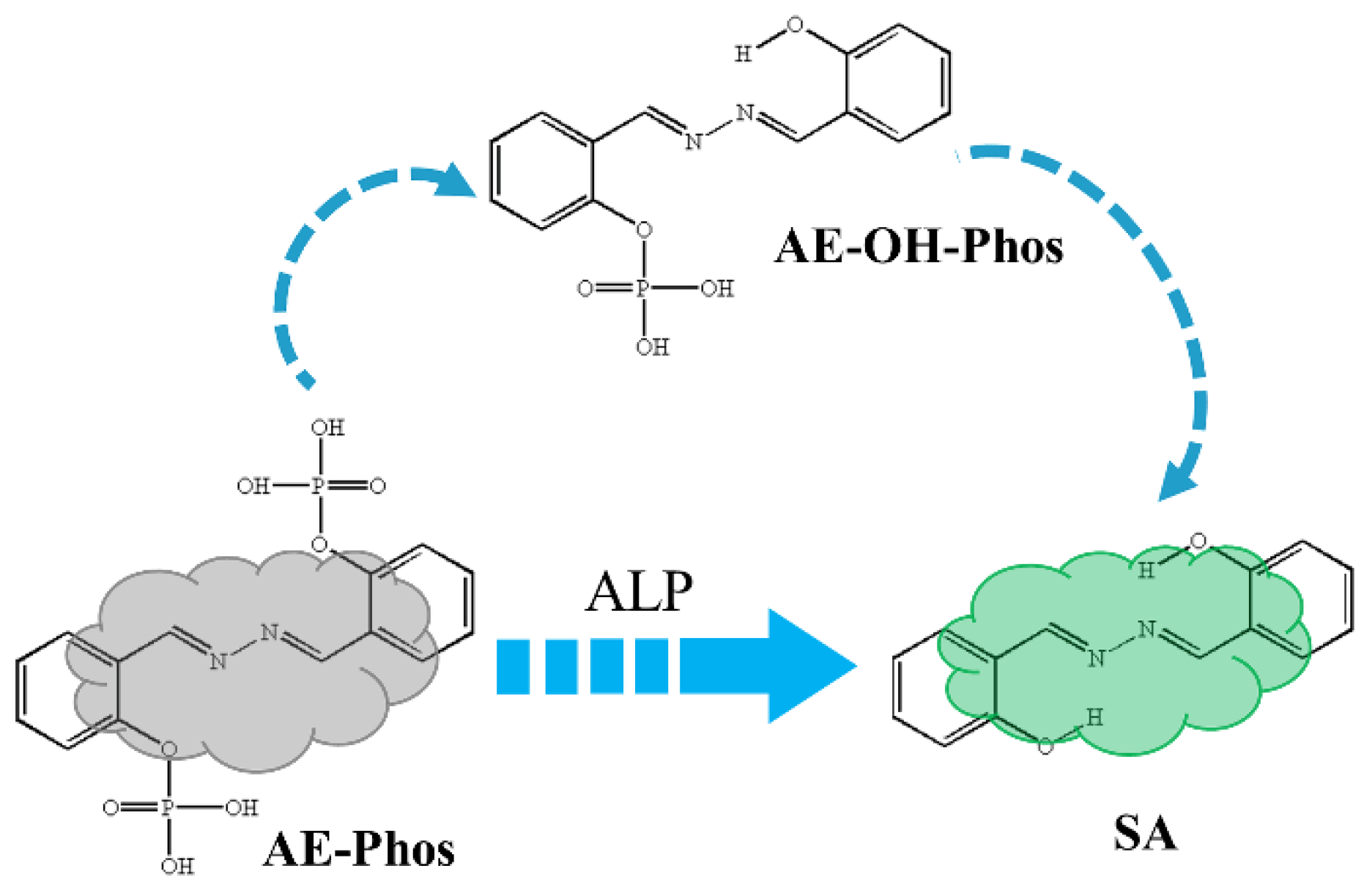
3.1. Intramolecular Hydrogen Bond
By analyzing the change in the strength of intramolecular hydrogen bonds (IHBs) upon photo-excitation, we can judge whether it is permissive for the ESIPT process and thus leads to isomerization. Therefore, the structures of all isomers for AE-OH-PHOs (enol and keto forms) and SA (named SA, SA-SPT, SA-DPT according to the proton transfer) have been optimized using the B3LYP/6-311G(d,p) basis set in the DMSO solvent, which is helpful to compare with the experiment. As shown in
Figure 1, all structures of the probe products were included, and the atomic numbers associated with the hydrogen bonds were marked on the molecular structure of SA. The bond length and bond angle parameters related to molecules AE-OH-PHOs and SA were presented in
Table 1 and
Table 2, respectively.
In general, the longer the bond length (O-H distance) for the proton donor, the shorter the bond length (H…N distance) for the proton acceptor, and the closer the bond angle δ(O-H…N) is to 180°, the more stable the IHB [
54,
55,
56]. For AE-OH-Phos, from the S
0 state to S
1 state (
Table 1), the shortening of the O
2-H
2, the elongation of H
2…N
2, and the decrease of δ(O
2-H
2…N
2) at the enol configuration indicate that the IHB is stronger and its structure is more stable at the S
0 state, which is detrimental to the ESIPT reaction. In addition, the probe product SA had a completely symmetrical planar structure, with the same parameters for the two hydrogen bonds. Taking O
2-H
2…N
2 as an example (
Table 2), the O
2-H
2 length increased from 0.99 Å (S
0) to 1.00 Å (S
1), and the H
2-N
2 diminished from 1.76 Å to 1.70 Å, accompanied by the O
2-H
2-N
2 angle, which enlarged from 146.1° to 148.3°. In addition, for SA-SPT that had undergone a single-step proton transfer process, it should be noted that the O
1-H
1, H
1…N
1 length and O
1-H
1…N
1 angle were separately changed from 0.98 Å, 1.80 Å, and 144.7° (S
0) to 0.99 Å, 1.77 Å, and 146.4° (S
1), respectively. Although there is a trend of hydrogen bond enhancement, based on the slight changes of these structural parameters, we think it is not enough to support us to analyze whether the ESIPT process could occur. Therefore, the previously reported infrared spectra and the RDG scatter plots are used to further analyze the change of hydron bond strength. In addition, for O
2…H
2-N
2 of which SA-SPT had undergone proton transfer, the change trend in the bond length and bond angle parameters indicates that the S
1-state SA-SPT is more stable than the S
0 state. However, the SA-DPT configuration is not stable in the S
0 state. The detailed reasons will be discussed in the potential energy surfaces section.
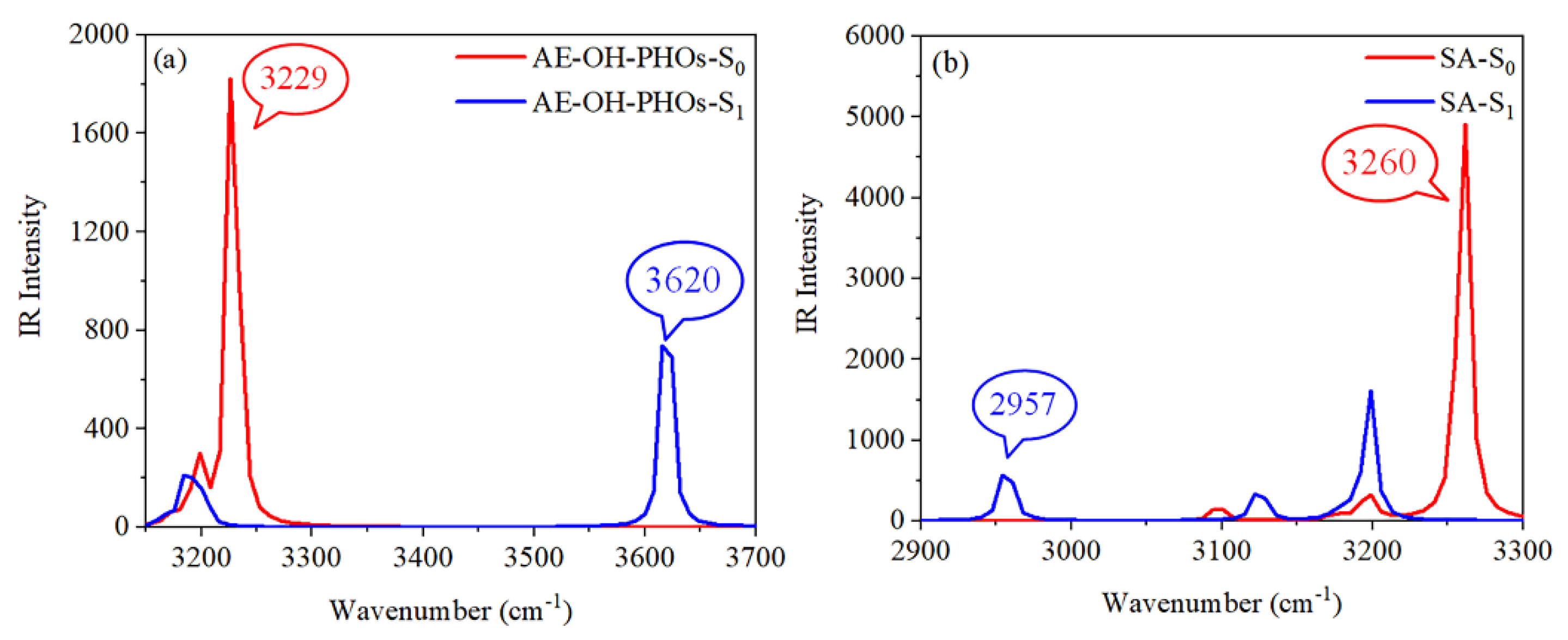
Infrared (IR) spectra is a frequently used method to analyze the change of hydrogen bond; the degree of change for hydrogen bond strength can be judged by comparing the red-shift or blue-shift of hydroxyl stretching vibration peaks in the S
0 and S
1 states. The IR spectra for AE-OH-PHOs and SA are displayed in
Figure 2. The calculated O
2-H
2 stretching vibration frequencies were separately located at 3229 cm
−1 and 3620 cm
−1 at the S
0 and S
1 states, respectively. Such a large blue-shift of 391 cm
−1 demonstrates that the IHB at the S
1 state is weaker than that at the S
0 state, which is unfavorable for the ESIPT process. However, the stretching vibration frequencies of O
1-H
1 and O
2-H
2 for SA both change from 3260 cm
−1 (S
0) to 2957 cm
−1 (S
1), respectively, with a 303 cm
−1 red-shift, which indicates that this is prone to ESIPT reaction. This result further validates our conjecture on the geometric structure.
For the sake of more intuitively showing whether the IHB is formed and the change of IHB strength, the IRI isosurfaces and RDG scatter plots were subjected to further analysis. The meaning of different colors in
Figure 3 are: red, green, and blue represent the steric effect, van der Waals interaction and hydrogen bond, respectively. Additionally, the bluer the IRI surface, the more negative the blue spike of RDG, indicating a stronger IHB. As shown in
Figure 3, the appearance of a blue isosurface between H
2 and N
2 atoms for the intermediate product AE-OH-Phos at the S
0 and S
1 states signifies that IHB interaction exists and its corresponding RDG blue spike was separately near −0.05 a.u. and −0.04 a.u.. This change in value clearly shows that the IHB strength in the S
1 state is weaker, which is in full agreement with the conclusions of the previous structure parameters and IR analysis. Therefore, it can be speculated that the ESIPT process cannot occur in AE-OH-Phos. Furthermore, SA, being a completely symmetric structure, has two IHBs of the same strength with the blue spikes of the S
0 and S
1 states located at −0.05 a.u. < ρ <−0.04 a.u. and −0.06 a.u. < ρ < −0.05 a.u.. The enhancement of IHB is very favorable for the ESIPT process after photoexcitation. However, whether the SA molecule underwent stepwise single proton transfer or concerted double proton transfer requires further analysis.
3.2. ESIPT Process Analysis
To calculate the precise reaction energy barrier for the proton transfer process occurring in SA and AE-OH-Phos at the S
1 state, the transition state (TS) structure of its different paths and the corresponding energies were calculated. The TS structure and energy for SA stepwise single proton transfer (path 1) and simultaneous double proton transfer (path 2), as well as AE-OH-Phos, are shown in
Figure 4. The energy barrier of AE-OH-Phos is up to 13.75 kcal/mol; this indicates that the proton transfer process was forbidden at the S
1 state. In addition, the reaction barrier of single proton transfer is 2.75 kcal/mol and the double proton transfer is 9.01 kcal/mol. This suggests that the SA is more inclined to undergo single proton transfer into the SA-SPT form than the process of double proton transfer. Moreover, the SA-SPT has the lowest energy, which means that its configuration is the most stable.
To be able to investigate the intrinsic mechanism of the ESIPT process and the possible proton transfer for AE-OH-Phos and SA, the potential energy surfaces (PESs) of SA and the potential energy curves (PECs) of AE-OH-Phos at the S
0 and S
1 states were scanned separately based on the optimized structure. The obtained PECs of AE-OH-Phos were as a function of O
2-H
2 distance and ranged from 0.99 Å(initial length)→1.99 Å and 0.97 Å(initial length)→1.97 Å at the S
0 and S
1 states, respectively. As depicted in
Figure 5, the reverse potential barrier of 1.93 kcal/mol is lower than the forward potential barrier (7.16 kcal/mol), indicating that it is difficult for the proton transfer process to occur at the S
0 state. This is consistent with the previously obtained result that weakening of the S
1-state hydrogen bond is unfavorable for the ESIPT reaction to proceed.
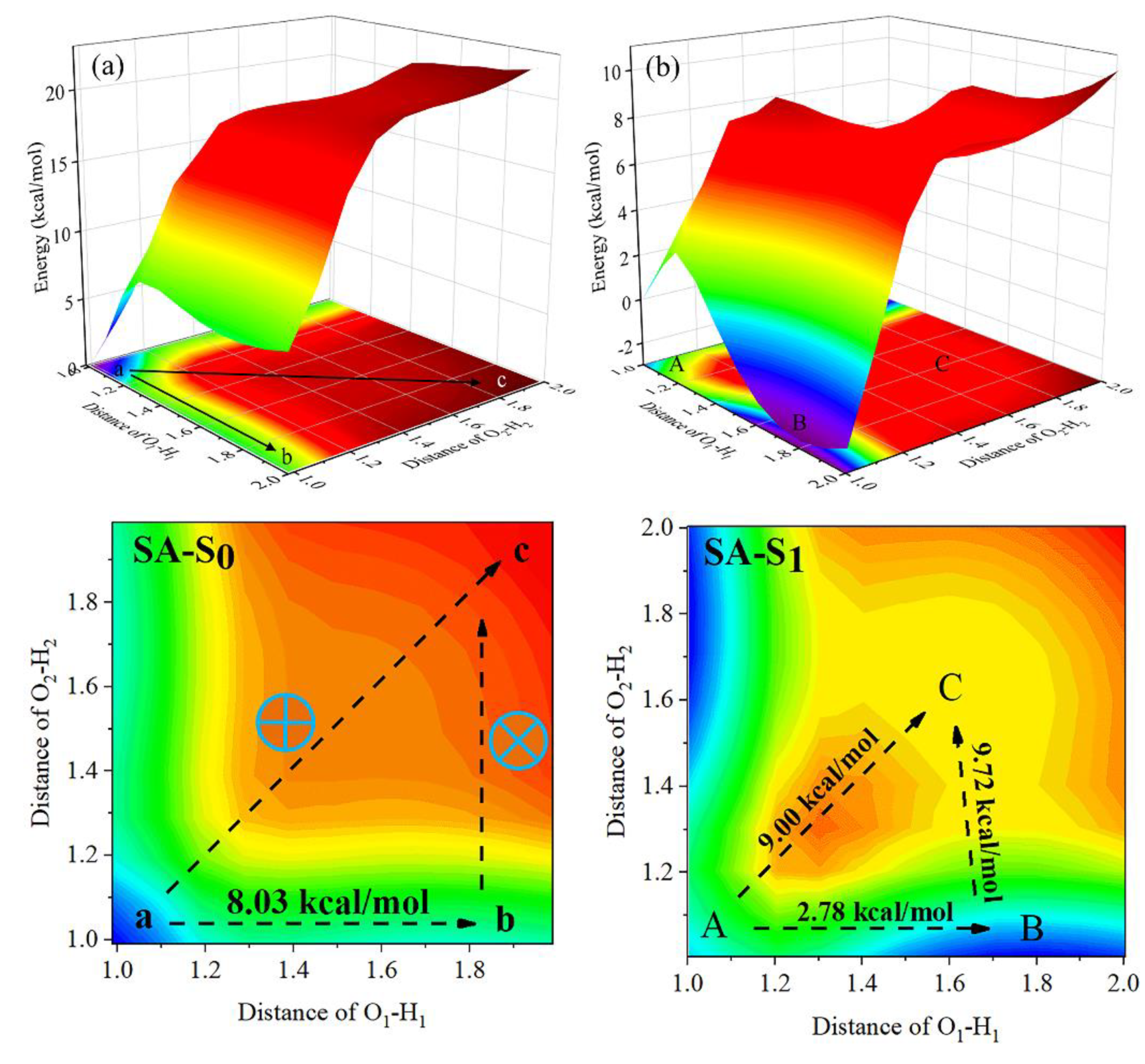
The PESs of SA were obtained by extending the distance of O
1-H
1 and O
2-H
2, as shown in
Figure 6. It is worth noting that the PESs of both the S
0 and S
1 states are symmetric, and the lowest energy points in the figures are marked for the convenience of analysis. In
Figure 6a, the coordinates of these corresponding points are a (0.99 Å, 0.99 Å), b (1.99 Å, 0.99 Å), and c (1.99 Å, 1.99 Å). In addition, the energy of all points on the S
0-state PES is higher than that of point a; this means that the structure of point a is the most stable at the S
0 state. Furthermore, the potential energy of paths a→c and b→c increases monotonically, which indicates that these two paths do not actually exist at the S
0 state. Moreover, the forward energy barrier of a→c is 8.03 kcal/mol, while the reverse energy barrier is only 0.31 kcal/mol, so it can be concluded that the isomerization process cannot happen at the S
0 state either.
Interestingly, the calculated potential energies of the minima points are in order of E
C > E
A > E
B at the S
1 state, as present in
Figure 6b. The coordinates of these corresponding points are A (1.00 Å, 1.00 Å), B (1.80 Å, 1.00 Å) and C (1.60 Å, 1.60 Å). It is clear that point B is the most stable configuration at the S
1 state, and the path from A to B only needs to cross the energy barrier of 2.78 kcal/mol, which means that the S
1-state SA molecule could easily undergo a single proton transfer process to form SA-SPT. Nevertheless, the energy barrier for the path A→C to cause a concerted double proton transfer process is 9.00 kcal/mol, and even though this energy barrier is not very high, the barrier of A→B is significantly lower than it, which demonstrates that the synchronous double proton transfer for SA is forbidden at the S
1 state. After reaching point B, the SA-SPT needs to cross the potential barrier of 9.72 kcal/mol to reach point C. To analyze whether the proton transfer process in the second step can occur, the reverse proton transfer energy barrier for all paths was calculated: C→B, C→A, and B→A are 0.12 kcal/mol, 2.11 kcal/mol, and 5.49 kcal/mol, respectively. The results prove that even if the proton transfer from B to C occurred, it would return to point B with an almost no-barrier process. It can be concluded that the SA-SPT conformation is stable to exist at point B. Based on the analysis of the ESIPT behavior of the intermediate product AE-OH-Phos and product SA, the following conclusions can be drawn: due to the presence of phosphate group AE-OH-Phos, which is unable to experience ESIPT process, SA can neither undergo stepwise nor synergistic double proton transfer, but can only proceed with a single proton transfer process, which is stable in the form of SA-SPT.
3.3. Electronic Spectra and Fluorescence Mechanism
Absorption and fluorescence spectra are often used to fit theoretical calculations to experiments to verify the reliability and accuracy of a theory. Therefore, the spectra of ALP probe product SA were simulated separately, employing different functions in a DMSO solvent of IEFPCM at 6-311G(d,p) basis set, as listed in
Table 3. The previous analysis can determine that the single proton transfer process of SA is definitely present, and the simulated fluorescence spectra are also the closest to the experimental value of SA-SPT. Hence, the Stokes-shift of SA-SPT was calculated using different functionals and found that the value (145 nm) of B3PW91 is the closest to the experimental value (180 nm). Therefore, the spectra of studied molecules were simulated by B3PW91 for subsequent discussion and the first three single excited state transition and fluorescence properties are separately gathered in
Table 4 and
Table 5.
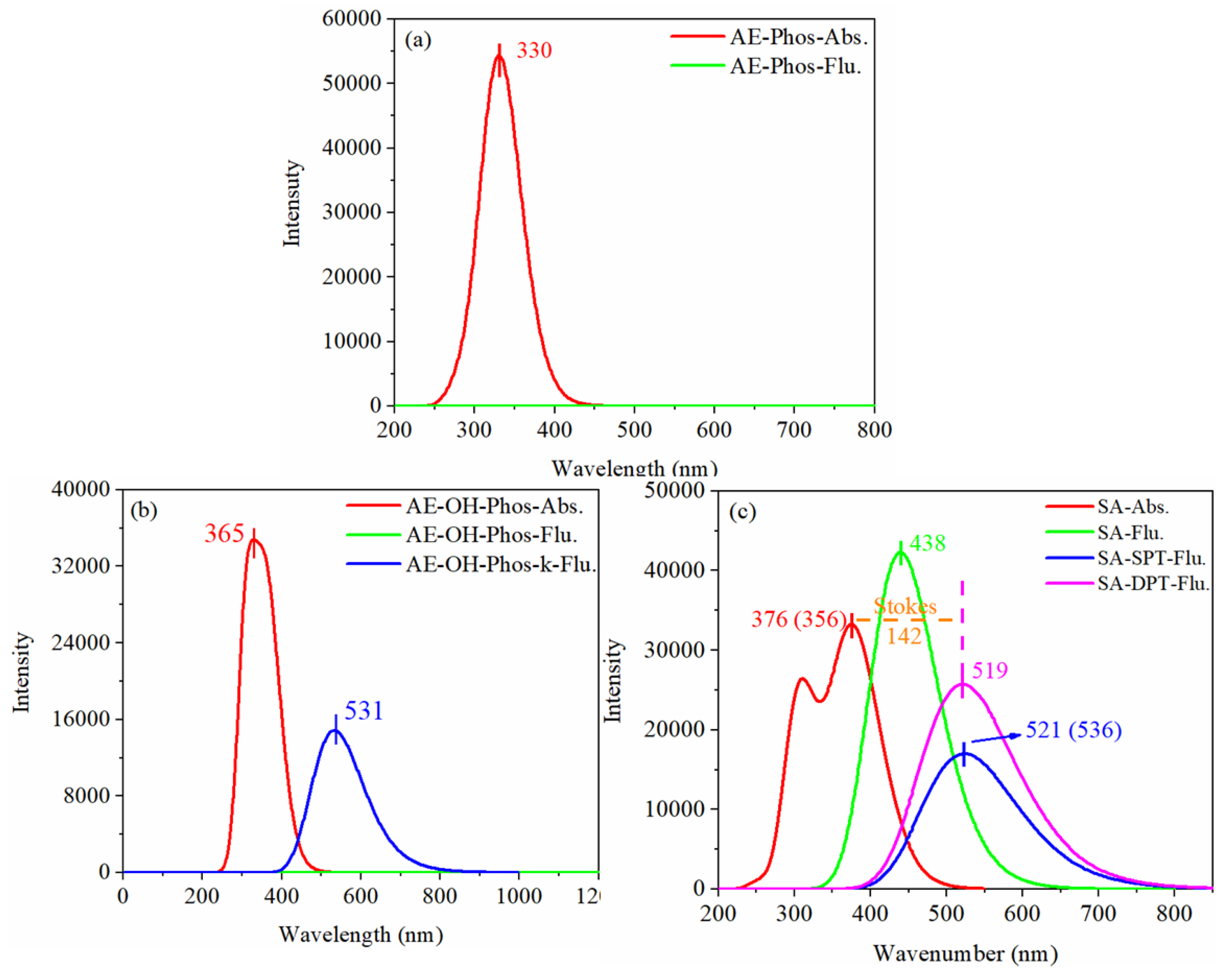
To compare the spectral changes more visually, their spectra are plotted in
Figure 7. It can be found that the fluorescence of probe AE-Phos and intermediate product AE-OH-Phos was quenched, which means that neither fluorescence can be observed experimentally. Moreover, the result is consistent with the experimentally measured spectra. As listed in
Table 4, the oscillator strength of S
1-state AE-Phos was 0.0010, which means that the fluorescence is almost unobservable experimentally. That is to say; the S
1 state is a dark state. The electrons of AE-Phos cannot be directly excited to the S
1 state, but are excited to the S
2 state. According to Kasha’s rule, the electrons of the S
2 state will return to the S
1 state through internal conversion, resulting in fluorescence quenching. However, the electrons of AE-OH-Phos were excited to the S
1 state but a fluorescence quenching also occurred, which we speculate may be due to the photo-induced electron transfer process; this will be further analyzed in the frontier molecular orbitals section. More importantly, the PEC shows that AE-OH-Phos is unable to undergo the ESIPT process into the keto form. As a consequence, we can conclude that AE-OH-Phos does not interfere with the experimental spectra by emitting fluorescence.
As shown in
Figure 7c, the two peaks of the absorption band of SA are consistent with the experimentally measured peaks. The highest peak in the absorption band at 376 nm is produced by the electron transitions to the S
1 state, which is in line with the experimental value (356 nm), and combined with
Table 4, we can know that another peak at a smaller wavelength is emitted from the S
0 state transitions to the S
4 state. In addition, the calculated fluorescence peak of SA-SPT (521 nm) is also in line with the experiment (536 nm), which is consistent with the conclusion that the SA-SPT form is stable the S
1 state obtained from our previous analysis. In a word, the probe AE-Phos produces the product SA when detecting the ALP activity, and the intermediate product AE-OH-PHOs may also be present, but since both AE-Phos and AE-OH-PHOs are fluorescence quenching, they will not affect the fluorescence spectra of the experiment. Moreover, the fluorescence of SA-SPT is almost identical to the experimental fluorescence; it is presumed that the fluorescence measured in the experiment is derived from SA-SPT, which is formed by SA undergoing a single proton transfer process.
3.4. Frontier Molecular Orbitals
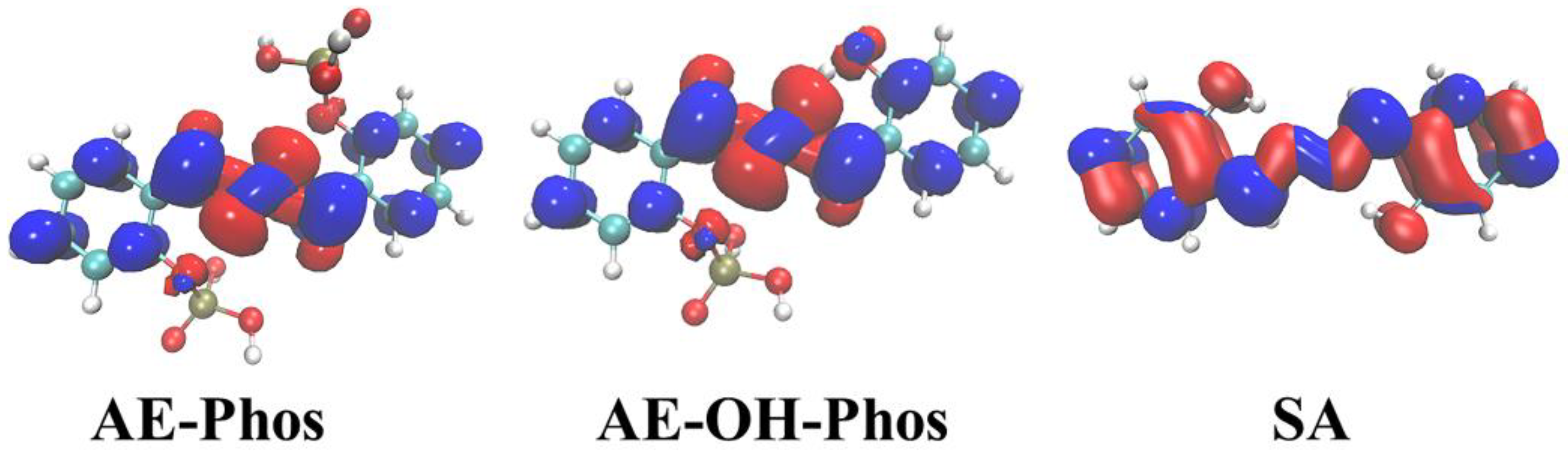
In the previous section, we mentioned that the fluorescence of AE-PHOs and AE-OH-PHOs is quenched, and this change in photophysical properties is attributed to the redistribution of electrons due to photoexcitation. To observe the change in charge distribution after photoexcitation, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) corresponding to their fluorescence emission are plotted in
Figure 8. When the electrons of AE-PHOs and AE-OH-PHOs fluoresce from LUMO to HOMO, there is an obvious concentration for charge from the benzene ring on both sides to the middle near the N-N bond. Such a charge transfer process could cause the chromophore part to fail to emit fluorescence, and this process is called the photo-induced electron transfer process. However, SA has almost no significant change in electron cloud distribution due to local excitation.
In order to better visualize the distribution of electrons and holes, and thus discuss the intrinsic cause of the fluorescence quenching for the probe and its intermediate products, the electrons and holes distribution of three molecules was investigated using the electron-hole analysis method. The relevant parameters were gathered in
Table 6. The D,
t, H, and Δσ index reflect the distance between the center of mass for electrons and holes, the degree of separation, the overall average distribution breadth, and the difference between the overall spatial distribution breadth, respectively. Generally speaking,
t > 0 and
t < 0 separately indicate whether the charge transfer makes the electron-hole sufficiently separated. Combined with
Table 6 and
Figure 9, the
t index of all three molecules is less than zero, but the figure shows an obvious separation of the electron-hole. In other words, the
t index is not perfect enough to analyze multidirectional charge transfer. The Sr index means the degree of electron-hole overlap; the larger the value, the higher the degree of overlap. The smaller hole and electron delocalization index (HDI and EDI) illustrates the higher delocalization degree of the electron-hole, that is, the greater the distribution and uniformity.
As depicted in
Figure 9, for AE-PHOs and AE-OH-PHOs, the holes are concentrated in the connecting part of the two benzene rings and the electrons are uniformly distributed throughout the framework of the molecule. The apparent movement of electrons from the middle part to the sides causes the fluorescence quenching in both molecules. The D index of the three molecules shows that the distance between their centers of mass is very short, again proving the opinion that the electrons are uniformly distributed on both sides. The Δσ index manifests that the electron distribution breadth for AE-PHOs and AE-OH-PHOs is larger than that of the holes, while SA is almost the same. The HDI of AE-PHOs and AE-OH-PHOs are approximately twice as high as the EDI, indicating that the electron delocalization of both molecules is greater than that of the holes. The electron-hole analysis verifies that AE-PHOs and AE-OH-PHOs produce a significant charge transfer, which is likely to lead to fluorescence quenching.