intracellular diffusive states and transition rates from single-molecule tracking data. Nat. Methods 2013, 10, 265–269. [Google Scholar] [CrossRef]
- Koo, P.K.; Mochrie, S.G. Systems-level approach to uncovering diffusive states and their transitions from single-particle trajectories. Phys. Rev. E 2016, 94, e052412. [Google Scholar] [CrossRef] [Green Version]
- Pinholt, H.D.; Bohr, S.S.R.; Iversen, J.F.; Boomsma, W.; Hatzakis, N.S. Single-particle diffusional fingerprinting: A machine-learning framework for quantitative analysis of heterogeneous diffusion. Proc. Natl. Acad. Sci. USA 2021, 118, e2104624118. [Google Scholar] [CrossRef]
- Heckert, A.; Dahal, L.; Tijan, R.; Darzacq, X. Recovering mixtures of fast-diffusing states from short single-particle trajectories. Elife 2022, 11, e70169. [Google Scholar] [CrossRef]
- de Jonge, W.J.; Patel, H.P.; Meeussen, J.V.; Lenstra, T.L. Following the tracks: How transcription factor binding dynamics control transcription. Biophys. J. 2022, 121, 1583–1592. [Google Scholar] [CrossRef]
- Wang, Z.H.; Deng, W.L. Dynamic transcription regulation at the single-molecule level. Dev. Biol. 2022, 482, 67–81. [Google Scholar] [CrossRef]
- Brown, K.; Andrianakos, H.; Ingersoll, S.; Ren, X. Single-molecule imaging of epigenetic complexes in living cells: Insights from studies on Polycomb group proteins. Nucleic Acids Res. 2021, 49, 6621–6637. [Google Scholar] [CrossRef]
- Mazzocca, M.; Fillot, T.; Loffreda, A.; Gnani, D.; Mazza, D. The needle and the haystack: Single molecule tracking to probe the transcription factor search in eukaryotes. Biochem. Soc. Trans. 2021, 49, 1121–1132. [Google Scholar] [CrossRef]
- Darzacq, X.; Tjian, R. Weak multivalent biomolecular interactions: A strength versus numbers tug of war with implications for phase partitioning. RNA 2022, 28, 48–51. [Google Scholar] [CrossRef]
- Baek, I.; Friedman, L.J.; Gelles, J.; Buratowski, S. Single-molecule studies reveal branched pathways for activator-dependent assembly of RNA polymerase II pre-initiation complexes. Mol. Cell 2021, 81, 3576–3588. [Google Scholar] [CrossRef]
- Chen, D.; Hinkley, C.S.; Henry, R.W.; Huang, S. TBP dynamics in living human cells: Constitutive association of TBP with mitotic chromosomes. Mol. Biol. Cell 2002, 13, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Sugaya, K.; Cook, P.R. The transcription cycle of RNA polymerase II in living cells. J. Cell Biol. 2002, 159, 777–782. [Google Scholar] [CrossRef]
- Patange, S.; Ball, D.A.; Wan, Y.; Karpova, T.S.; Girvan, M.; Levens, D.; Larson, D.R. MYC amplifies gene expression through global changes in transcription factor dynamics. Cell Rep. 2022, 38, e110292. [Google Scholar] [CrossRef]
- Jeronimo, C.; Angel, A.; Nguyen, V.Q.; Kim, J.M.; Poitras, C.; Lambert, E.; Collin, P.; Mellor, J.; Wu, C.; Robert, F. FACT is recruited to the+1 nucleosome of transcribed genes and spreads in a Chd1-dependent manner. Mol. Cell 2021, 81, 3542–3559. [Google Scholar] [CrossRef]
- Aoi, Y.; Takahashi, Y.H.; Shah, A.P.; Iwanaszko, M.; Rendleman, E.J.; Khan, N.H.; Cho, B.K.; Goo, Y.A.; Ganesan, S.; Kelleher, N.L.; et al. SPT5 stabilization of promoter-proximal RNA polymerase II. Mol. Cell 2021, 81, 4413–4424. [Google Scholar] [CrossRef]
- Rosen, G.A.; Baek, I.; Friedman, L.J.; Joo, Y.J.; Buratowski, S.; Gelles, J. Dynamics of RNA polymerase II and elongation factor Spt4/5 recruitment during activator-dependent transcription. Proc. Natl. Acad. Sci. USA 2020, 117, 32348–32357. [Google Scholar] [CrossRef]
- Ferro, L.S.; Fang, Q.; Eshun-Wilson, L.; Fernandes, J.; Jack, A.; Farrell, D.P.; Golcuk, M.; Huijben, T.; Costa, K.; Gur, M.; et al. Structural and functional insight into regulation of kinesin-1 by microtubule-associated protein MAP7. Science 2022, 375, 326–331. [Google Scholar] [CrossRef]
- Larson, D.R.; Fritzsch, C.; Sun, L.; Meng, X.; Lawrence, D.S.; Singer, R.H. Direct observation of frequency modulated transcription in single cells using light activation. Elife 2013, 2, e00750. [Google Scholar] [CrossRef]
- Senecal, A.; Munsky, B.; Proux, F.; Ly, N.; Braye, F.E.; Zimmer, C.; Mueller, F.; Darzacq, X. Transcription Factors Modulate c-Fos Transcriptional Bursts. Cell Rep. 2014, 8, 75–83. [Google Scholar] [CrossRef]
- Rullan, M.; Benzinger, D.; Schmidt, G.W.; Milias-Argeitis, A.; Khammash, M. An Optogenetic Platform for Real-Time, Single-Cell Interrogation of Stochastic Transcriptional Regulation. Mol. Cell 2018, 70, 745–756. [Google Scholar] [CrossRef]
- Stavreva, D.A.; Garcia, D.A.; Fettweis, G.; Gudla, P.R.; Zaki, G.F.; Soni, V.; McGowan, A.; Williams, G.; Huynh, A.; Palangat, M.; et al. Transcriptional Bursting and Co-bursting Regulation by Steroid Hormone Release Pattern and Transcription Factor Mobility. Mol. Cell 2019, 75, 1161–1177. [Google Scholar] [CrossRef]
- Popp, A.P.; Hettich, J.; Gebhardt, J.C.M. Altering transcription factor binding reveals comprehensive transcriptional kinetics of a basic gene. Nucleic Acids Res. 2021, 49, 6249–6266. [Google Scholar] [CrossRef]
- von Hippel, P.H.; Berg, O.G. Facilitated target location in biological systems. J. Biol. Chem. 1989, 264, 675–678. [Google Scholar] [CrossRef]
- Blainey, P.C.; van Oijen, A.M.; Banerjee, A.; Verdine, G.L.; Xie, X.S. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc. Natl. Acad. Sci. USA 2006, 103, 5752–5757. [Google Scholar] [CrossRef] [Green Version]
- Elf, J.; Li, G.W.; Xie, X.S. Probing transcription factor dynamics at the single-molecule level in a living cell. Science 2007, 316, 1191–1194. [Google Scholar] [CrossRef] [Green Version]
- Normanno, D.; Boudarène, L.; Dugast-Darzacq, C.; Chen, J.; Richter, C.; Proux, F.; Bénichou, O.; Voituriez, R.; Darzacq, X.; Dahan, M. Probing the target search of DNA-binding proteins in mammalian cells using TetR as model searcher. Nat. Commun. 2015, 6, 7357. [Google Scholar] [CrossRef] [Green Version]
- Gorman, J.; Plys, A.J.; Visnapuu, M.L.; Alani, E.; Greene, E.C. Visualizing one-dimensional diffusion of eukaryotic DNA repair factors along a chromatin lattice. Nat. Struct. Mol. Biol. 2010, 17, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Carcamo, C.C.; Poyton, M.F.; Ranjan, A.; Park, G.; Louder, R.K.; Feng, X.A.; Kim, J.M.; Dzu, T.; Wu, C.; Ha, T. ATP binding facilitates target search of SWR1 chromatin remodeler by promoting one-dimensional diffusion on DNA. Elife 2022, 11, e77352. [Google Scholar] [CrossRef]
- Friedman, L.J.; Mumm, J.P.; Gelles, J. RNA polymerase approaches its promoter without long-range sliding along DNA. Proc. Natl. Acad. Sci. USA 2013, 110, 9740–9745. [Google Scholar] [CrossRef] [Green Version]
- Izeddin, I.; Récamier, V.; Bosanac, L.; Cissé, I.I.; Boudarene, L.; Dugast-Darzacq, C.; Proux, F.; Bénichou, O.; Voituriez, R.; Bensaude, O.; et al. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. Elife 2014, 3, e02230. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.S.; Amitai, A.; Cattoglio, C.; Tjian, R.; Darzacq, X. Guided nuclear exploration increases CTCF target search efficiency. Nat. Chem. Biol. 2020, 16, 257–266. [Google Scholar] [CrossRef]
- Larson, D.R.; Zenklusen, D.; Wu, B.; Chao, J.A.; Singer, R.H. Real-Time Observation of Transcription Initiation and Elongation on an Endogenous Yeast Gene. Science 2011, 332, 475–478. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Maheshri, N. A regulatory role for repeated decoy transcription factor binding sites in target gene expression. Mol. Syst. Biol. 2012, 8, e576. [Google Scholar] [CrossRef]
- Cisse, I.I.; Izeddin, I.; Causse, S.Z.; Boudarene, L.; Senecal, A.; Muresan, L.; Dugast-Darzacq, C.; Hajj, B.; Dahan, M.; Darzacq, X. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 2013, 341, 664–667. [Google Scholar] [CrossRef]
- Cho, W.K.; Spille, J.H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef] [Green Version]
- Chong, S.; Graham, T.G.; Dugast-Darzacq, C.; Dailey, G.M.; Darzacq, X.; Tjian, R. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Mol. Cell 2022, 82, 2084–2097. [Google Scholar] [CrossRef]
- Wagh, K.; Ishikawa, M.; Garcia, D.A.; Stavreva, D.A.; Upadhyaya, A.; Hager, G.L. Mechanical regulation of transcription: Recent advances. Trends Cell Biol. 2021, 31, 457–472. [Google Scholar] [CrossRef]
- Quintero-Cadena, P.; Lenstra, T.L.; Sternberg, P.W. RNA Pol II Length and Disorder Enable Cooperative Scaling of Transcriptional Bursting. Mol. Cell 2020, 79, 207–220. [Google Scholar] [CrossRef]
- Mir, M.; Reimer, A.; Haines, J.E.; Li, X.Y.; Stadler, M.; Garcia, H.; Eisen, M.B.; Darzacq, X. Dense Bicoid hubs accentuate binding along the morphogen gradient. Genes Dev. 2017, 31, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Loffreda, A.; Jacchetti, E.; Antunes, S.; Rainone, P.; Daniele, T.; Morisaki, T.; Bianchi, M.E.; Tacchetti, C.; Mazza, D. Live-cell p53 single-molecule binding is modulated by C-terminal acetylation and correlates with transcriptional activity. Nat. Commun. 2017, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Paakinaho, V.; Presman, D.M.; Ball, D.A.; Johnson, T.A.; Schiltz, R.L.; Levitt, P.; Mazza, D.; Morisaki, T.; Karpova, T.S.; Hager, G.L. Single-molecule analysis of steroid receptor and cofactor action in living cells. Nat. Commun. 2017, 8, 15896. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Ren, G.; Day, C.R.; Zhao, K.; Chow, C.C.; Larson, D.R. Intrinsic dynamics of a human gene reveal the basis of expression heterogeneity. Cell 2019, 176, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Karr, J.P.; Ferrie, J.J.; Tjian, R.; Darzacq, X. The transcription factor activity gradient (TAG) model: Contemplating a contact-independent mechanism for enhancer-promoter communication. Genes Dev. 2022, 36, 7–16. [Google Scholar] [CrossRef]
- Hao, N.; O’Shea, E.K. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 2012, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ball, D.A.; Jalloh, B.; Karpova, T.S. Impact of Saccharomyces cerevisiae on the Field of Single-Molecule Biophysics. Int. J. Mol. Sci. 2022, 23, 15895. https://doi.org/10.3390/ijms232415895
Ball DA, Jalloh B, Karpova TS. Impact of Saccharomyces cerevisiae on the Field of Single-Molecule Biophysics. International Journal of Molecular Sciences. 2022; 23(24):15895. https://doi.org/10.3390/ijms232415895
Chicago/Turabian StyleBall, David A., Binta Jalloh, and Tatiana S. Karpova. 2022. "Impact of Saccharomyces cerevisiae on the Field of Single-Molecule Biophysics" International Journal of Molecular Sciences 23, no. 24: 15895. https://doi.org/10.3390/ijms232415895




