Silk Sericin Enrichment through Electrodeposition and Carbonous Materials for the Removal of Methylene Blue from Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of SS/CMCS Hydrogel and SC
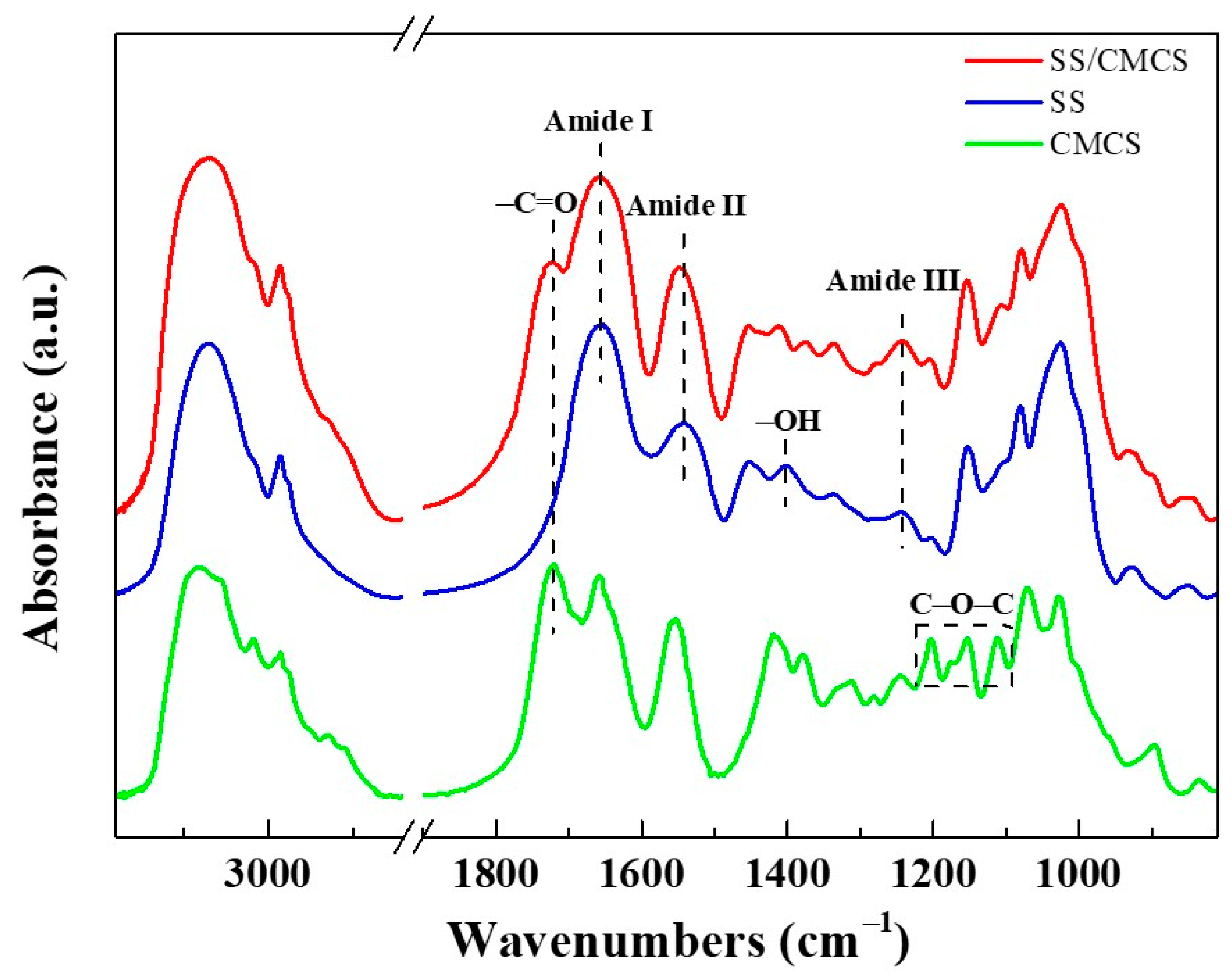
2.1.1. Determination of SS Content and Intermolecular Interactions in SS/CMCS Hydrogel
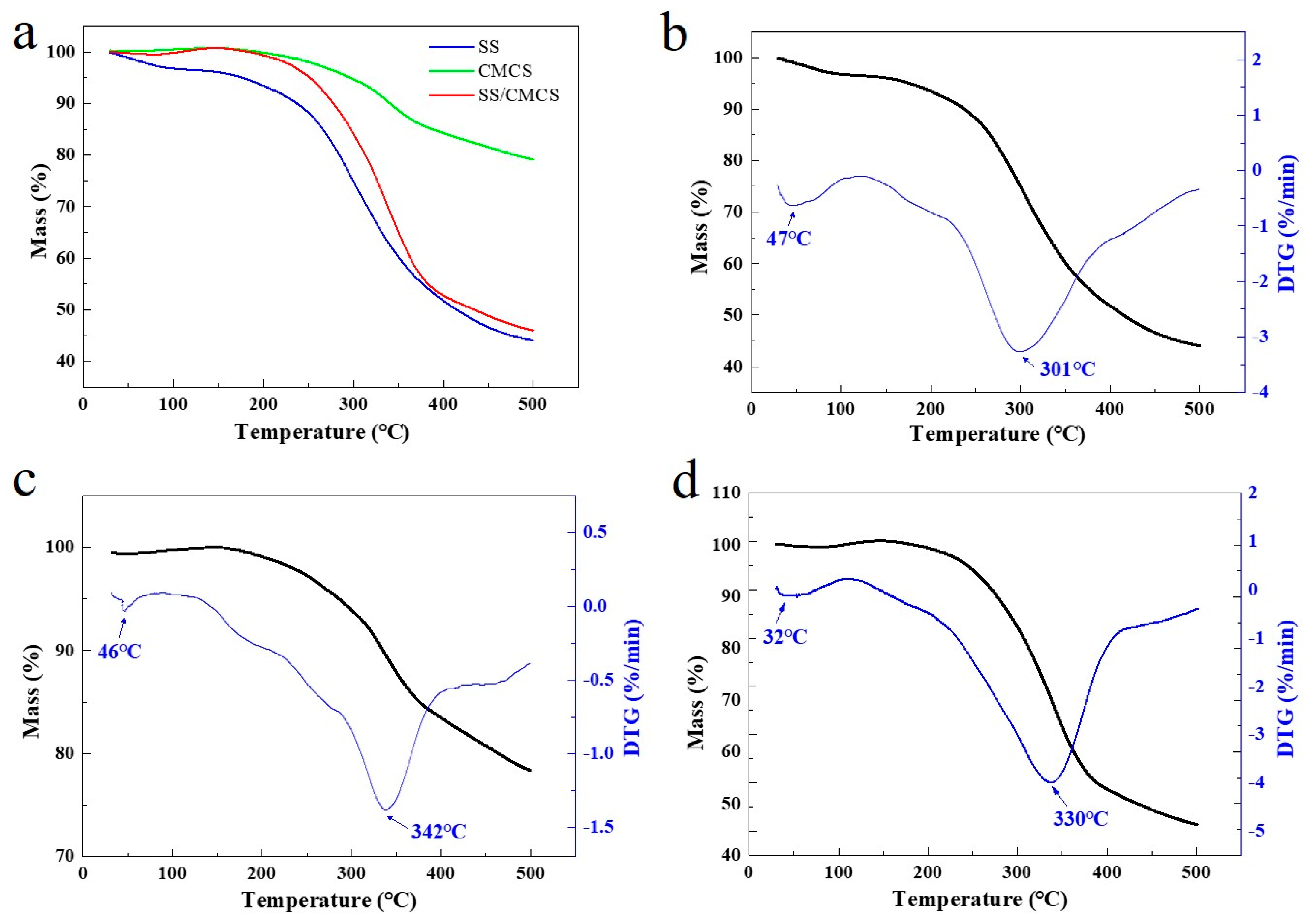
2.1.2. Evaluation of Thermal Stability
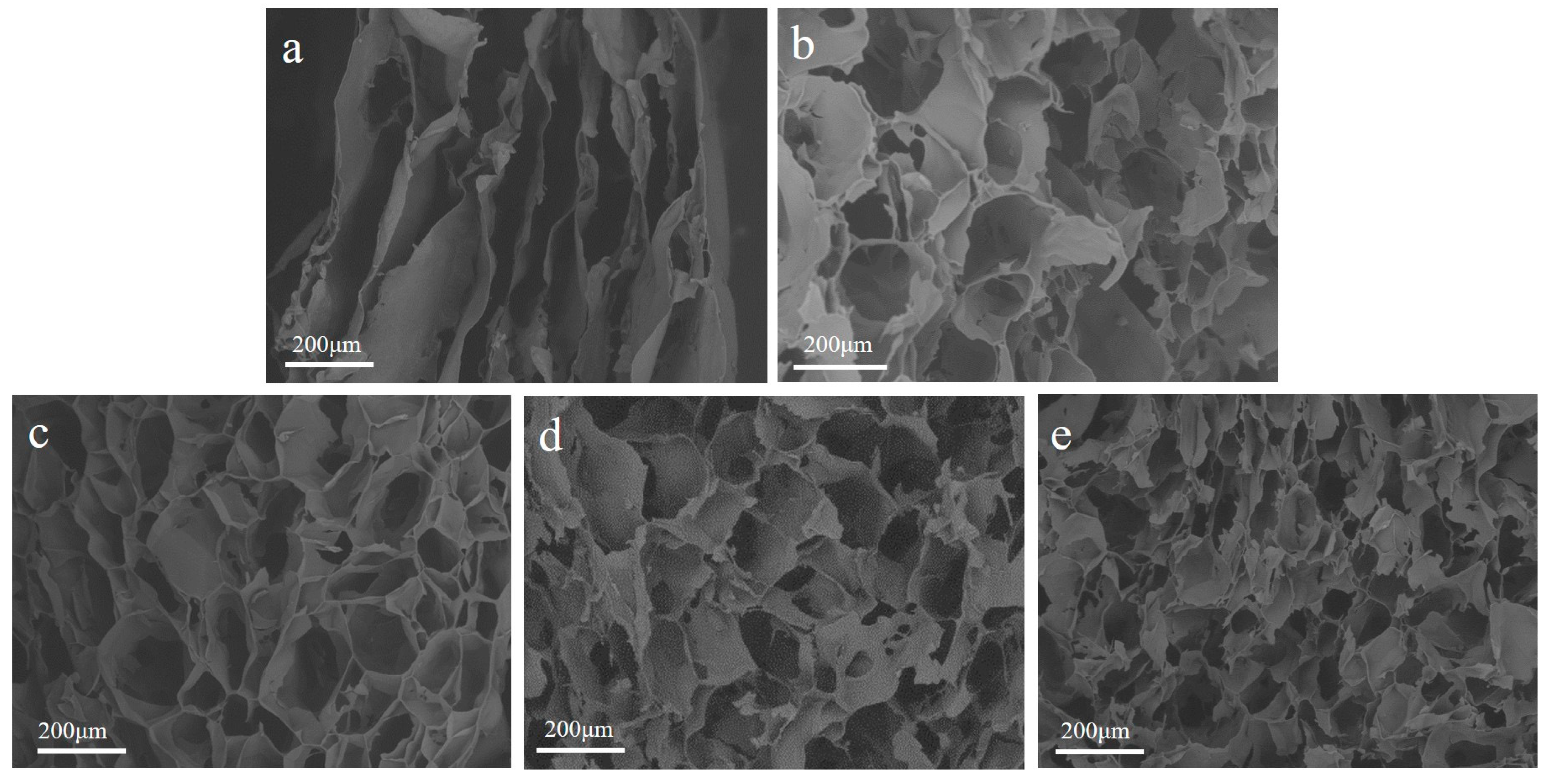
2.1.3. SEM Surface Morphology Characterization
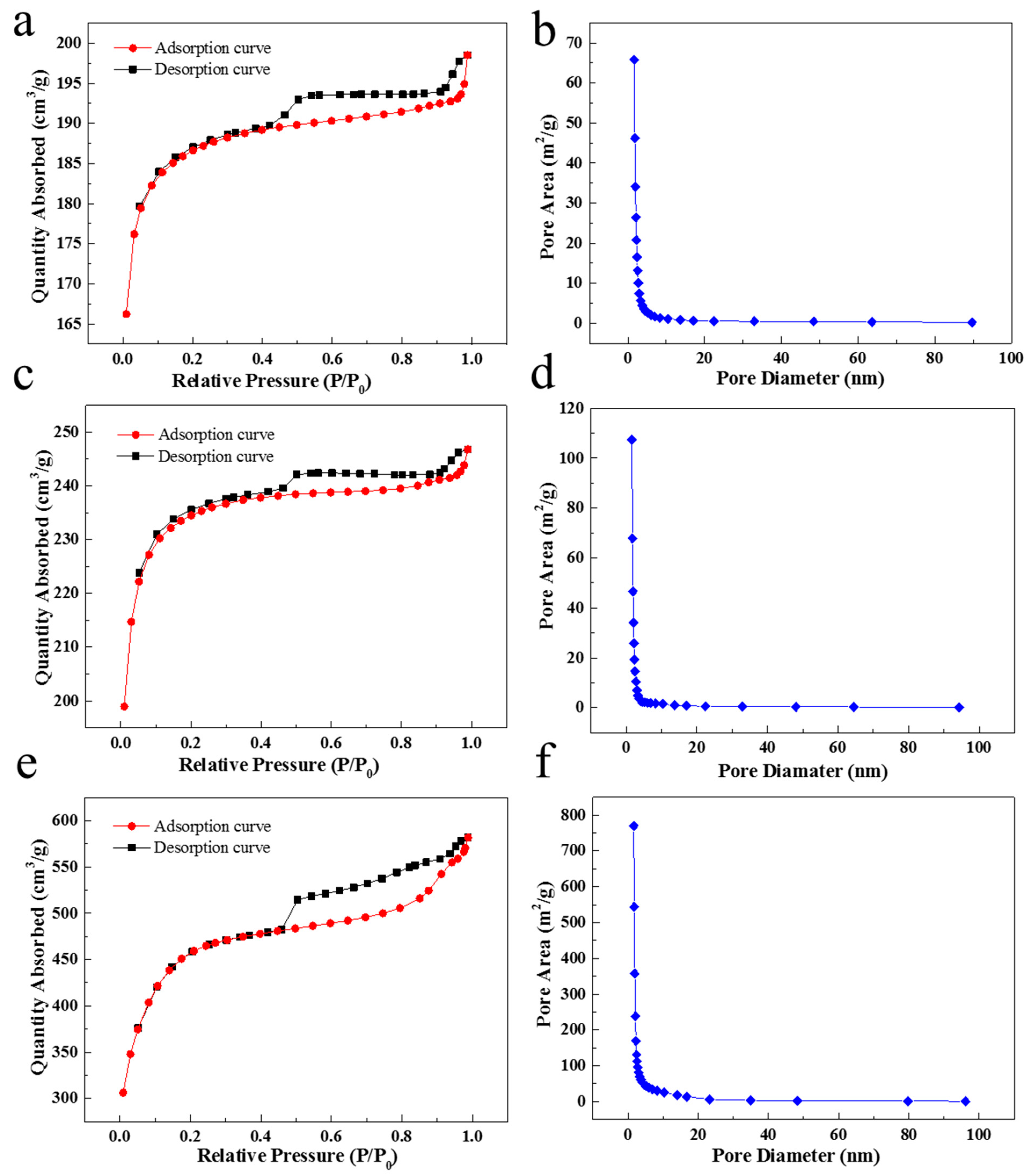
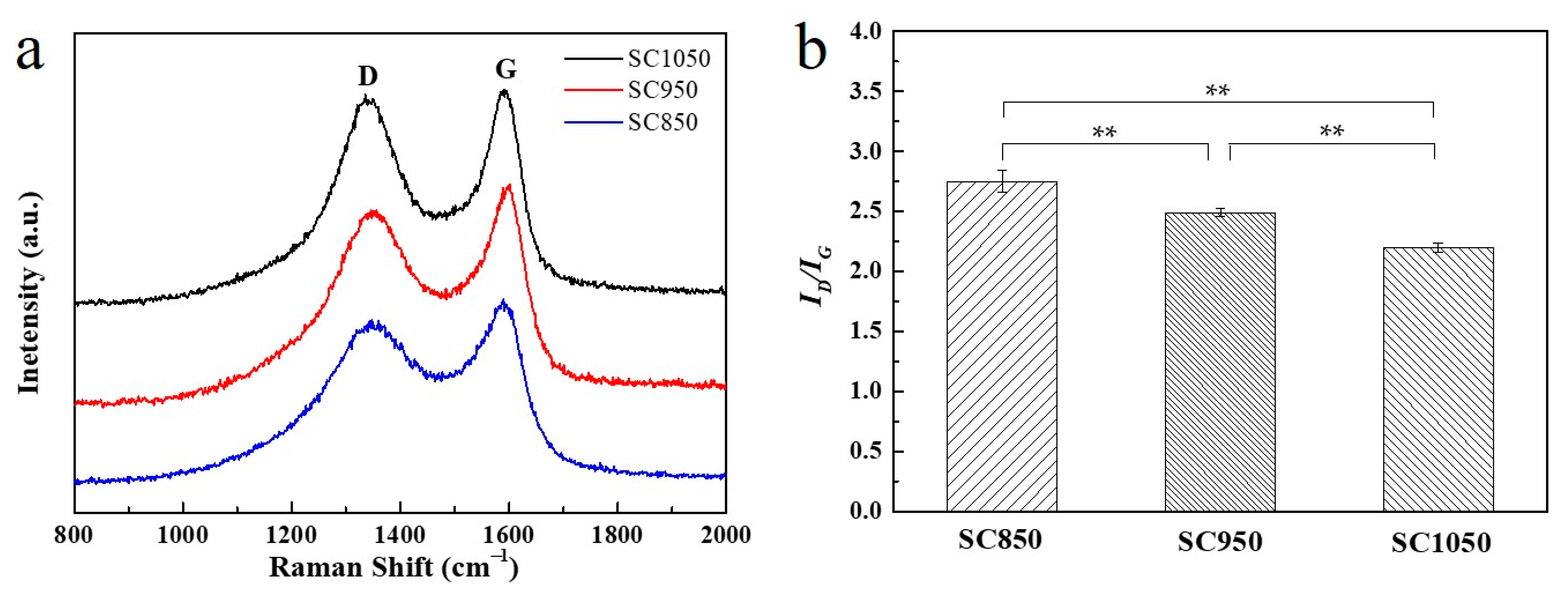
2.1.4. The Effect of Carbonization Temperature on Pore Development and Graphitization
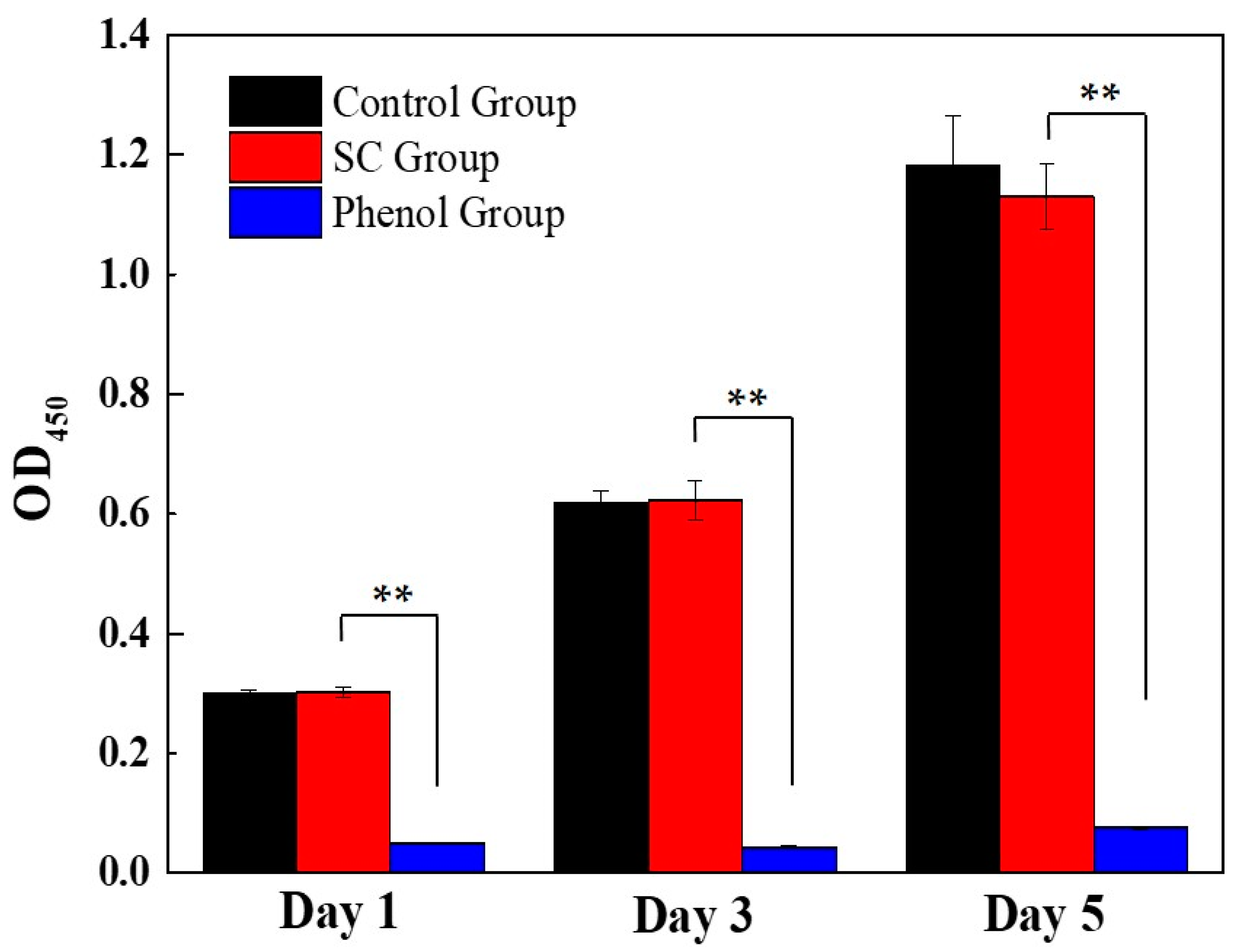
2.1.5. Cytotoxicity Evaluation
2.2. Dye Adsorption Experiments
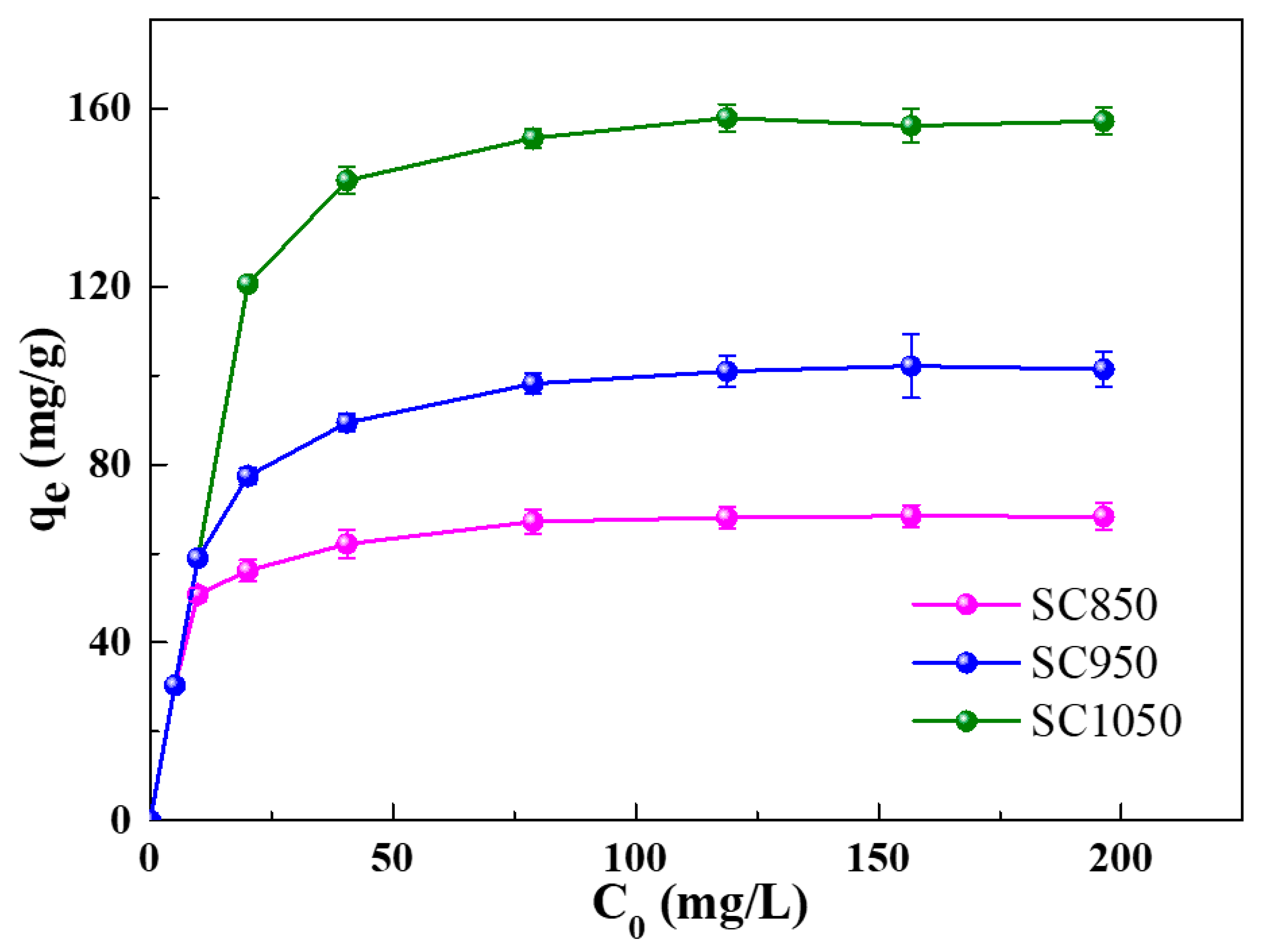
2.2.1. Effect of MB Initial Concentration
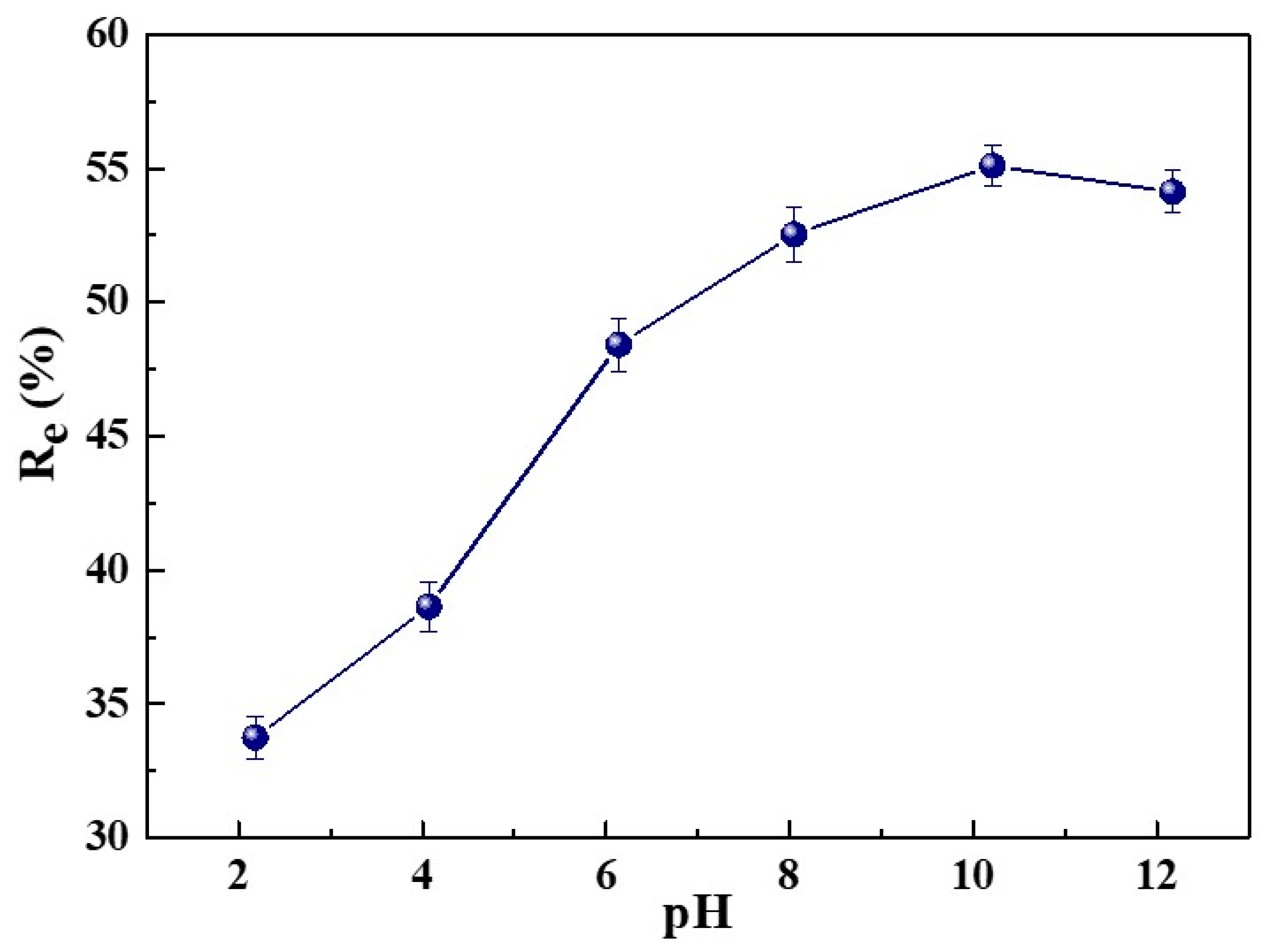
2.2.2. Effect of Solution Initial pH
2.2.3. The Effect of Adsorption Temperature and the Study for Adsorption Thermodynamics
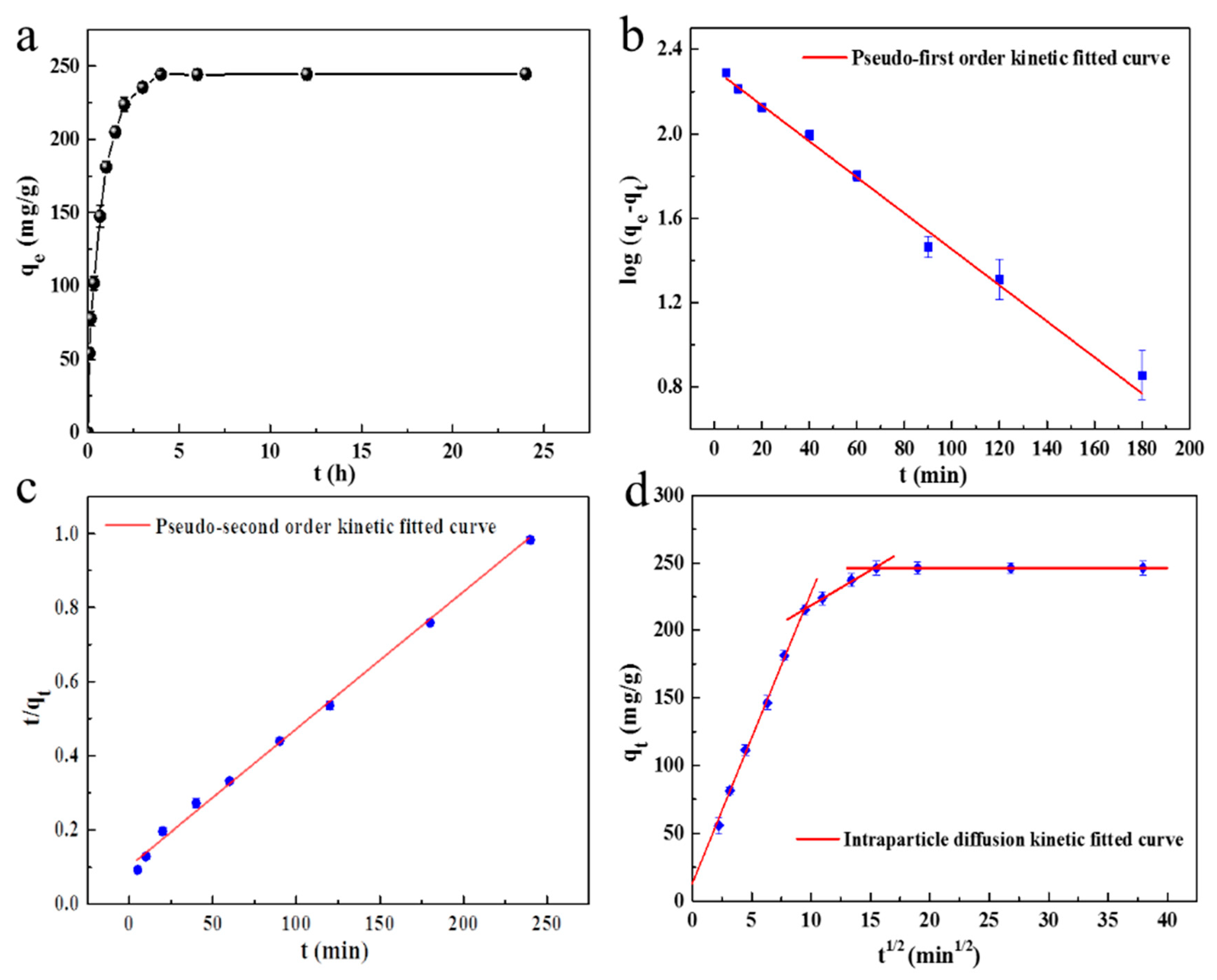
2.2.4. The Effect of Adsorption Time and the Kinetics of Adsorption
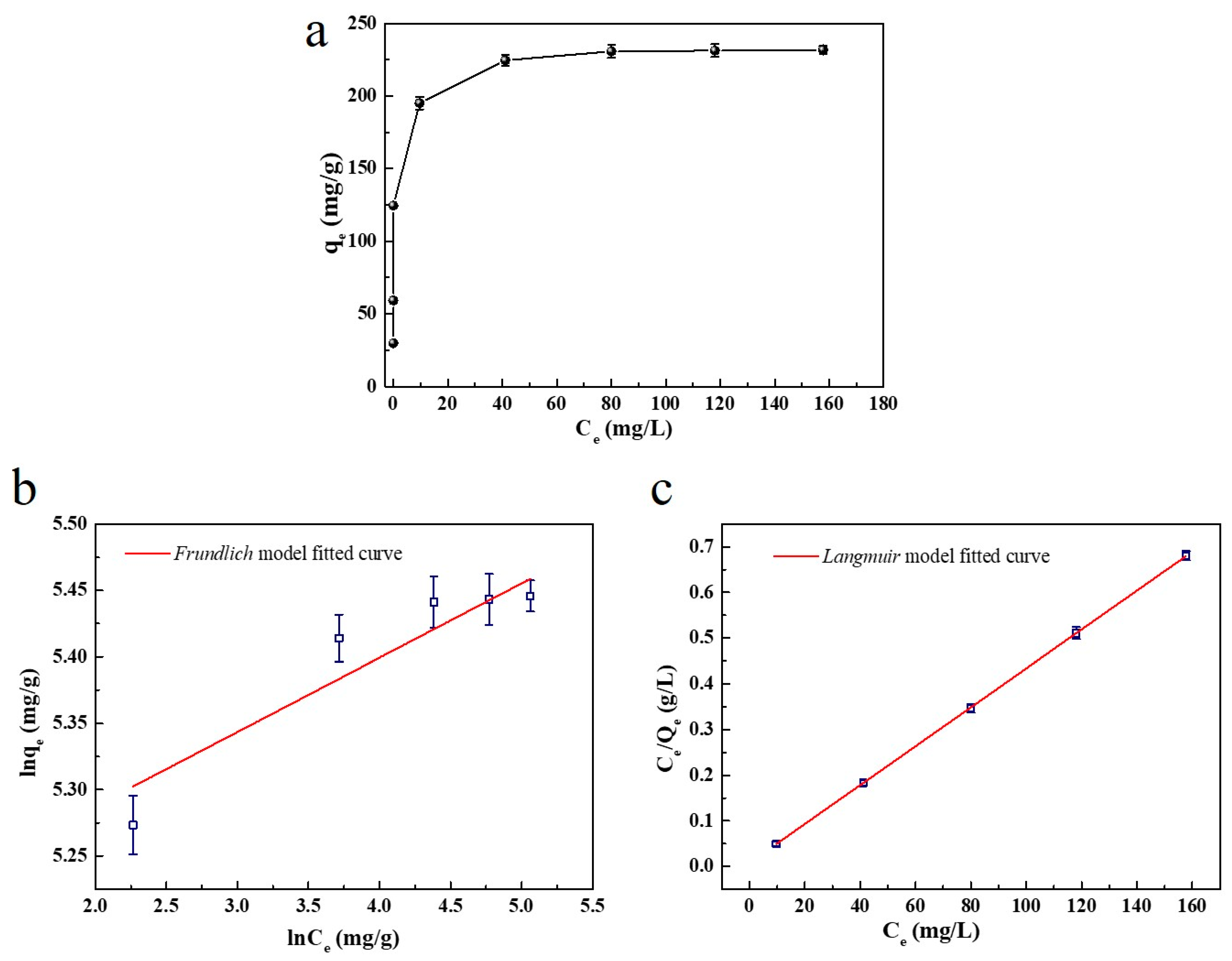
2.2.5. Study of Adsorption Isotherms
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Sericin Derived Carbon (SC)
3.2.1. Fabrication of SS/CMCS Hydrogel
3.2.2. Carbonization of the SS/CMCS Hydrogel
3.3. Characterization
3.3.1. Evaluation of SS Content in SS/CMCS Hydrogel
3.3.2. Viscosity Evaluation
3.3.3. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
3.3.4. Thermogravimetric (TGA) Analysis
3.3.5. Scanning Electron Microscopy (SEM) Surface Morphology Characterization
3.3.6. BET-BJH Analyses
3.3.7. Raman Spectrum Measurement
3.3.8. Determination of Zero Point Charge (pHpzc)
3.3.9. Cytotoxicity Assay
3.4. Dye Adsorption Behavior of SC Samples
3.4.1. The Effect of MB Initial Concentration
3.4.2. The Effect of Initial pH
3.4.3. The Effect of Adsorption Temperature and Adsorption Thermodynamics
3.4.4. The Effect of Adsorption Time and Adsorption Kinetics
3.4.5. Adsorption Isotherm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naushad, M.; Alqadami, A.A.; Alothman, Z.A.; Alsohaimi, I.H.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Xiong, J.H.; Guo, S.C.; Zhao, T.Y.; Liang, Y.N.; Chen, G.N. Degradation of methylene blue by intimate coupling photocatalysis and biodegradation with bagasse cellulose composite carrier. Cellulose 2020, 27, 3391–3404. [Google Scholar] [CrossRef]
- Shi, G.B.; Ruan, C.C.; He, S.; Pan, H.J.; Yang, X.B. Zr-based mof @ carboxymethylated filter paper: Insight into construction and methylene blue removal mechanism. Collolds Surf. A Physicochem. Eng. Asp. 2021, 613, 126053. [Google Scholar] [CrossRef]
- Parthipan, P.; Cheng, L.; Rajasekar, A.; Govarthanan, M.; Subramania, A. Biologically reduced graphene oxide as green and easily available photocatalyst for degradation of organic dyes. Environ. Res. 2021, 196, 110983. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.U.; Le, H.S.; Hoang, H.Y.; Tran, V.A.; Le, V.T. Natural core-shell structure activated carbon beads derived from litsea glutinosa seeds for removal of methylene blue: Facile preparation, characterization, and adsorption properties. Environ. Res. 2020, 198, 110481. [Google Scholar] [CrossRef]
- Aramwit, P.; Napavichayanun, S.; Pienpinijtham, P.; Rasmi, Y. Antibiofilm activity and cytotoxicity of silk sericin against streptococcus mutans bacteria in biofilm: An in vitro study. J. Wound Care 2020, 29, S25–S35. [Google Scholar] [CrossRef]
- Saha, J.; Mondal, M.I.H.; Sheikh, M.R.K.; Habib, M.A. Extraction, structural and functional properties of silk sericin biopolymer from bombyx mori silk cocoon waste. J. Text. Sci. Eng. 2019, 9, 1000390. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2011, 30, 217–224. [Google Scholar] [CrossRef]
- Lanzutti, A.; Lekka, M.; De Leitenburg, C.; Fedrizzi, L. Effect of pulse current on wear behavior of Ni matrix micro-and nano-SiC composite coatings at room and elevated temperature. Tribol. Int. 2018, 132, 50–61. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Koreshkova, A.A. Electrochemical deposition and properties of composite coatings consisting of zinc and carbon nanotubes. Russ. J. Appl. Chem. 2015, 88, 272–274. [Google Scholar] [CrossRef]
- Gyawali, G.; Joshi, B.; Tripathi, K.; Lee, S.W. Effect of ultrasonic nanocrystal surface modification on properties of electrodeposited Ni and Ni-SiC composite coatings. J. Mater. Eng. Perform. 2017, 26, 4462–4469. [Google Scholar] [CrossRef]
- Zou, Y.; Zhong, Y.; Li, H.; Ding, F.; Shi, X. Electrodeposition of polysaccharide and protein hydrogels for biomedical applications. Curr. Med. Chem. 2020, 27, 2610–2630. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Liang, J.; Ji, Y.; Fang, H. Preparation of silk fibroin/carboxymethyl chitosan hydrogel under low voltage as a wound dressing. Int. J. Mol. Sci. 2021, 22, 7610. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, F.F.; Kiparissides, C. Prediction of viscoelastic properties of enzymatically crosslinkable tyramine–modified hyaluronic acid solutions using a dynamic monte carlo kinetic approach. Int. J. Mol. Sci. 2021, 22, 7317. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Flor, S.D.I.; Kozlowska, J. From supramolecular hydrogels to multifunctional carriers for biologically active substances. Int. J. Mol. Sci. 2021, 22, 7402. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Zhang, X.M.; Pan, Y.J.; Li, S.K.; Xing, L.; Niu, X. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 2020, 164, 2204–2214. [Google Scholar] [CrossRef]
- Hong, Y.; Jin, H.J.; Kwak, H.W. Nitrogen-rich magnetic bio-activated carbon from sericin: A fast removable and easily separable superadsorbent for anionic dye removal. Macromol. Res. 2020, 28, 1–11. [Google Scholar] [CrossRef]
- Kwak, H.W.; Hong, Y.; Lee, M.E.; Jin, H.J. Sericin-derived activated carbon-loaded alginate bead: An effective and recyclable natural polymer-based adsorbent for methylene blue removal. Int. J. Biol. Macromol. 2018, 120, 906–914. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Isotherm, kinetics, thermodynamics studies and effects of carbonization temperature on adsorption of indigo carmine (ic) dye using c. Odorata biochar. Chem. Data Collect. 2021, 33, 100673. [Google Scholar] [CrossRef]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Kalliola, S.; Repo, E.; Srivastava, V.; Zhao, F.P.; Sillanpaa, M. Carboxymethyl chitosan and its hydrophobically modified derivative as pH-switchable emulsifiers. Langmuir 2018, 34, 2800–2806. [Google Scholar] [CrossRef] [Green Version]
- Morandim-Giannetti, A.; Wecchi, P.; Silvério, P.; Carlstron, R.; Bersanetti, P.A. Attainment and characterization of carboxymethyl chitosan hydrogels by enzymatic cross-linking. J. Therm. Anal. Calorim. 2019, 138, 3635–3643. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhao, Y.Y.; He, X.B.; Fang, A.; Zhang, G.Z. A sterile self-assembled sericin hydrogel via a simple two-step process. Polym. Test. 2019, 80, 106016. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Su, H.S.; Wang, L.; Zhang, J.L. Relationship between hydrogen bond and viscosity for a series of pyridinium ionic liquids: Molecular dynamics and quantum chemistry. J. Mol. Liq. 2018, 255, 176–184. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W.; Tao, K.X.; Song, Y.; Wang, L. Sustained local release of ngf from a chitosan-sericin composite scaffold for treating chronic nerve compression. ACS Appl. Mater. Interfaces 2017, 9, 3432–3444. [Google Scholar] [CrossRef]
- Song, J.H.; Wang, H.R.; Yang, Y.Q.; Xiao, Z.H.; Zhang, X. Nanogels of carboxymethyl chitosan and lysozyme encapsulated amorphous calcium phosphate to occlude dentinal tubules. J. Mater. Sci. Mater. Med. 2018, 29, 84. [Google Scholar] [CrossRef]
- Vaghani, S.S.; Patel, M.M.; Satish, C.S.; Patel, K.M.; Jivani, N.P. Synthesis and characterization of carboxymethyl chitosan hydrogel: Application as site specific delivery for lercanidipine hydrochloride. Bull. Mater. Sci. 2012, 35, 1133–1142. [Google Scholar] [CrossRef]
- Dara, P.K.; Mahadevan, R.; Digita, P.A.; Visnuvinayagam, S.; Anandan, R. Synthesis and biochemical characterization of silver nanoparticles grafted chitosan (Chi-Ag-NPs): In vitro studies on antioxidant and antibacterial applications. SN Appl. Sci. 2020, 2, 665. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Płanecka, A. Surface properties of thin films based on the mixtures of chitosan and silk fibroin. J. Mol. Liq. 2013, 186, 157–162. [Google Scholar] [CrossRef]
- Amit, T.A.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for high-value applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar] [CrossRef]
- Wang, S.H.; Hwang, Y.K.; Choi, S.W.; Yuan, X.Z.; Chang, F.C. Developing self-activated lignosulfonate-based porous carbon material for ethylene adsorption. J. Taiwan Inst. Chem. Eng. 2020, 115, 315–320. [Google Scholar] [CrossRef]
- Stirling, R.J.; Snape, C.E.; Meredith, W. The impact of hydrothermal carbonisation on the char reactivity of biomass. Fuel Process. Technol. 2018, 177, 152–158. [Google Scholar] [CrossRef]
- Abbasi, Z.; Shamsaei, E.; Leong, S.K.; Ladewig, B.; Wang, H.T. Effect of carbonization temperature on adsorption property of zif-8 derived nanoporous carbon for water treatment. Microporous Mesoporous Mater. 2016, 236, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.W.; Xiong, Z.; Meng, X.; Zhu, H.S.; Xia, Y.Z. Broad-spectrum adsorption property of chondrus crispus activated carbon for ionic and solvent dyes. Water Air Soil Pollut. 2020, 231, 64. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.H.; Dong, W.P.; Zhang, L.I.; Wang, W.L. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017, 7, 12437. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Zhao, C.W.; Chen, D.; Yang, W. Ammonium-functionalized hollow polymer particles as a ph-responsive adsorbent for selective removal of acid dye. ACS Appl. Mater. Interfaces 2016, 8, 16690–16698. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Z.M.; Yang, C.X.; Ning, J.L.; Li, G.Y. Novel magnetic graphene oxide decorated with persimmon tannins for efficient adsorption of malachite green from aqueous solutions. Collolds Surf. A Physicochem. Eng. Asp. 2019, 566, 48–57. [Google Scholar] [CrossRef]
- Ma, T.; Chang, P.R.; Zheng, P.; Zhao, F.; Ma, X. Fabrication of ultra-light graphene-based gels and their adsorption of methylene blue. Chem. Eng. J. 2014, 240, 595–600. [Google Scholar] [CrossRef]
- Altenor, S.; Carene, B.; Emmanuel, E.; Lambert, J.; Ehrhardt, J.J.; Gaspard, S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J. Hazard. Mater. 2009, 165, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, M.; Asaduzzaman, M.; Kabir, M.S.; Mostofa, M.G.; Miyazawa, T. Chemical and structural evaluation of activated carbon prepared from jute sticks for brilliant green dye removal from aqueous solution. J. Hazard. Mater. 2010, 174, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, W.; Qiu, J.; Li, J.; Zhou, W.; Fang, Y.; Zhang, S.; Li, X. Enhanced photocatalytic degradation and adsorption of methylene blue via TiO2 nanocrystals supported on graphene-like bamboo charcoal. Appl. Surf. Sci. 2015, 358, 425–435. [Google Scholar] [CrossRef]
- Adamčíková, L.; Pavlíková, K.; Ševčík, P. The decay of methylene blue in alkaline solution. React. Kinet. Catal. Lett. 2000, 69, 91–94. [Google Scholar] [CrossRef]
- Rashid, R.A.; Jawad, A.H.; Ishak, M.a.M.; Kasim, N.N. Koh-activated carbon developed from biomass waste: Adsorption equilibrium, kinetic and thermodynamic studies for methylene blue uptake. Desalination Water Treat. 2016, 57, 1–11. [Google Scholar] [CrossRef]
- Arumugam, T.K.; Krishnamoorthy, P.; Rajagopalan, N.R.; Nanthini, S.; Vasudevan, D. Removal of malachite green from aqueous solutions using a modified chitosan composite. Int. J. Biol. Macromol. 2019, 128, 655–664. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Azeez, M.A.; Ndungu, P. Theoretical and experimental adsorption studies of phenol and crystal violet dye on carbon nanotube functionalized with deep eutectic solvent. J. Mol. Liq. 2019, 288, 110895. [Google Scholar] [CrossRef]
- Zhao, S.M.; Zhan, Y.Q.; Wan, X.Y.; He, S.J.; Zhang, G.Y. Selective and efficient adsorption of anionic dyes by core/shell magnetic mwcnts nano-hybrid constructed through facial polydopamine tailored graft polymerization: Insight of adsorption mechanism, kinetic, isotherm and thermodynamic study. J. Mol. Liq. 2020, 319, 114289. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.N.; Chang, C.Y.; Cheng, G.Z.; Chu, B. Hydrogen-bond-induced inclusion complex in aqueous cellulose/lioh/urea solution at low temperature. ChemPhysChem 2007, 3, 1572–1579. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.Y.; Zhang, M.C.; Jian, M.Q.; Zhang, Y.Y. Feeding single-walled carbon nanotubes or graphene to silkworms for reinforced silk fibers. Nano Lett. 2016, 16, 6695–6700. [Google Scholar] [CrossRef] [PubMed]
- Salinas, O.; Ma, X.H.; Wang, Y.G.; Han, Y.; Pinnau, I. Carbon molecular sieve membrane from a microporous spirobisindane-based polyimide precursor with enhanced ethylene/ethane mixed-gas selectivity. RSC Adv. 2017, 7, 3265–3272. [Google Scholar] [CrossRef] [Green Version]
- Giusto, L.a.R.; Pissetti, F.L.; Castro, T.S.; Magalhaes, F. Preparation of activated carbon from sugarcane bagasse soot and methylene blue adsorption. Water Air Soil Pollut. 2017, 228, 249. [Google Scholar] [CrossRef]
- Tang, Z.M.; Fang, J.; Zhang, Y.H.; Zhang, Y.; Yang, Y.; Huang, X.L.; Wang, Y.Y.; Zhang, D.D.; Ni, N.; Liu, F.; et al. Mussel-inspired injectable hydrogel and its counterpart for actuating proliferation and neuronal differentiation of retinal progenitor cells. Biomaterials 2018, 194, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.I.; Luo, C.N.; Li, X.J.; Lu, F.G.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef]
- Mansha, M.; Waheed, A.; Ahmad, T.; Kazi, I.W.; Ullah, N. Synthesis of a novel polysuccinimide based resin for the ultrahigh removal of anionic azo dyes from aqueous solution. Environ. Res. 2020, 184, 109337. [Google Scholar] [CrossRef]
- Ouachtak, H.; Guerdaoui, A.E.; Haounati, R.; Akhouairi, S.; Taha, M.L. Highly efficient and fast batch adsorption of orange g dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 2021, 335, 116560. [Google Scholar] [CrossRef]
- Mu, W.N.; Bao, D.C.; Chang, C.; Lian, F. Adsorption of methyl blue by maize waste based biochar: Adsorption kinetics and isotherms. J. Phys.: Conf. Ser. 2020, 1622, 012081. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.Y.; Pei, Z.G.; Li, C.M.; Zhang, S.Z. Adsorption kinetics, isotherms and thermodynamics of cr(iii) on graphene oxide. Collolds Surf. A Physicochem. Eng. Asp. 2014, 457, 100–106. [Google Scholar] [CrossRef]
- Boukoussa, B.; Mokhtar, A.; Guerdaoui, A.E.; Hachemoui, M.; Hamacha, R. Adsorption behavior of cationic dye on mesoporous silica sba-15 carried by calcium alginate beads: Experimental and molecular dynamics study. J. Mol. Liq. 2021, 333, 115976. [Google Scholar] [CrossRef]

| Samples | Viscosity (mpa·s) |
|---|---|
| 8% SS solution | 1.82 ± 0.02 |
| 1% CMCS solution | 5.04 ± 0.02 |
| 4% SS/0.5% CMCS mixture | 3.72 ± 0.01 |
| Samples | Pore Size (μm) |
|---|---|
| SS/CMCS |  |
| SC850 | |
| SC950 | |
| SC1050 |
| Samples | BET Surface Area (cm2/g) | BJH Average Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| SC850 | 566.07 | 2.94 | 0.05 |
| SC950 | 713.66 | 2.79 | 0.07 |
| SC1050 | 1494.96 | 2.63 | 0.54 |
| T (K) | ∆H (kJ/mol) | ||
|---|---|---|---|
| 288.15 | −0.98 ± 0.53 | −5.01 ± 0.53 | −3.39 ± 1.74 |
| 298.15 | −1.02 ± 0.74 | ||
| 308.15 | −1.05 ± 0.75 | ||
| 318.15 | −1.08 ± 0.77 |
| Sample | Pseudo-First Order | Pseudo-Second Order | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| q1 (mg/g) | k1 (1/min) | R2 (%) | q2 (mg/g) | k2 (g·mg−1·min−1) | R2 (%) | ki (mg·g−1·min−1/2) | Ci | R2 (%) | |
| SC1050 | 202.21 | 0.02 | 98.63 | 269.54 | 1.32 × 10−4 | 99.73 | 21.49 | 12.85 | 99.81 |
| Sample | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| RL | KL (L/mg) | R2 (%) | n | KF (L/mg) | R2 (%) | |
| SC1050 | 0.19 | 0.52 | 99.99 | 17.89 | 1.64 | 81.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhang, X.; Chen, Z.; Xiao, Y.; Li, S.; Gu, J.; Hu, H.; Cheng, G. Silk Sericin Enrichment through Electrodeposition and Carbonous Materials for the Removal of Methylene Blue from Aqueous Solution. Int. J. Mol. Sci. 2022, 23, 1668. https://doi.org/10.3390/ijms23031668
Ji Y, Zhang X, Chen Z, Xiao Y, Li S, Gu J, Hu H, Cheng G. Silk Sericin Enrichment through Electrodeposition and Carbonous Materials for the Removal of Methylene Blue from Aqueous Solution. International Journal of Molecular Sciences. 2022; 23(3):1668. https://doi.org/10.3390/ijms23031668
Chicago/Turabian StyleJi, Yansong, Xiaoning Zhang, Zhenyu Chen, Yuting Xiao, Shiwei Li, Jie Gu, Hongmei Hu, and Guotao Cheng. 2022. "Silk Sericin Enrichment through Electrodeposition and Carbonous Materials for the Removal of Methylene Blue from Aqueous Solution" International Journal of Molecular Sciences 23, no. 3: 1668. https://doi.org/10.3390/ijms23031668
APA StyleJi, Y., Zhang, X., Chen, Z., Xiao, Y., Li, S., Gu, J., Hu, H., & Cheng, G. (2022). Silk Sericin Enrichment through Electrodeposition and Carbonous Materials for the Removal of Methylene Blue from Aqueous Solution. International Journal of Molecular Sciences, 23(3), 1668. https://doi.org/10.3390/ijms23031668






