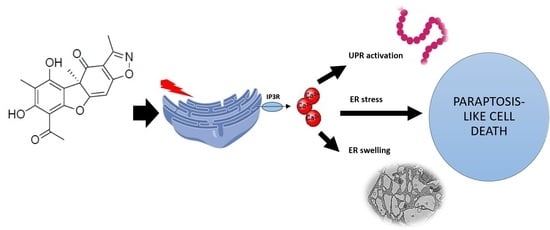
The Isoxazole Derivative of Usnic Acid Induces an ER Stress Response in Breast Cancer Cells That Leads to Paraptosis-like Cell Death
Abstract
:1. Introduction
2. Results
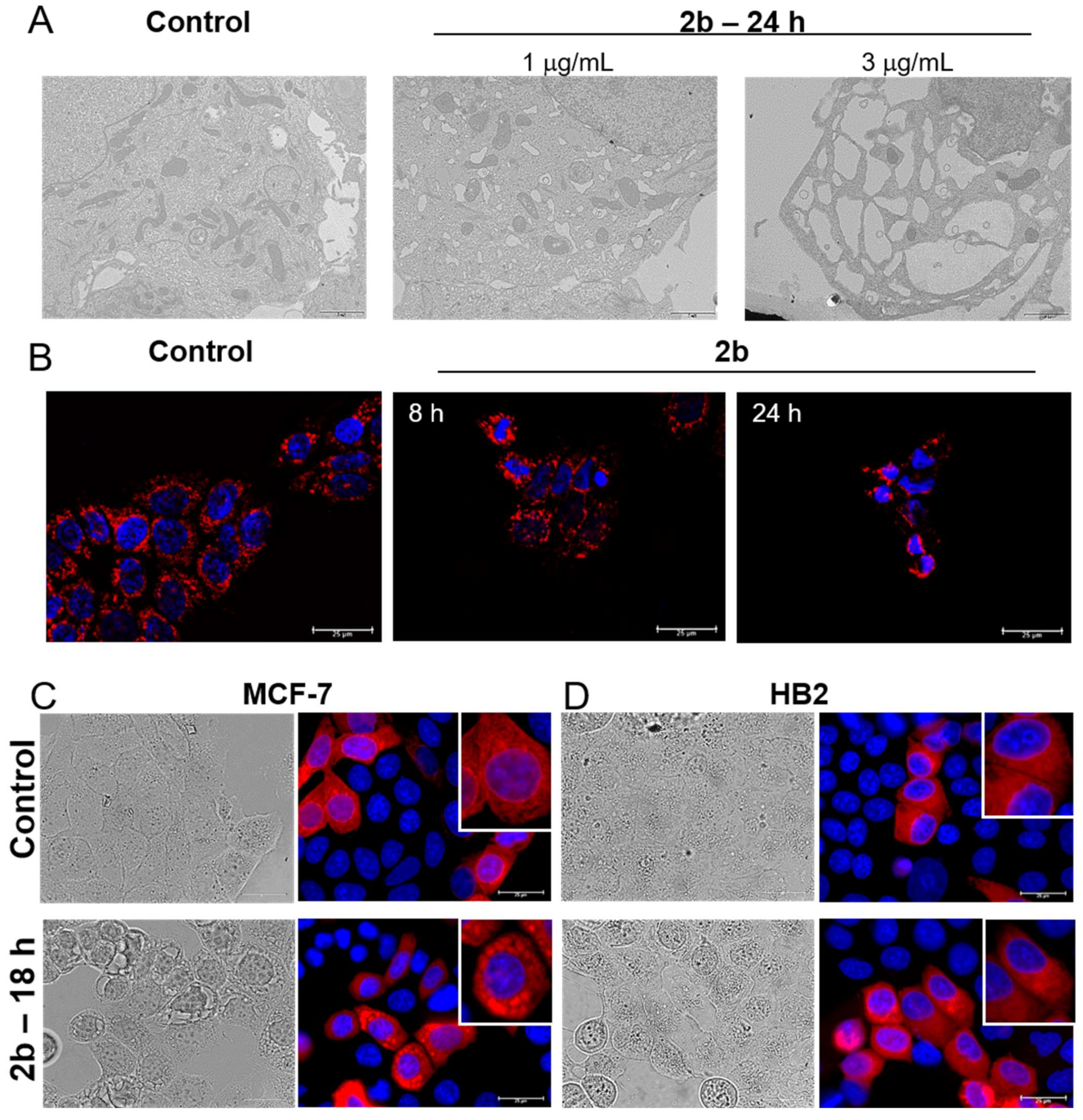
2.1. UA Isoxazole Derivative-Induced Vacuoles in Cancer Cells Are of Endoplasmic Reticulum Origin
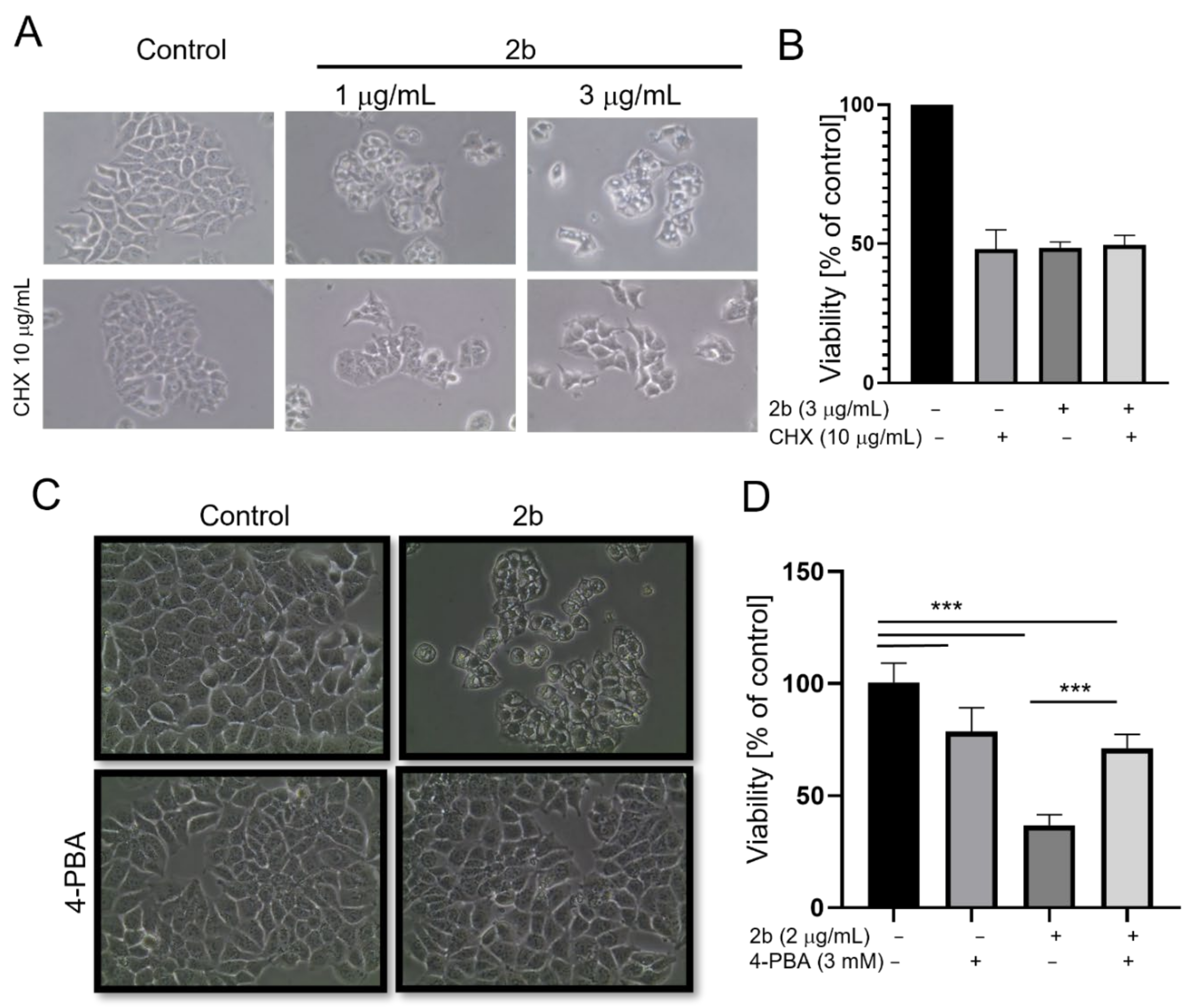
2.2. Derivative 2b Induces ER Stress Which Leads to Cell Death
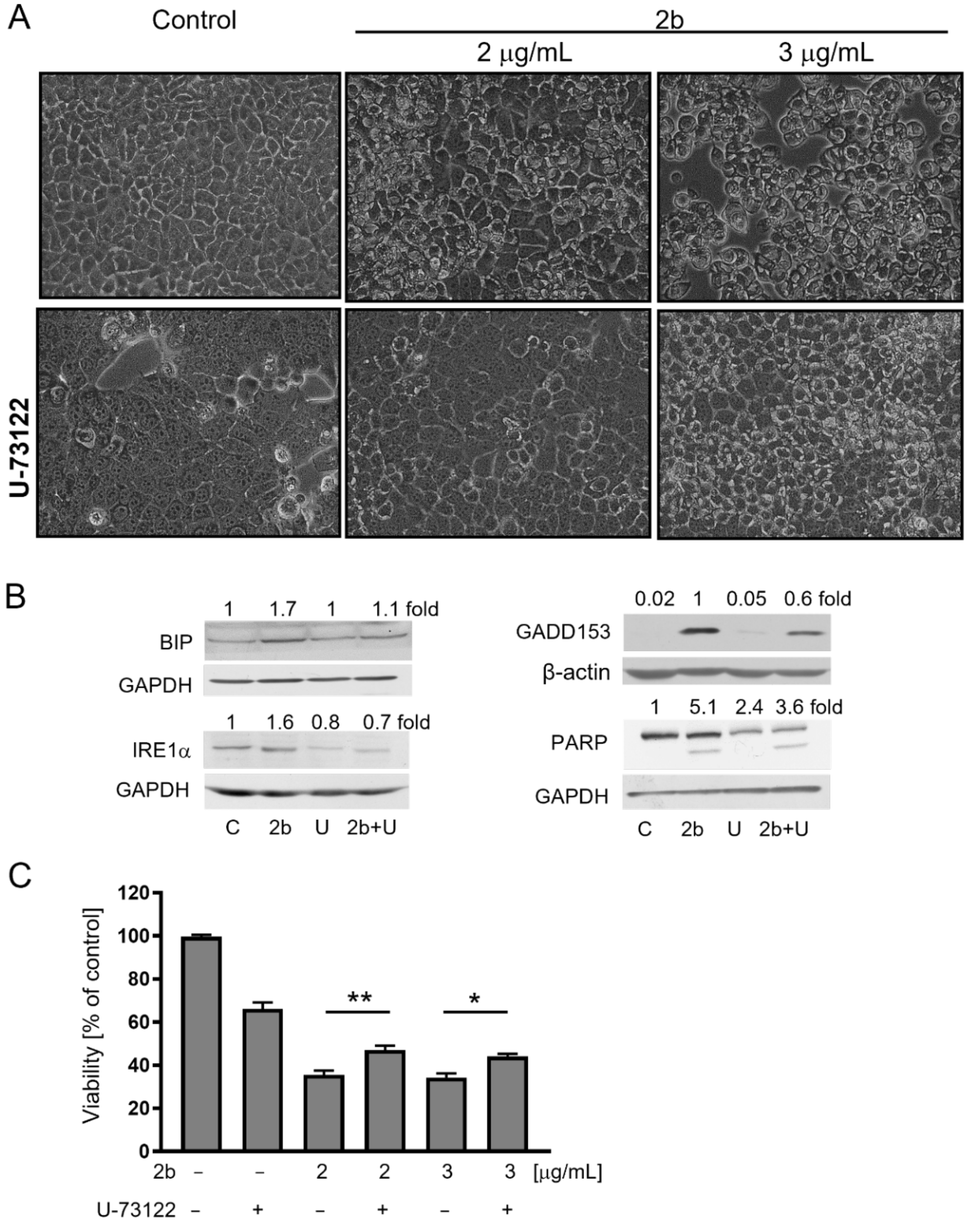
2.3. 2b-Induced ER Stress Is Accompanied by the Release of Calcium Ions
2.4. Inhibition of IP3 Synthesis by PLC Protects against Vacuolization and Cell Death Induced by 2b
2.5. Orally Administered 2b Retards MCF-7 Xenografts Growth in Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture Conditions
4.3. Cell Viability Assay
4.4. Immunofluorescence and Light Microscopy
4.5. Transmission Electron Microscopy (TEM)
4.6. RNA Library Preparation and High-Throughput Sequencing
4.7. Read Mapping and Gene Expression Profiling
4.8. Gene Ontology and KEGG Pathway Enrichment Analyses
4.9. Immunoblotting
4.10. Measurement of Ca2+ Level
4.11. Animal Studies
4.12. Histopathology
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, H.J.; Chambers, J.E.; Liniker, E.; Marciniak, S.J. Endoplasmic reticulum stress in malignancy. Cancer Cell 2014, 25, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, U.; Ozcan, L.; Yilmaz, E.; Duvel, K.; Sahin, M.; Manning, B.D.; Hotamisligil, G.S. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 2008, 29, 541–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. Chop induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [Green Version]
- Riha, R.; Gupta-Saraf, P.; Bhanja, P.; Badkul, S.; Saha, S. Stressed out—Therapeutic implications of ER stress related cancer research. Oncomedicine 2017, 2, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Melchior, W.B., Jr.; Guo, L. Endoplasmic reticulum stress in drug- and environmental toxicant-induced liver toxicity. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 83–104. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [Green Version]
- Carafoli, E.; Krebs, J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, G.; Brostrom, M.A.; Brostrom, C.O. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J. Biol. Chem. 1992, 267, 3932–3939. [Google Scholar] [CrossRef]
- Ingolfsdottir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Araujo, A.A.; de Melo, M.G.; Rabelo, T.K.; Nunes, P.S.; Santos, S.L.; Serafini, M.R.; Santos, M.R.; Quintans-Junior, L.J.; Gelain, D.P. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef]
- Galanty, A.; Pasko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef] [Green Version]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 2. Effects on higher organisms. Molecular and physicochemical aspects. Rus. J. Bioorg. Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Backorova, M.; Jendzelovsky, R.; Kello, M.; Backor, M.; Mikes, J.; Fedorocko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. In Vitro 2012, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Luo, J.; Liu, M.; Yi, Z. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated Akt and ERK1/2 signaling pathways. Angiogenesis 2012, 15, 421–432. [Google Scholar] [CrossRef]
- Sanchez, W.; Maple, J.T.; Burgart, L.J.; Kamath, P.S. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin. Proc. 2006, 81, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Neff, G.W.; Reddy, K.R.; Durazo, F.A.; Meyer, D.; Marrero, R.; Kaplowitz, N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J. Hepatol. 2004, 41, 1062–1064. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Matsumaru, K.; Rettori, D.; Kaplowitz, N. Usnic acid-induced necrosis of cultured mouse hepatocytes: Inhibition of mitochondrial function and oxidative stress. Biochem. Pharmacol. 2004, 67, 439–451. [Google Scholar] [CrossRef]
- da Silva Santos, N.P.; Nascimento, S.C.; Wanderley, M.S.; Pontes-Filho, N.T.; da Silva, J.F.; de Castro, C.M.; Pereira, E.C.; da Silva, N.H.; Honda, N.K.; Santos-Magalhaes, N.S. Nanoencapsulation of usnic acid: An attempt to improve antitumour activity and reduce hepatotoxicity. Eur. J. Pharm. Biopharm. 2006, 64, 154–160. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Kopperman, H.L. L-usnic acid: Tumor inhibitor isolated from lichens. Experientia 1975, 31, 625. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Uehara, Y.; Beisler, J.A. Usnic acid derivatives as potential antineoplastic agents. J. Med. Chem. 1979, 22, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Guzow-Krzemińska, B.; Guzow, K.; Herman-Antosiewicz, A. Usnic acid derivatives as cytotoxic agents against cancer cells and the mechanisms of their activity. Curr. Pharmacol. Rep. 2019, 5, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Pyrczak-Felczykowska, A.; Narlawar, R.; Pawlik, A.; Guzow-Krzeminska, B.; Artymiuk, D.; Hac, A.; Rys, K.; Rendina, L.M.; Reekie, T.A.; Herman-Antosiewicz, A.; et al. Synthesis of usnic acid derivatives and evaluation of their antiproliferative activity against cancer cells. J. Nat. Prod. 2019, 82, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.S.; Ayaub, E.A.; Zhou, W.; Yum, V.; Dickhout, J.G.; Ask, K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Wu, Y.; Shi, Q.; Yan, H.; Mei, N.; Tolleson, W.H.; Guo, L. Endoplasmic reticulum stress and store-operated calcium entry contribute to usnic acid-induced toxicity in hepatic cells. Toxicol. Sci. 2015, 146, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Mikoshiba, K. Role of IP3 receptor signaling in cell functions and diseases. Adv. Bio. Regul. 2015, 57, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, L.F.; Hirdes, W.; Suh, B.C.; Hilgemann, D.W.; Mackie, K.; Hille, B. Phospholipase C in living cells: Activation, inhibition, Ca2+ requirement, and regulation of m current. J. Gen. Physiol. 2005, 126, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Gregory, R.B.; Rychkov, G.; Barritt, G.J. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem. J. 2001, 354, 285–290. [Google Scholar] [CrossRef]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; De Smedt, H.; Missiaen, L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef]
- Marks, A.R. Intracellular calcium-release channels: Regulators of cell life and death. Am. J. Physiol. 1997, 272, H597–H605. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, C.; Mikoshiba, K. IP3 receptor mutations and brain diseases in human and rodents. J. Neurochem. 2017, 141, 790–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.G. Inositol phospholipids-specific phospholipase C: Interaction of the gamma 1 isoform with tyrosine kinase. Trends Biochem. Sci. 1991, 16, 297–301. [Google Scholar] [CrossRef]
- Kiviluoto, S.; Vervliet, T.; Ivanova, H.; Decuypere, J.P.; De Smedt, H.; Missiaen, L.; Bultynck, G.; Parys, J.B. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim. Biophys. Acta 2013, 1833, 1612–1624. [Google Scholar] [CrossRef] [Green Version]
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 receptor-mediated calcium signaling and its role in autophagy in cancer. Front. Oncol. 2017, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Wasik, A.M.; Almestrand, S.; Wang, X.; Hultenby, K.; Dackland, A.L.; Andersson, P.; Kimby, E.; Christensson, B.; Sander, B. Win55,212-2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell. Death Dis. 2011, 2, e225. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandio, S.; Poksay, K.; de Belle, I.; Lafuente, M.J.; Liu, B.; Nasir, J.; Bredesen, D.E. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004, 11, 1066–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandio, S.; Poksay, K.S.; Schilling, B.; Crippen, D.; Gibson, B.W.; Bredesen, D.E. Identification of new modulators and protein alterations in non-apoptotic programmed cell death. J. Cell. Biochem. 2010, 111, 1401–1412. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K.; Nakanishi, H.; Maeda, H.; Nishioku, T.; Hashimoto, K.; Liou, S.Y.; Akamine, A.; Yamamoto, K. A predominant apoptotic death pathway of neuronal PC12 cells induced by activated microglia is displaced by a non-apoptotic death pathway following blockage of caspase-3-dependent cascade. J. Biol. Chem. 1999, 274, 15725–15731. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Lee, D.M.; Seo, M.J.; Lee, H.J.; Choi, K.S. Intracellular Ca2+ imbalance critically contributes to paraptosis. Front. Cell Dev. Biol. 2020, 8, 607844. [Google Scholar] [CrossRef]
- Zhang, F.J.; Yang, J.Y.; Mou, Y.H.; Sun, B.S.; Wang, J.M.; Wu, C.F. Oligomer procyanidins from grape seeds induce a paraptosis-like programmed cell death in human glioblastoma U-87 cells. Pharm. Biol. 2010, 48, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.Y.; Shim, M.J.; Lee, D.M.; Lee, A.R.; Kim, M.A.; Yoon, M.J.; Kwon, M.R.; Lee, H.I.; Seo, M.J.; Choi, Y.W.; et al. Loperamide overcomes the resistance of colon cancer cells to bortezomib by inducing chop-mediated paraptosis-like cell death. Biochem. Pharmacol. 2019, 162, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Prins, D.; Michalak, M. Organellar calcium buffers. Cold Spring Harb. Perspect. Biol. 2011, 3, a004069. [Google Scholar] [CrossRef] [Green Version]
- Mimnaugh, E.G.; Xu, W.; Vos, M.; Yuan, X.; Neckers, L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol. Cancer Res. 2006, 4, 667–681. [Google Scholar] [CrossRef] [Green Version]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.A.; Kim, I.Y.; Lee, A.R.; Yoon, M.J.; Cho, H.; Lee, J.S.; Choi, K.S. Ca2+ influx-mediated dilation of the endoplasmic reticulum and c-FLIPL downregulation trigger CDDO-Me-induced apoptosis in breast cancer cells. Oncotarget 2015, 6, 21173–21192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; De, S.; Das, S.; Mukherjee, S.; Sengupta Bandyopadhyay, S. Withaferin A induces ROS-mediated paraptosis in human breast cancer cell-lines MCF-7 and MDA-MB-231. PLoS ONE 2016, 11, e0168488. [Google Scholar] [CrossRef]
- Wang, W.B.; Feng, L.X.; Yue, Q.X.; Wu, W.Y.; Guan, S.H.; Jiang, B.H.; Yang, M.; Liu, X.; Guo, D.A. Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J. Cell Physiol. 2012, 227, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Star: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Brouwer, C. Pathview: An r/bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G., Jr.; Brouwer, C. Pathview web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef] [PubMed]
- Ubezio, P. Beyond the T/C ratio: Old and new anticancer activity scores in vivo. Cancer Manag. Res. 2019, 11, 8529–8538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
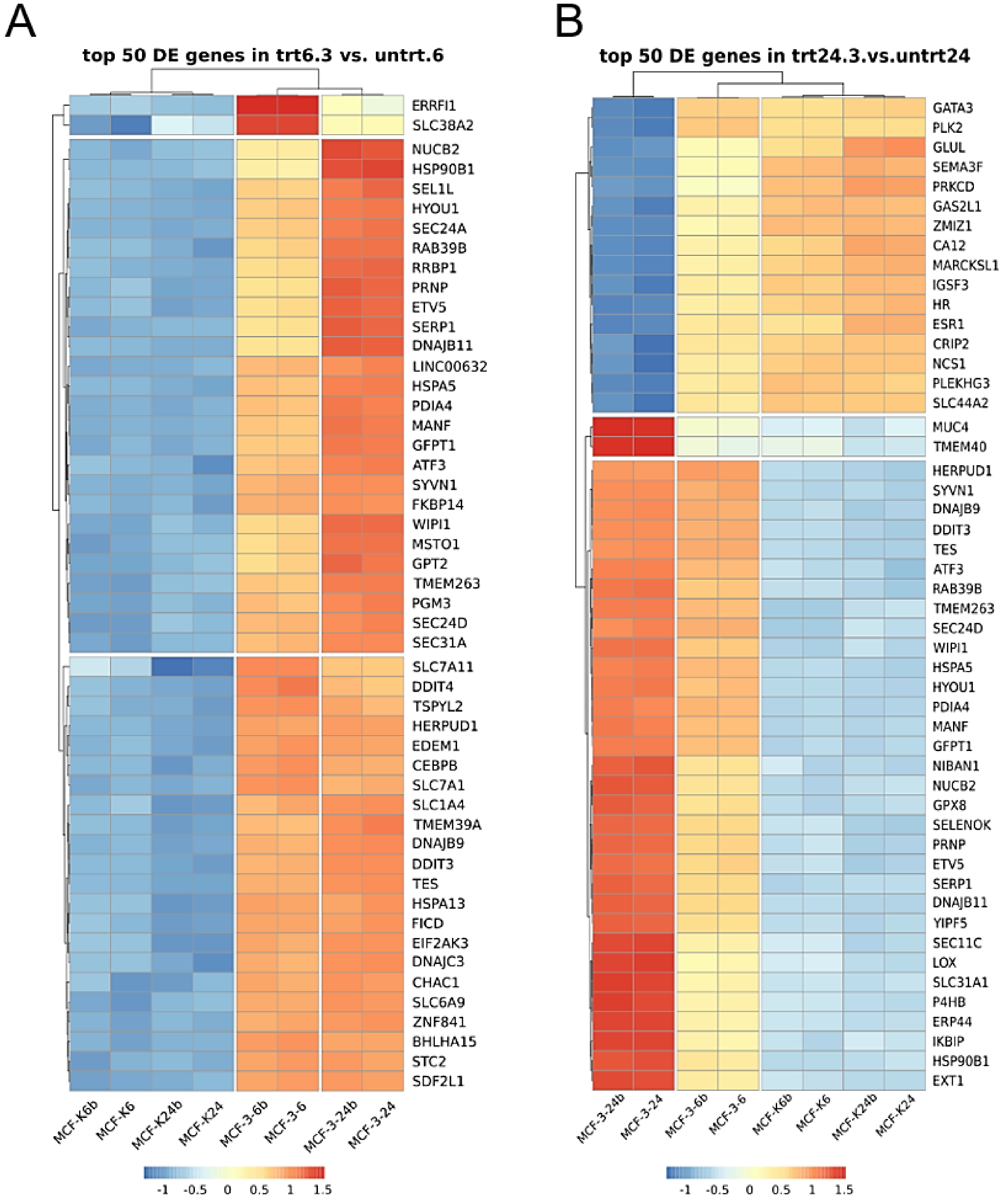
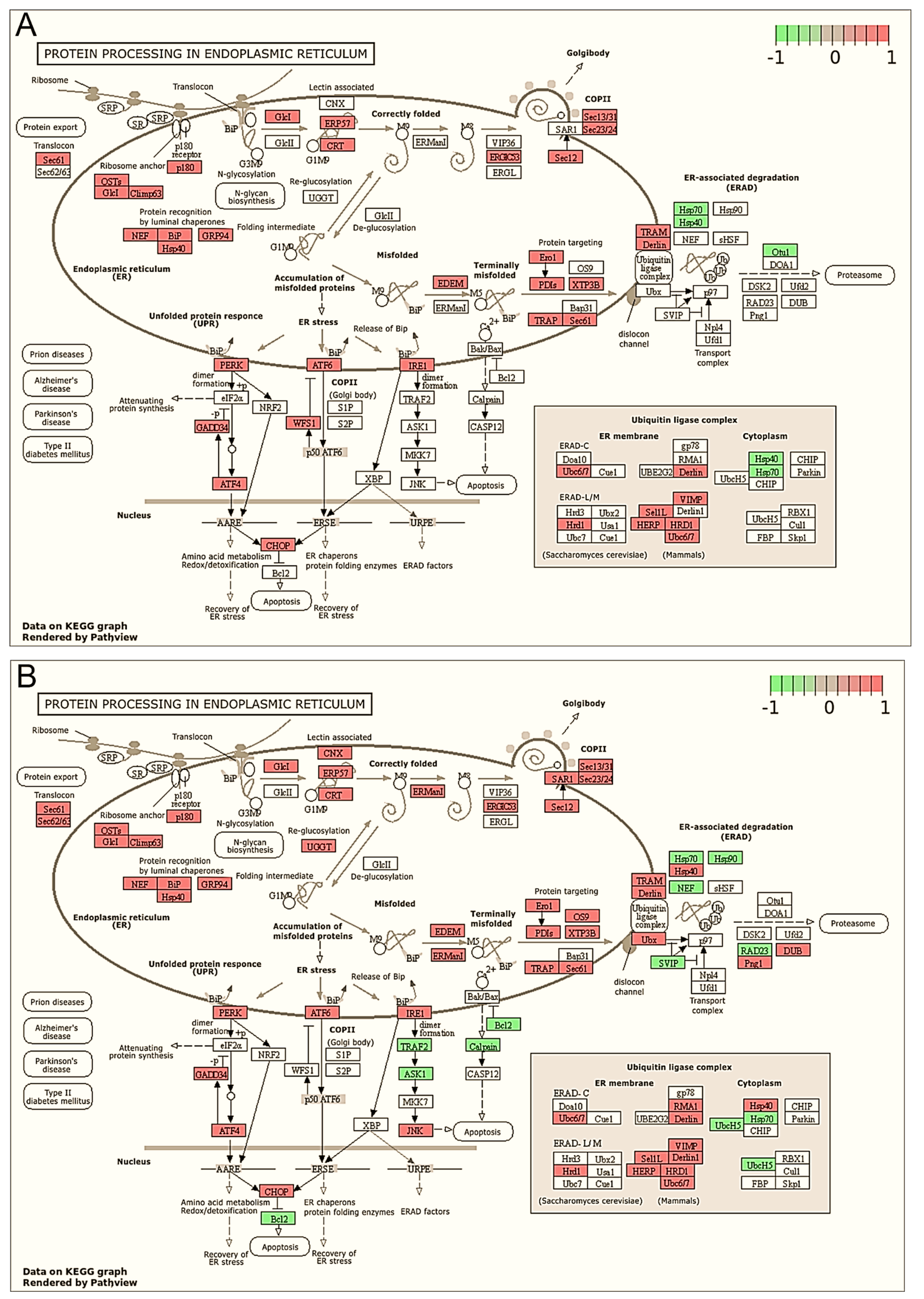



| No. | Pathway | N | Up | Down | P.Up | P.Down |
|---|---|---|---|---|---|---|
| hsa04141 | Protein processing in endoplasmic reticulum | 151 | 87 | 20 | 8.22 × 10−19 | 1 |
| hsa04015 | Rap1 signalling pathway | 139 | 23 | 61 | 0.99 | 4.86 × 10−9 |
| hsa04390 | Hippo signalling pathway | 123 | 15 | 53 | 1 | 1.02 × 10−7 |
| hsa04360 | Axon guidance | 146 | 14 | 60 | 1 | 1.19 × 10−7 |
| hsa03060 | Protein export | 23 | 16 | 0 | 5.09 × 10−6 | 1 |
| hsa04550 | Signalling pathways regulating the pluripotency of stem cells | 104 | 19 | 43 | 0.94 | 5.88 × 10−6 |
| hsa03008 | Ribosome biogenesis in eukaryotes | 70 | 33 | 8 | 2.09 × 10−5 | 0.99 |
| hsa00510 | N-glycan biosynthesis | 45 | 24 | 8 | 2.17 × 10−5 | 0.80 |
| hsa04750 | Inflammatory mediator regulation of TRP channels | 67 | 9 | 30 | 0.99 | 2.39 × 10−5 |
| hsa00970 | Aminoacyl-tRNA biosynthesis | 44 | 22 | 3 | 1.68 × 10−4 | 1 |
| hsa05200 | Pathways in cancer | 368 | 78 | 109 | 0.92 | 2.60 × 10−4 |
| hsa00513 | Various types of N-glycan biosynthesis | 35 | 18 | 3 | 4.19 × 10−4 | 0.99 |
| hsa04916 | Melanogenesis | 71 | 12 | 28 | 0.95 | 6.02 × 10−4 |
| hsa05418 | Fluid shear stress and atherosclerosis | 100 | 24 | 36 | 0.55 | 8.44 × 10−4 |
| hsa04914 | Progesterone-mediated oocyte maturation | 76 | 13 | 29 | 0.95 | 9.11 × 10−4 |
| No. | Pathway | N | Up | Down | P.Up | P.Down |
|---|---|---|---|---|---|---|
| hsa04141 | Protein processing in endoplasmic reticulum | 151 | 109 | 30 | 6.97 × 10−15 | 1 |
| hsa04015 | Rap1 signalling pathway | 139 | 22 | 98 | 1 | 1.64 × 10−10 |
| hsa04810 | Regulation of actin cytoskeleton | 152 | 31 | 101 | 1 | 1.44 × 10−8 |
| hsa04360 | Axon guidance | 146 | 27 | 97 | 1 | 2.80 × 10−8 |
| hsa04390 | Hippo signalling pathway | 123 | 23 | 84 | 1 | 3.54 × 10−8 |
| hsa05168 | Herpes simplex virus 1 infection | 372 | 204 | 111 | 3.96 × 10−8 | 1 |
| hsa05200 | Pathways in cancer | 368 | 108 | 208 | 1 | 5.26 × 10-7 |
| hsa04750 | Inflammatory mediator regulation of TRP channels | 67 | 13 | 47 | 1 | 1.20 × 10−5 |
| hsa05205 | Proteoglycans in cancer | 156 | 37 | 95 | 1 | 1.28 × 10−5 |
| hsa05206 | MicroRNAs in cancer | 141 | 43 | 86 | 1 | 2.98 × 10−5 |
| hsa01100 | Metabolic pathways | 1091 | 385 | 541 | 1 | 4.27 × 10−5 |
| hsa03040 | Spliceosome | 129 | 75 | 32 | 6.43 × 10−5 | 1 |
| hsa05322 | Systemic lupus erythematosus | 47 | 9 | 34 | 1 | 7.18 × 10−5 |
| hsa04611 | Platelet activation | 83 | 18 | 54 | 1 | 7.85 × 10−5 |
| hsa05100 | Bacterial invasion of epithelial cells | 65 | 17 | 44 | 1 | 8.89 × 10−5 |
| hsa05225 | Hepatocellular carcinoma | 138 | 41 | 82 | 1 | 1.62 × 10−4 |
| hsa05412 | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 43 | 9 | 31 | 1 | 1.66 × 10−4 |
| hsa00970 | Aminoacyl-tRNA biosynthesis | 44 | 30 | 9 | 2.52 × 10−4 | 1 |
| hsa04916 | Melanogenesis | 71 | 15 | 46 | 1 | 3.02 × 10−4 |
| hsa04713 | Circadian entrainment | 60 | 10 | 40 | 1 | 3.04 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrczak-Felczykowska, A.; Reekie, T.A.; Jąkalski, M.; Hać, A.; Malinowska, M.; Pawlik, A.; Ryś, K.; Guzow-Krzemińska, B.; Herman-Antosiewicz, A. The Isoxazole Derivative of Usnic Acid Induces an ER Stress Response in Breast Cancer Cells That Leads to Paraptosis-like Cell Death. Int. J. Mol. Sci. 2022, 23, 1802. https://doi.org/10.3390/ijms23031802
Pyrczak-Felczykowska A, Reekie TA, Jąkalski M, Hać A, Malinowska M, Pawlik A, Ryś K, Guzow-Krzemińska B, Herman-Antosiewicz A. The Isoxazole Derivative of Usnic Acid Induces an ER Stress Response in Breast Cancer Cells That Leads to Paraptosis-like Cell Death. International Journal of Molecular Sciences. 2022; 23(3):1802. https://doi.org/10.3390/ijms23031802
Chicago/Turabian StylePyrczak-Felczykowska, Agnieszka, Tristan A. Reekie, Marcin Jąkalski, Aleksandra Hać, Marcelina Malinowska, Anna Pawlik, Kamil Ryś, Beata Guzow-Krzemińska, and Anna Herman-Antosiewicz. 2022. "The Isoxazole Derivative of Usnic Acid Induces an ER Stress Response in Breast Cancer Cells That Leads to Paraptosis-like Cell Death" International Journal of Molecular Sciences 23, no. 3: 1802. https://doi.org/10.3390/ijms23031802