CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer
Abstract
:1. Introduction
2. CXCR2 Gene and Regulation of Expression
2.1. CXCR2 Gene
2.2. Regulation of CXCR2 Expression
2.3. Involvement of MicroRNAs in the Regulation of CXCR2 Expression
3. CXCR2 Ligands
4. CXCR2 Signal Transduction
4.1. Heterotrimeric G Proteins and CXCR2
4.2. Phosphorylation of CXCR2
4.3. CXCR2 Chemosynapse
4.4. Other Pathways Activated by CXCR2
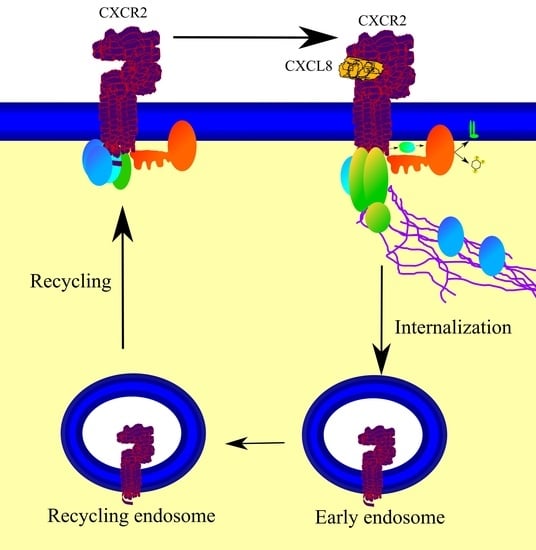
4.5. Internalization of CXCR2
4.6. CXCR2 Recycling or Degradation
5. The Role of CXCR2 in Cancer
6. Perspective for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.J.; Locati, M.; Mantovani, A.; Rot, A.; Thelen, M. The biochemistry and biology of the atypical chemokine receptors. Immunol. Lett. 2012, 145, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.J.; Li, Y.B.; Yang, L.X.; Cheng, H.J.; Liu, X.; Chen, H. Roles of the CXCL8-CXCR1/2 axis in the tumor microenvironment and immunotherapy. Molecules 2021, 27, 137. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M.; McDermott, D. Functional expression of the human formyl peptide receptor in Xenopus oocytes requires a complementary human factor. J. Biol. Chem. 1991, 266, 12560–12567. [Google Scholar] [CrossRef]
- Lee, J.; Horuk, R.; Rice, G.C.; Bennett, G.L.; Camerato, T.; Wood, W.I. Characterization of two high affinity human interleukin-8 receptors. J. Biol. Chem. 1992, 267, 16283–16287. [Google Scholar] [CrossRef]
- Loetscher, P.; Seitz, M.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Both interleukin-8 receptors independently mediate chemotaxis. Jurkat cells transfected with IL-8R1 or IL-8R2 migrate in response to IL-8, GRO alpha and NAP-2. FEBS Lett. 1994, 341, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.G.; Schraw, W.P.; Richmond, A. Melanoma growth stimulatory activity enhances the phosphorylation of the class II interleukin-8 receptor in non-hematopoietic cells. J. Biol. Chem. 1994, 269, 1973–1980. [Google Scholar] [CrossRef]
- Ahuja, S.K.; Ozçelik, T.; Milatovitch, A.; Francke, U.; Murphy, P.M. Molecular evolution of the human interleukin-8 receptor gene cluster. Nat. Genet. 1992, 2, 31–36. [Google Scholar] [CrossRef]
- Mollereau, C.; Muscatelli, F.; Mattei, M.G.; Vassart, G.; Parmentier, M. The high-affinity interleukin 8 receptor gene (IL8RA) maps to the 2q33-q36 region of the human genome: Cloning of a pseudogene (IL8RBP) for the low-affinity receptor. Genomics 1993, 16, 248–251. [Google Scholar] [CrossRef]
- Sprenger, H.; Lloyd, A.R.; Kelvin, D.J. Promoter analysis of the human interleukin-8 receptor genes, IL-8RA and IL-8RB. Immunobiology 1995, 193, 334–340. [Google Scholar] [CrossRef]
- Sprenger, H.; Lloyd, A.R.; Lautens, L.L.; Bonner, T.I.; Kelvin, D.J. Structure, genomic organization, and expression of the human interleukin-8 receptor B gene. J. Biol. Chem. 1994, 269, 11065–11072. [Google Scholar] [CrossRef]
- Ahuja, S.K.; Shetty, A.; Tiffany, H.L.; Murphy, P.M. Comparison of the genomic organization and promoter function for human interleukin-8 receptors A and B. J. Biol. Chem. 1994, 269, 26381–26389. [Google Scholar] [CrossRef]
- Maxwell, P.J.; Gallagher, R.; Seaton, A.; Wilson, C.; Scullin, P.; Pettigrew, J.; Stratford, I.J.; Williams, K.J.; Johnston, P.G.; Waugh, D.J. HIF-1 and NF-κB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene 2007, 26, 7333–7345. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, H.; Yashiro, M.; Fukuoka, T.; Hasegawa, T.; Morisaki, T.; Kasashima, H.; Masuda, G.; Noda, S.; Hirakawa, K. Diffuse-type gastric cancer cells switch their driver pathways from FGFR2 signaling to SDF1/CXCR4 axis in hypoxic tumor microenvironments. Carcinogenesis 2015, 36, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-Jackson, H.; Panopoulos, A.D.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood 2010, 115, 3354–3363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Z.; Zhou, Z.J.; Xia, G.K.; Zhang, X.H.; Wei, Z.W.; Zhu, J.T.; Yu, J.; Chen, W.; He, Y.; Schwarz, R.E.; et al. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene 2017, 36, 5122–5133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xia, G.; Xiang, Z.; Liu, M.; Wei, Z.; Yan, J.; Chen, W.; Zhu, J.; Awasthi, N.; Sun, X.; et al. A C-X-C chemokine receptor type 2-dominated cross-talk between tumor cells and macrophages drives gastric cancer metastasis. Clin. Cancer Res. 2019, 25, 3317–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.Y.; Shi, X.; Liu, P.; Sun, Y.; Sahbaie, P.; Li, W.W.; Yeomans, D.C.; Clark, J.D. The chemokine receptor CXCR2 supports nociceptive sensitization after traumatic brain injury. Mol. Pain 2017, 13, 1744806917730212. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Zhang, Y.; Yang, R.; Wang, X.; Ji, G.; Huo, L.; Shao, Z.; Li, X. MiR-940 suppresses tumor cell invasion and migration via regulation of CXCR2 in hepatocellular carcinoma. Biomed. Res. Int. 2016, 2016, 7618342. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Zhao, Z.; Wang, Z.; Wang, C.; Zhang, L. MiR-940 inhibits migration and invasion of tongue squamous cell carcinoma via regulatingCXCR2/NF-κB system-mediated epithelial-mesenchymal transition. Naunyn Schmiedebergs Arch. Pharm. 2019, 392, 1359–1369. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, X.; Xiang, Y.; Chen, Y.; Shen, C.X.; Zhang, Y.C.; Li, Y.G. The lncRNA MALAT1 protects the endothelium against ox-LDL-induced dysfunction via upregulating the expression of the miR-22-3p target genes CXCR2 and AKT. FEBS Lett. 2015, 589, 3189–3196. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Kitaura, H.; Liu, L.; Mizoguchi, I.; Liu, S. The miR-155-5p inhibits osteoclast differentiation through targeting CXCR2 in orthodontic root resorption. J. Periodontal Res. 2021, 56, 761–773. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, X.; Zhou, B.; Teng, J.; Zhang, C.; Liu, S.; Lian, J.; Luo, B.; Zhao, G.; Lu, H.; et al. Interleukin 8 (CXCL8)-CXC chemokine receptor 2 (CXCR2) axis contributes to MiR-4437-associated recruitment of granulocytes and natural killer cells in ischemic stroke. Mol. Immunol. 2018, 101, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Wei, J. Identification of potential novel biomarkers and therapeutic targets involved in human atrial fibrillation based on bioinformatics analysis. Kardiol. Pol. 2020, 78, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, H.Q.; Zhao, H.J.; Luan, S.X.; Zhang, C.G. Identification of candidate genes and miRNAs associated with neuropathic pain induced by spared nerve injury. Int. J. Mol. Med. 2019, 44, 1205–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, T.R.; Elisei, L.S.; Malta, I.H.; Galdino, G. Participation of CXCL1 in the glial cells during neuropathic pain. Eur. J. Pharmacol. 2020, 875, 173039. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, J.; Huang, L.; Xu, Q.; Ling, X.; Liu, J. Cordyceps militaris alleviates severity of murine acute lung injury through miRNAs-mediated CXCR2 inhibition. Cell. Physiol. Biochem. 2015, 36, 2003–2011. [Google Scholar] [CrossRef]
- Li, W.; Jia, X.; Shen, C.; Zhang, M.; Xu, J.; Shang, Y.; Zhu, K.; Hu, M.; Yan, Q.; Qin, D.; et al. A KSHV microRNA enhances viral latency and induces angiogenesis by targeting GRK2 to activate the CXCR2/AKT pathway. Oncotarget 2016, 7, 32286–32305. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Sônego, F.; Souto, F.O.; Freitas, A.; Verri, W.A., Jr.; Auxiliadora-Martins, M.; Basile-Filho, A.; McKenzie, A.N.; Xu, D.; Cunha, F.Q.; et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 2010, 16, 708–712. [Google Scholar] [CrossRef]
- Ahuja, S.K.; Murphy, P.M. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996, 271, 20545–20550. [Google Scholar] [CrossRef] [Green Version]
- Wuyts, A.; Proost, P.; Lenaerts, J.P.; Ben-Baruch, A.; van Damme, J.; Wang, J.M. Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur. J. Biochem. 1998, 255, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Li, D.; Xu, P.; Tian, S.; Sun, H.; Liu, H.; Hou, T. Exploring the binding mechanisms of MIF to CXCR2 using theoretical approaches. Phys. Chem. Chem. Phys. 2015, 17, 3370–3382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, Y.L.; Li, M.X.; Ye, S.B.; Huang, W.R.; Cai, T.T.; He, J.; Peng, J.Y.; Duan, T.H.; Cui, J.; et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017, 36, 2095–2104. [Google Scholar] [CrossRef]
- LaRosa, G.J.; Thomas, K.M.; Kaufmann, M.E.; Mark, R.; White, M.; Taylor, L.; Gray, G.; Witt, D.; Navarro, J. Amino terminus of the interleukin-8 receptor is a major determinant of receptor subtype specificity. J. Biol. Chem. 1992, 267, 25402–25406. [Google Scholar] [CrossRef]
- Katancik, J.A.; Sharma, A.; de Nardin, E. Interleukin 8, neutrophil-activating peptide-2 and GRO-alpha bind to and elicit cell activation via specific and different amino acid residues of CXCR2. Cytokine 2000, 12, 1480–1488. [Google Scholar] [CrossRef]
- Lowman, H.B.; Slagle, P.H.; DeForge, L.E.; Wirth, C.M.; Gillece-Castro, B.L.; Bourell, J.H.; Fairbrother, W.J. Exchanging interleukin-8 and melanoma growth-stimulating activity receptor binding specificities. J. Biol. Chem. 1996, 271, 14344–14352. [Google Scholar] [CrossRef] [Green Version]
- Trettel, F.; di Bartolomeo, S.; Lauro, C.; Catalano, M.; Ciotti, M.T.; Limatola, C. Ligand-independent CXCR2 dimerization. J. Biol. Chem. 2003, 278, 40980–40988. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.; Wilkinson, G.; Milligan, G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J. Biol. Chem. 2005, 280, 28663–28674. [Google Scholar] [CrossRef] [Green Version]
- Martínez Muñoz, L.; Lucas, P.; Navarro, G.; Checa, A.I.; Franco, R.; Martínez-A, C.; Rodríguez-Frade, J.M.; Mellado, M. Dynamic regulation of CXCR1 and CXCR2 homo- and heterodimers. J. Immunol. 2009, 183, 7337–7346. [Google Scholar] [CrossRef] [Green Version]
- Weis, W.I.; Kobilka, B.K. The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Damaj, B.B.; McColl, S.R.; Neote, K.; Songqing, N.; Ogborn, K.T.; Hébert, C.A.; Naccache, P.H. Identification of G-protein binding sites of the human interleukin-8 receptors by functional mapping of the intracellular loops. FASEB J. 1996, 10, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Damaj, B.B.; McColl, S.R.; Mahana, W.; Crouch, M.F.; Naccache, P.H. Physical association of Gi2α with interleukin-8 receptors. J. Biol. Chem. 1996, 271, 12783–12789. [Google Scholar] [CrossRef] [Green Version]
- Zarbock, A.; Deem, T.L.; Burcin, T.L.; Ley, K. Gαi2 is required for chemokine-induced neutrophil arrest. Blood 2007, 110, 3773–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Raghuwanshi, S.K.; Smith, N.; Rivers, E.J.; Richardson, R.M. G protein-coupled receptor kinase-6 interacts with activator of G protein signaling-3 to regulate CXCR2-mediated cellular functions. J. Immunol. 2014, 192, 2186–2194. [Google Scholar] [CrossRef] [Green Version]
- Kuwano, Y.; Adler, M.; Zhang, H.; Groisman, A.; Ley, K. Gαi2 and Gαi3 differentially regulate arrest from flow and chemotaxis in mouse neutrophils. J. Immunol. 2016, 196, 3828–3833. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.A.; Beresford, I.J.; Browning, C.; Giles, H. Signalling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: A comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPγS binding induced by IL-8 and GROα. Br. J. Pharmacol. 1999, 126, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [Green Version]
- Stephens, L.R.; Eguinoa, A.; Erdjument-Bromage, H.; Lui, M.; Cooke, F.; Coadwell, J.; Smrcka, A.S.; Thelen, M.; Cadwallader, K.; Tempst, P.; et al. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 1997, 89, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Boyer, J.L.; Graber, S.G.; Waldo, G.L.; Harden, T.K.; Garrison, J.C. Selective activation of phospholipase C by recombinant G-protein alpha- and beta gamma-subunits. J. Biol. Chem. 1994, 269, 2814–2819. [Google Scholar] [CrossRef]
- Metzner, B.; Elsner, J.; Dobos, G.; Kownatzki, E.; Parlow, F.; Schraufstätter, I.; Norgauer, J. [Ca2+]i-transients and actin polymerization in human neutrophils under stimulation with GROα and complement fragment C5a. Agents Actions 1994, 42, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Shyamala, V.; Khoja, H. Interleukin-8 receptors R1 and R2 activate mitogen-activated protein kinases and induce c-fos, independent of Ras and Raf-1 in Chinese hamster ovary cells. Biochemistry 1998, 37, 15918–15924. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Neamati, N. Pyrimidine-based compounds modulate CXCR2-mediated signaling and receptor turnover. Mol. Pharm. 2014, 11, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, Z.F.; Wang, D.K.; Sun, M.; Qi, W.; Ma, Q.; Zhang, L.; Chu, C.; Chan, E.Y.M.; Lee, S.S.T.; et al. Molecular and functional characterization of tumor-induced factor (TIF): Hamster homolog of CXCL3 (GROγ) displays tumor suppressive activity. Cytokine 2018, 102, 62–75. [Google Scholar] [CrossRef]
- Chan, E.C.; Ren, C.; Xie, Z.; Jude, J.; Barker, T.; Koziol-White, C.A.; Ma, M.; Panettieri, R.A., Jr.; Wu, D.; Rosenberg, H.F.; et al. Regulator of G protein signaling 5 restricts neutrophil chemotaxis and trafficking. J. Biol. Chem. 2018, 293, 12690–12702. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.G.; White, J.R.; Schraw, W.P.; Lam, V.; Richmond, A. Ligand-induced desensitization of the human CXC chemokine receptor-2 is modulated by multiple serine residues in the carboxyl-terminal domain of the receptor. J. Biol. Chem. 1997, 272, 8207–8214. [Google Scholar] [CrossRef] [Green Version]
- Schraufstätter, I.U.; Burger, M.; Hoch, R.C.; Oades, Z.G.; Takamori, H. Importance of the carboxy-terminus of the CXCR2 for signal transduction. Biochem. Biophys. Res. Commun. 1998, 244, 243–248. [Google Scholar] [CrossRef]
- Raghuwanshi, S.K.; Su, Y.; Singh, V.; Haynes, K.; Richmond, A.; Richardson, R.M. The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions. J. Immunol. 2012, 189, 2824–2832. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wang, D.; Richmond, A. Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J. Biol. Chem. 1999, 274, 11328–11333. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.H.; Yang, W.; Sai, J.; Richmond, A. Phosphorylation-independent association of CXCR2 with the protein phosphatase 2A core enzyme. J. Biol. Chem. 2001, 276, 16960–16968. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.G.; Schraw, W.P.; Richmond, A. Activation of protein kinase C enhances the phosphorylation of the type B interleukin-8 receptor and stimulates its degradation in non-hematopoietic cells. J. Biol. Chem. 1995, 270, 10439–10448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, M.W.; Marjoram, R.J.; Brown, S.L.; Richardson, R.M. Cross-desensitization among CXCR1, CXCR2, and CCR5: Role of protein kinase C-epsilon. J. Immunol. 2005, 174, 6927–6933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neel, N.F.; Sai, J.; Ham, A.J.; Sobolik-Delmaire, T.; Mernaugh, R.L.; Richmond, A. IQGAP1 is a novel CXCR2-interacting protein and essential component of the “chemosynapse”. PLoS ONE 2011, 6, e23813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, D.; Neel, N.F.; Sai, J.; Mernaugh, R.L.; Ham, A.J.; Richmond, A.J. Characterization of chemokine receptor CXCR2 interacting proteins using a proteomics approach to define the CXCR2 “chemosynapse”. Methods Enzymol. 2009, 460, 315–330. [Google Scholar] [PubMed] [Green Version]
- Holcomb, J.; Jiang, Y.; Guan, X.; Trescott, L.; Lu, G.; Hou, Y.; Wang, S.; Brunzelle, J.; Sirinupong, N.; Li, C.; et al. Crystal structure of the NHERF1 PDZ2 domain in complex with the chemokine receptor CXCR2 reveals probable modes of PDZ2 dimerization. Biochem. Biophys. Res. Commun. 2014, 448, 169–174. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Farooq, S.M.; Castelvetere, M.P.; Hou, Y.; Gao, J.L.; Navarro, J.V.; Oupicky, D.; Sun, F.; Li, C. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J. Biol. Chem. 2012, 287, 5744–5755. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wang, S.; Holcomb, J.; Trescott, L.; Guan, X.; Hou, Y.; Brunzelle, J.; Sirinupong, N.; Li, C.; Yang, Z. Crystallographic analysis of NHERF1-PLCβ3 interaction provides structural basis for CXCR2 signaling in pancreatic cancer. Biochem. Biophys. Res. Commun. 2014, 446, 638–643. [Google Scholar] [CrossRef]
- Swart-Mataraza, J.M.; Li, Z.; Sacks, D.B. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 2002, 277, 24753–24763. [Google Scholar] [CrossRef] [Green Version]
- Neel, N.F.; Barzik, M.; Raman, D.; Sobolik-Delmaire, T.; Sai, J.; Ham, A.J.; Mernaugh, R.L.; Gertler, F.B.; Richmond, A. VASP is a CXCR2-interacting protein that regulates CXCR2-mediated polarization and chemotaxis. J. Cell Sci. 2009, 122, 1882–1894. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, Y.; Mizuno, S.; Komenoi, S.; Sakai, H.; Sakane, F. Activation of conventional and novel protein kinase C isozymes by different diacylglycerol molecular species. Biochem. Biophys. Rep. 2016, 7, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, S.F. Distinctive activation mechanisms and functions for protein kinase Cδ. Biochem. J. 2004, 384, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sai, J.; Walker, G.; Wikswo, J.; Richmond, A. The IL sequence in the LLKIL motif in CXCR2 is required for full ligand-induced activation of Erk, Akt, and chemotaxis in HL60 cells. J. Biol. Chem. 2006, 281, 35931–35941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sai, J.; Fan, G.H.; Wang, D.; Richmond, A. The C-terminal domain LLKIL motif of CXCR2 is required for ligand-mediated polarization of early signals during chemotaxis. J. Cell Sci. 2004, 117, 5489–5496. [Google Scholar] [CrossRef] [Green Version]
- Raman, D.; Sai, J.; Neel, N.F.; Chew, C.S.; Richmond, A. LIM and SH3 protein-1 modulates CXCR2-mediated cell migration. PLoS ONE 2010, 5, e10050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sai, J.; Raman, D.; Liu, Y.; Wikswo, J.; Richmond, A. Parallel phosphatidylinositol 3-kinase (PI3K)-dependent and Src-dependent pathways lead to CXCL8-mediated Rac2 activation and chemotaxis. J. Biol. Chem. 2008, 283, 26538–26547. [Google Scholar] [CrossRef] [Green Version]
- Schraw, W.; Richmond, A. Melanoma growth stimulatory activity signaling through the class II interleukin-8 receptor enhances the tyrosine phosphorylation of Crk-associated substrate, p130, and a 70-kilodalton protein. Biochemistry 1995, 34, 13760–13767. [Google Scholar] [CrossRef]
- Gavard, J.; Hou, X.; Qu, Y.; Masedunskas, A.; Martin, D.; Weigert, R.; Li, X.; Gutkind, J.S. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol. Cell. Biol. 2009, 29, 2469–2480. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Wong, M.M.; Potter, C.M.; Simpson, R.M.; Karamariti, E.; Zhang, Z.; Zeng, L.; Warren, D.; Hu, Y.; Wang, W.; et al. Vascular stem/progenitor cell migration induced by smooth muscle cell-derived chemokine (C-C motif) ligand 2 and chemokine (C-X-C motif) ligand 1 contributes to neointima formation. Stem Cells 2016, 34, 2368–2380. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sai, J.; Carter, G.; Sachpatzidis, A.; Lolis, E.; Richmond, A. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry 2002, 41, 7100–7107. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.H.; Yang, W.; Sai, J.; Richmond, A. Hsc/Hsp70 interacting protein (hip) associates with CXCR2 and regulates the receptor signaling and trafficking. J. Biol. Chem. 2002, 277, 6590–6597. [Google Scholar] [CrossRef] [Green Version]
- Raman, D.; Sai, J.; Hawkins, O.; Richmond, A. Adaptor protein2 (AP2) orchestrates CXCR2-mediated cell migration. Traffic 2014, 15, 451–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagnac, G.; Meas-Yedid, V.; Irondelle, M.; Castro-Castro, A.; Franco, M.; Shida, T.; Nachury, M.V.; Benmerah, A.; Olivo-Marin, J.C.; Chavrier, P. αTAT1 catalyses microtubule acetylation at clathrin-coated pits. Nature 2013, 502, 567–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.A.; Moser, B.; Thelen, M. A comparison of post-receptor signal transduction events in Jurkat cells transfected with either IL-8R1 or IL-8R2. Chemokine mediated activation of p42/p44 MAP-kinase (ERK-2). FEBS Lett. 1995, 364, 211–214. [Google Scholar] [PubMed] [Green Version]
- Zhao, M.; Wimmer, A.; Trieu, K.; Discipio, R.G.; Schraufstatter, I.U. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J. Biol. Chem. 2004, 279, 49259–49267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatakrishnan, G.; Salgia, R.; Groopman, J.E. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J. Biol. Chem. 2000, 275, 6868–6875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolitho, C.; Hahn, M.A.; Baxter, R.C.; Marsh, D.J. The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr.-Relat. Cancer 2010, 17, 929–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.; Li, G.; Wu, H.; Sun, K.; Chen, J.; Feng, Y.; Chen, C.; Cai, S.; Xu, J.; et al. CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett. 2017, 385, 28–38. [Google Scholar] [CrossRef]
- Artz, A.; Butz, S.; Vestweber, D. GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-βRII heterodimer. Blood 2016, 128, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Antonosante, A.; Brandolini, L.; D’Angelo, M.; Benedetti, E.; Castelli, V.; Maestro, M.D.; Luzzi, S.; Giordano, A.; Cimini, A.; Allegretti, M. Autocrine CXCL8-dependent invasiveness triggers modulation of actin cytoskeletal network and cell dynamics. Aging (Albany NY) 2020, 12, 1928–1951. [Google Scholar] [CrossRef]
- Wei, Z.W.; Xia, G.K.; Wu, Y.; Chen, W.; Xiang, Z.; Schwarz, R.E.; Brekken, R.A.; Awasthi, N.; He, Y.L.; Zhang, C.H. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015, 359, 335–343. [Google Scholar] [CrossRef]
- Wei, L.; Liu, Y.; Ma, Y.; Ding, C.; Zhang, H.; Lu, Z.; Gu, Z.; Zhu, C. C-X-C chemokine receptor 2 correlates with unfavorable prognosis and facilitates malignant cell activities via activating JAK2/STAT3 pathway in non-small cell lung cancer. Cell Cycle 2019, 18, 3456–3471. [Google Scholar] [CrossRef]
- Han, X.; Shi, H.; Sun, Y.; Shang, C.; Luan, T.; Wang, D.; Ba, X.; Zeng, X. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions contributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis. 2019, 10, 598. [Google Scholar] [CrossRef] [Green Version]
- Moldobaeva, A.; Baek, A.; Wagner, E.M. MIP-2 causes differential activation of RhoA in mouse aortic versus pulmonary artery endothelial cells. Microvasc. Res. 2008, 75, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.H.; Yang, W.; Wang, X.J.; Qian, Q.; Richmond, A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry 2001, 40, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclair, H.M.; Dubois, S.M.; Azzi, S.; Dwyer, J.; Bidère, N.; Gavard, J. Control of CXCR2 activity through its ubiquitination on K327 residue. BMC Cell Biol. 2014, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, J.J.; Foley, J.F.; Murphy, P.M.; Venkatesan, S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J. Biol. Chem. 2004, 279, 24372–24386. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.M.; Pridgen, B.C.; Haribabu, B.; Ali, H.; Snyderman, R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J. Biol. Chem. 1998, 273, 23830–23836. [Google Scholar] [CrossRef] [Green Version]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef]
- Fan, G.H.; Lapierre, L.A.; Goldenring, J.R.; Richmond, A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood 2003, 101, 2115–2124. [Google Scholar] [CrossRef] [Green Version]
- Neel, N.F.; Lapierre, L.A.; Goldenring, J.R.; Richmond, A. RhoB plays an essential role in CXCR2 sorting decisions. J. Cell Sci. 2007, 120, 1559–1571. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Li, Z.; Li, X.; Huo, Z. High CXCR2 expression predicts poor prognosis in adult patients with acute myeloid leukemia. Ther. Adv. Hematol. 2020, 11, 2040620720958586. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.; Di, G.; Yang, G. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018, 412, 69–80. [Google Scholar] [CrossRef]
- Zhao, J.; Ou, B.; Feng, H.; Wang, P.; Yin, S.; Zhu, C.; Wang, S.; Chen, C.; Zheng, M.; Zong, Y.; et al. Overexpression of CXCR2 predicts poor prognosis in patients with colorectal cancer. Oncotarget 2017, 8, 28442–28454. [Google Scholar] [CrossRef] [Green Version]
- Sui, P.; Hu, P.; Zhang, T.; Zhang, X.; Liu, Q.; Du, J. High expression of CXCR-2 correlates with lymph node metastasis and predicts unfavorable prognosis in resected esophageal carcinoma. Med. Oncol. 2014, 31, 809. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Shen, Z.; Wang, X.; Zhang, H.; Qin, J.; Xu, J.; Sun, Y.; Qin, X. The prognostic value of CXC-chemokine receptor 2 (CXCR2) in gastric cancer patients. BMC Cancer 2015, 15, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasashima, H.; Yashiro, M.; Nakamae, H.; Masuda, G.; Kinoshita, H.; Morisaki, T.; Fukuoka, T.; Hasegawa, T.; Nakane, T.; Hino, M.; et al. Clinicopathologic significance of the CXCL1-CXCR2 axis in the tumor microenvironment of gastric carcinoma. PLoS ONE 2017, 12, e0178635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sueoka, H.; Hirano, T.; Uda, Y.; Iimuro, Y.; Yamanaka, J.; Fujimoto, J. Blockage of CXCR2 suppresses tumor growth of intrahepatic cholangiocellular carcinoma. Surgery 2014, 155, 640–649. [Google Scholar] [CrossRef]
- Han, L.; Jiang, B.; Wu, H.; Wang, X.; Tang, X.; Huang, J.; Zhu, J. High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis of laryngeal squamous cell carcinoma. Med. Oncol. 2012, 29, 2466–2472. [Google Scholar] [CrossRef]
- Saintigny, P.; Massarelli, E.; Lin, S.; Ahn, Y.H.; Chen, Y.; Goswami, S.; Erez, B.; O’Reilly, M.S.; Liu, D.; Lee, J.J.; et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. 2013, 73, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Rosen, D.G.; Liu, G.; Yang, F.; Guo, X.; Xiao, X.; Xue, F.; Mercado-Uribe, I.; Huang, J.; Lin, S.H.; et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin. Cancer Res. 2010, 16, 3875–3886. [Google Scholar] [CrossRef] [Green Version]
- Henriques, T.B.; dos Santos, D.Z.; dos Santos Guimarães, I.; Tessarollo, N.G.; Lyra-Junior, P.C.M.; Mesquita, P.; Pádua, D.; Amaral, A.L.; Cavadas, B.; Pereira, L.; et al. Inhibition of CXCR2 plays a pivotal role in re-sensitizing ovarian cancer to cisplatin treatment. Aging (Albany NY) 2021, 13, 13405–13420. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissière-Michot, F.; Jacot, W.; Massol, O.; Mollevi, C.; Lazennec, G. CXCR2 levels correlate with immune infiltration and a better prognosis of triple-negative breast cancers. Cancers Basel 2021, 13, 2328. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, O.; Conlon, S.; O’Grady, A.; Purcell, C.; Wilson, C.; Maxwell, P.J.; Johnston, P.G.; Stevenson, M.; Kay, E.W.; Wilson, R.H.; et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br. J. Cancer 2011, 104, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Y.; Chen, X.; Tan, W.; He, L.; Peng, L. Characterization of the prognostic values of the CXCR1-7 in clear cell renal cell carcinoma (ccRCC) microenvironment. Front. Mol. Biosci. 2020, 7, 601206. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y. Characterization of the prognostic values of CXCR family in gastric cancer. Cytokine 2019, 123, 154785. [Google Scholar] [CrossRef]
- Maeda, S.; Kuboki, S.; Nojima, H.; Shimizu, H.; Yoshitomi, H.; Furukawa, K.; Miyazaki, M.; Ohtsuka, M. Duffy antigen receptor for chemokines (DARC) expressing in cancer cells inhibits tumor progression by suppressing CXCR2 signaling in human pancreatic ductal adenocarcinoma. Cytokine 2017, 95, 12–21. [Google Scholar] [CrossRef]
- Timaxian, C.; Vogel, C.F.A.; Orcel, C.; Vetter, D.; Durochat, C.; Chinal, C.; NGuyen, P.; Aknin, M.L.; Mercier-Nomé, F.; Davy, M.; et al. Pivotal role for Cxcr2 in regulating tumor-associated neutrophil in breast cancer. Cancers 2021, 13, 2584. [Google Scholar] [CrossRef]
- Yang, J.; Richmond, A. Constitutive IκB kinase activity correlates with nuclear factor-κB activation in human melanoma cells. Cancer Res. 2001, 61, 4901–4909. [Google Scholar]
- Wang, D.; Richmond, A. Nuclear factor-κB activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 2001, 276, 3650–3659. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.L.; Kabir, S.M.; Lee, E.S.; Son, D.S. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling. PLoS ONE 2013, 8, e83789. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.; Tang, H.W.; Cai, P.C.; Leung, T.H.; Ngu, S.F.; Chan, K.K.; Xu, D.; Yang, H.; Ngan, H.Y.; Chan, D.W. GRO-α and IL-8 enhance ovarian cancer metastatic potential via the CXCR2-mediated TAK1/NFκB signaling cascade. Theranostics 2018, 8, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Kveiborg, M.; Instrell, R.; Rowlands, C.; Howell, M.; Parker, P.J. PKCα and PKCδ regulate ADAM17-mediated ectodomain shedding of heparin binding-EGF through separate pathways. PLoS ONE 2011, 6, e17168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; D’Amore, M.; de Lucro, R.; Ribatti, D. GRO-α/CXCR2 system and ADAM17 correlated expression in Sjögren’s syndrome. Inflammation 2013, 36, 759–766. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Trieu, K.; Zhao, M.; Rose, D.M.; Terkeltaub, R.A.; Burger, M. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J. Immunol. 2003, 171, 6714–6722. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Khachigian, L.M.; Esau, L.; Birrer, M.J.; Zhao, X.; Parker, M.I.; Hendricks, D.T. A key role for early growth response-1 and nuclear factor-κB in mediating and maintaining GRO/CXCR2 proliferative signaling in esophageal cancer. Mol. Cancer Res. 2009, 7, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Ignacio, R.M.C.; Dong, Y.L.; Kabir, S.M.; Choi, H.; Lee, E.S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.S. CXCR2 is a negative regulator of p21 in p53-dependent and independent manner via Akt-mediated Mdm2 in ovarian cancer. Oncotarget 2018, 9, 9751–9765. [Google Scholar] [CrossRef] [Green Version]
- Midgley, C.A.; Desterro, J.M.; Saville, M.K.; Howard, S.; Sparks, A.; Hay, R.T.; Lane, D.P. An N-terminal p14ARF peptide blocks Mdm2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene 2000, 19, 2312–2323. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.C.; O’Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; da Costa, M.; Brown, C.; Popov, N.; et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Liu, Z.; Xu, B.; Hu, H.; Wei, Z.; Liu, Q.; Zhang, X.; Ding, X.; Wang, Y.; Zhao, M.; et al. Chemokine receptor CXCR2 is transactivated by p53 and induces p38-mediated cellular senescence in response to DNA damage. Aging Cell 2013, 12, 1110–1121. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, P.M.; Jackman, J.; Bae, I.; Myers, T.G.; Fan, S.; Mutoh, M.; Scudiero, D.A.; Monks, A.; Sausville, E.A.; Weinstein, J.N.; et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997, 57, 4285–4300. [Google Scholar] [PubMed]
- Yang, G.; Rosen, D.G.; Zhang, Z.; Bast, R.C., Jr.; Mills, G.B.; Colacino, J.A.; Mercado-Uribe, I.; Liu, J. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16472–16477. [Google Scholar] [CrossRef] [Green Version]
- Sano, M.; Ijichi, H.; Takahashi, R.; Miyabayashi, K.; Fujiwara, H.; Yamada, T.; Kato, H.; Nakatsuka, T.; Tanaka, Y.; Tateishi, K.; et al. Blocking CXCLs-CXCR2 axis in tumor-stromal interactions contributes to survival in a mouse model of pancreatic ductal adenocarcinoma through reduced cell invasion/migration and a shift of immune-inflammatory microenvironment. Oncogenesis 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Awaji, M.; Saxena, S.; Wu, L.; Prajapati, D.R.; Purohit, A.; Varney, M.L.; Kumar, S.; Rachagani, S.; Ly, Q.P.; Jain, M.; et al. CXCR2 signaling promotes secretory cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. FASEB J. 2020, 34, 9405–9418. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, X.W.; Huang, H.; Gao, Y.; Yang, Q.W.; Wang, L.H.; Cui, X.G.; Xu, D.F. Epithelial-mesenchymal transition induced by GRO-α-CXCR2 promotes bladder cancer recurrence after intravesical chemotherapy. Oncotarget 2017, 8, 45274–45285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.C.; Lee, C.W.; Chang, T.M.; Chen, P.C.; Liu, J.F. CXCL1/CXCR2 paracrine axis contributes to lung metastasis in osteosarcoma. Cancers Basel 2020, 12, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.; Nannuru, K.C.; Saxena, S.; Varney, M.L.; Singh, R.K. CXCR2: A novel mediator of mammary tumor bone metastasis. Int. J. Mol. Sci. 2019, 20, 1237. [Google Scholar] [CrossRef] [Green Version]
- Grassi, F.; Piacentini, A.; Cristino, S.; Toneguzzi, S.; Cavallo, C.; Facchini, A.; Lisignoli, G. Human osteoclasts express different CXC chemokines depending on cell culture substrate: Molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem. Cell Biol. 2003, 120, 391–400. [Google Scholar] [CrossRef]
- Onan, D.; Allan, E.H.; Quinn, J.M.; Gooi, J.H.; Pompolo, S.; Sims, N.A.; Gillespie, M.T.; Martin, T.J. The chemokine Cxcl1 is a novel target gene of parathyroid hormone (PTH)/PTH-related protein in committed osteoblasts. Endocrinology 2009, 150, 2244–2253. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhu, M.D.; Zhang, X.; Tian, H.; Zhang, J.H.; Wu, X.B.; Gao, Y.J. NFκB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J. Neuroinflamm. 2014, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Wang, Y.; An, K.; Liu, Q.; Xu, L.; Zhu, C.; Deng, H.; He, Q.; Wang, T.; Xu, M.; et al. Crosstalk between NFκB-dependent astrocytic CXCL1 and neuron CXCR2 plays a role in descending pain facilitation. J. Neuroinflamm. 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, H.; Xu, M.; Xie, K.; Fei, Y.; Deng, H.; He, Q.; Wang, T.; Liu, S.; Zhu, J.; Xu, L.; et al. Liquiritin alleviates pain through inhibiting CXCL1/CXCR2 signaling pathway in bone cancer pain rat. Front. Pharmacol. 2020, 11, 436. [Google Scholar] [CrossRef]
- Addison, C.L.; Daniel, T.O.; Burdick, M.D.; Liu, H.; Ehlert, J.E.; Xue, Y.Y.; Buechi, L.; Walz, A.; Richmond, A.; Strieter, R.M. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 2000, 165, 5269–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J. Immunol. 2005, 175, 5351–5357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Goodison, S.; Urquidi, V.; Gomes Giacoia, E.; Rosser, C.J. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab. Investig. 2013, 93, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, M.P.; Belperio, J.A.; Xue, Y.Y.; Burdick, M.D.; Strieter, R.M. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 2004, 172, 2853–2860. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, C.; He, Y.; Wu, H.; Wang, Z.; Song, W.; Li, W.; He, W.; Cai, S.; Zhan, W. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. Int. J. Cancer 2012, 130, 787–797. [Google Scholar] [CrossRef]
- Karl, E.; Warner, K.; Zeitlin, B.; Kaneko, T.; Wurtzel, L.; Jin, T.; Chang, J.; Wang, S.; Wang, C.Y.; Strieter, R.M.; et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-κB and CXC chemokines. Cancer Res. 2005, 65, 5063–5069. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Zhang, Z.; Mantellini, M.G.; Karl, E.; Zeitlin, B.; Verhaegen, M.; Soengas, M.S.; Lingen, M.; Strieter, R.M.; Nunez, G.; et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007, 67, 9685–9693. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Berk, R.; Kosir, M.A. CXCL7-mediated stimulation of lymphangiogenic factors VEGF-C, VEGF-D in human breast cancer cells. J. Oncol. 2010, 2010, 939407. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhang, M.; Chen, G.; Wang, W.; Zhang, P.; Yue, Y.; Guan, Z.; Wang, X.; Fan, J. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int. J. Oncol. 2019, 54, 1555–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Cheng, X.J.; Moro, A.; Singh, R.K.; Hines, O.J.; Eibl, G. CXCR2-dependent endothelial progenitor cell mobilization in pancreatic cancer growth. Transl. Oncol. 2011, 4, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.; Nannuru, K.C.; Varney, M.L.; Singh, R.K. Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin. Exp. Metastasis 2015, 32, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mitri, D.; Mirenda, M.; Vasilevska, J.; Calcinotto, A.; Delaleu, N.; Revandkar, A.; Gil, V.; Boysen, G.; Losa, M.; Mosole, S.; et al. Re-education of tumor-associated macrophages by CXCR2 blockade drives senescence and tumor inhibition in advanced prostate cancer. Cell Rep. 2019, 28, 2156–2168.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Huang, L.; Ding, G.; Huang, H.; Cao, G.; Sun, X.; Lou, N.; Wei, Q.; Shen, T.; Xu, X.; et al. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J. Immunother. Cancer 2020, 8, e000308. [Google Scholar] [CrossRef] [Green Version]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Chao, T.; Furth, E.E.; Vonderheide, R.H. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 2016, 4, 968–982. [Google Scholar] [CrossRef] [Green Version]
- Ohms, M.; Möller, S.; Laskay, T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front. Immunol 2020, 11, 532. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Ren, X.; Gorska, A.E.; Chytil, A.; Aakre, M.; Carbone, D.P.; Matrisian, L.M.; Richmond, A.; Lin, P.C.; et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 2008, 13, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P.G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V.E.; et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012, 150, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, M.; Xu, Y.; Tang, R.; Ren, J.; Shen, S.; Chen, Y.; Liu, B.; Hou, Y.; Wang, T. MiR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol. Cancer Ther. 2014, 13, 3152–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasashima, H.; Yashiro, M.; Nakamae, H.; Kitayama, K.; Masuda, G.; Kinoshita, H.; Fukuoka, T.; Hasegawa, T.; Nakane, T.; Hino, M.; et al. CXCL1-chemokine (C-X-C motif) receptor 2 signaling stimulates the recruitment of bone marrow-derived mesenchymal cells into diffuse-type gastric cancer stroma. Am. J. Pathol. 2016, 186, 3028–3039. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Sun, Y.; Shang, C.; Wei, M.; Ba, X.; Zeng, X. Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells. Cancer Sci. 2018, 109, 3826–3839. [Google Scholar] [CrossRef] [PubMed]
- Ijichi, H.; Chytil, A.; Gorska, A.E.; Aakre, M.E.; Bierie, B.; Tada, M.; Mohri, D.; Miyabayashi, K.; Asaoka, Y.; Maeda, S.; et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J. Clin. Investig. 2011, 121, 4106–4117. [Google Scholar] [CrossRef]
- Ning, Y.; Labonte, M.J.; Zhang, W.; Bohanes, P.O.; Gerger, A.; Yang, D.; Benhaim, L.; Paez, D.; Rosenberg, D.O.; Venkata, K.C.N.; et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol. Cancer Ther. 2012, 11, 1353–1364. [Google Scholar] [CrossRef] [Green Version]
- Angara, K.; Borin, T.F.; Rashid, M.H.; Lebedyeva, I.; Ara, R.; Lin, P.C.; Iskander, A.; Bollag, R.J.; Achyut, B.R.; Arbab, A.S. CXCR2-expressing tumor cells drive vascular mimicry in antiangiogenic therapy-resistant glioblastoma. Neoplasia 2018, 20, 1070–1082. [Google Scholar] [CrossRef]
- Cheng, Y.; Mo, F.; Li, Q.; Han, X.; Shi, H.; Chen, S.; Wei, Y.; Wei, X. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol. Cancer 2021, 20, 62. [Google Scholar] [CrossRef]
- Devapatla, B.; Sharma, A.; Woo, S. CXCR2 inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian cancer. PLoS ONE 2015, 10, e0139237. [Google Scholar]
- Sapoznik, S.; Ortenberg, R.; Galore-Haskel, G.; Kozlovski, S.; Levy, D.; Avivi, C.; Barshack, I.; Cohen, C.J.; Besser, M.J.; Schachter, J.; et al. CXCR1 as a novel target for directing reactive T cells toward melanoma: Implications for adoptive cell transfer immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1833–1847. [Google Scholar] [CrossRef]
- Peng, W.; Ye, Y.; Rabinovich, B.A.; Liu, C.; Lou, Y.; Zhang, M.; Whittington, M.; Yang, Y.; Overwijk, W.W.; Lizée, G.; et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin. Cancer Res. 2010, 16, 5458–5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, V.; Ligtenberg, M.A.; Zendehdel, R.; Seitz, C.; Duivenvoorden, A.; Wennerberg, E.; Colón, E.; Scherman-Plogell, A.H.; Lundqvist, A. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J. Immunother. Cancer 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rui, W.; Zheng, H.; Huang, D.; Yu, F.; Zhang, Y.; Dong, J.; Zhao, X.; Lin, X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur. J. Immunol. 2020, 50, 712–724. [Google Scholar] [CrossRef] [PubMed]





| Type of Cancer | Impact on Prognosis | Number of Patients Selected for Testing | Notes | Source |
|---|---|---|---|---|
| Breast cancer: invasive ductal breast cancer | Worse prognosis | 225 | disease-free survival and overall survival | [102] |
| Breast cancer: triple-negative breast cancer | Better prognosis | 290 | recurrence-free survival and overall survival | [113] |
| Colorectal cancer | Not associated with patient prognosis | 254 | recurrence-free survival and overall survival | [114] |
| Colorectal cancer | Worse prognosis | 134 | overall survival and disease-free survival | [103] |
| Esophageal cancer | Worse prognosis | 95 | overall survival | [104] |
| Gastric cancer | Better prognosis | 593 | from TCGA database, overall survival | [116] |
| Gastric cancer | Worse prognosis | 115 | cumulative survival | [16] |
| Gastric cancer | Worse prognosis | 269 | overall survival | [106] |
| Gastric cancer | Worse prognosis | 357 | overall survival | [105] |
| Gastric cancer | Worse prognosis | 155 | overall survival | [17] |
| Intrahepatic cholangiocellular carcinoma | Worse prognosis | 34 | overall survival | [107] |
| Kidney cancer: clear cell renal cell carcinoma | Better prognosis | 530 | from TCGA database, overall survival | [115] |
| Laryngeal squamous cell carcinoma | Worse prognosis | 109 | overall survival | [108] |
| Leukemia: acute myeloid leukemia | Worse prognosis | 45 | recurrence-free survival and overall survival | [101] |
| Lung cancer: Lung adenocarcinoma | Worse prognosis | 173 | recurrence-free survival and overall survival | [109] |
| Lung cancer: non-small cell lung cancer | Worse prognosis | 340 | disease-free survival and overall survival | [91] |
| Ovarian cancer | Worse prognosis | 240 | disease-free survival | [110] |
| Ovarian cancer | Worse prognosis | 370 | from TCGA database, overall survival | [111] |
| Pancreatic ductal adenocarcinoma | Not associated with patient prognosis | 102 | recurrence-free survival and overall survival | [117] |
| Pancreatic ductal adenocarcinoma | Worse prognosis | 44 | overall survival | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, J.; Kupnicka, P.; Chlubek, M.; Gorący, J.; Gutowska, I.; Baranowska-Bosiacka, I. CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer. Int. J. Mol. Sci. 2022, 23, 2168. https://doi.org/10.3390/ijms23042168
Korbecki J, Kupnicka P, Chlubek M, Gorący J, Gutowska I, Baranowska-Bosiacka I. CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer. International Journal of Molecular Sciences. 2022; 23(4):2168. https://doi.org/10.3390/ijms23042168
Chicago/Turabian StyleKorbecki, Jan, Patrycja Kupnicka, Mikołaj Chlubek, Jarosław Gorący, Izabela Gutowska, and Irena Baranowska-Bosiacka. 2022. "CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer" International Journal of Molecular Sciences 23, no. 4: 2168. https://doi.org/10.3390/ijms23042168