Water-Based Graphene Oxide–Silicon Hybrid Nanofluids—Experimental and Theoretical Approach
Abstract
:1. Introduction
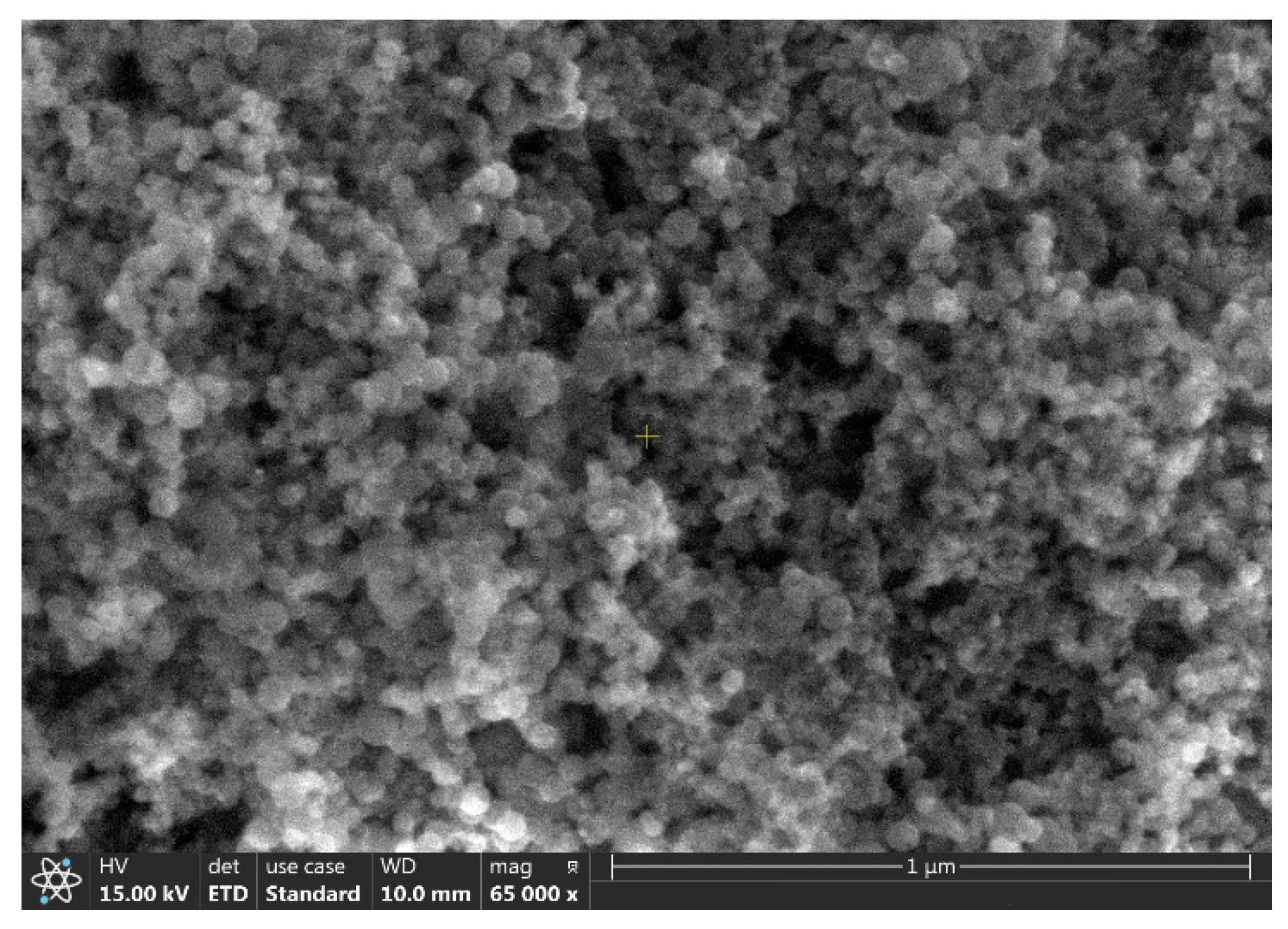
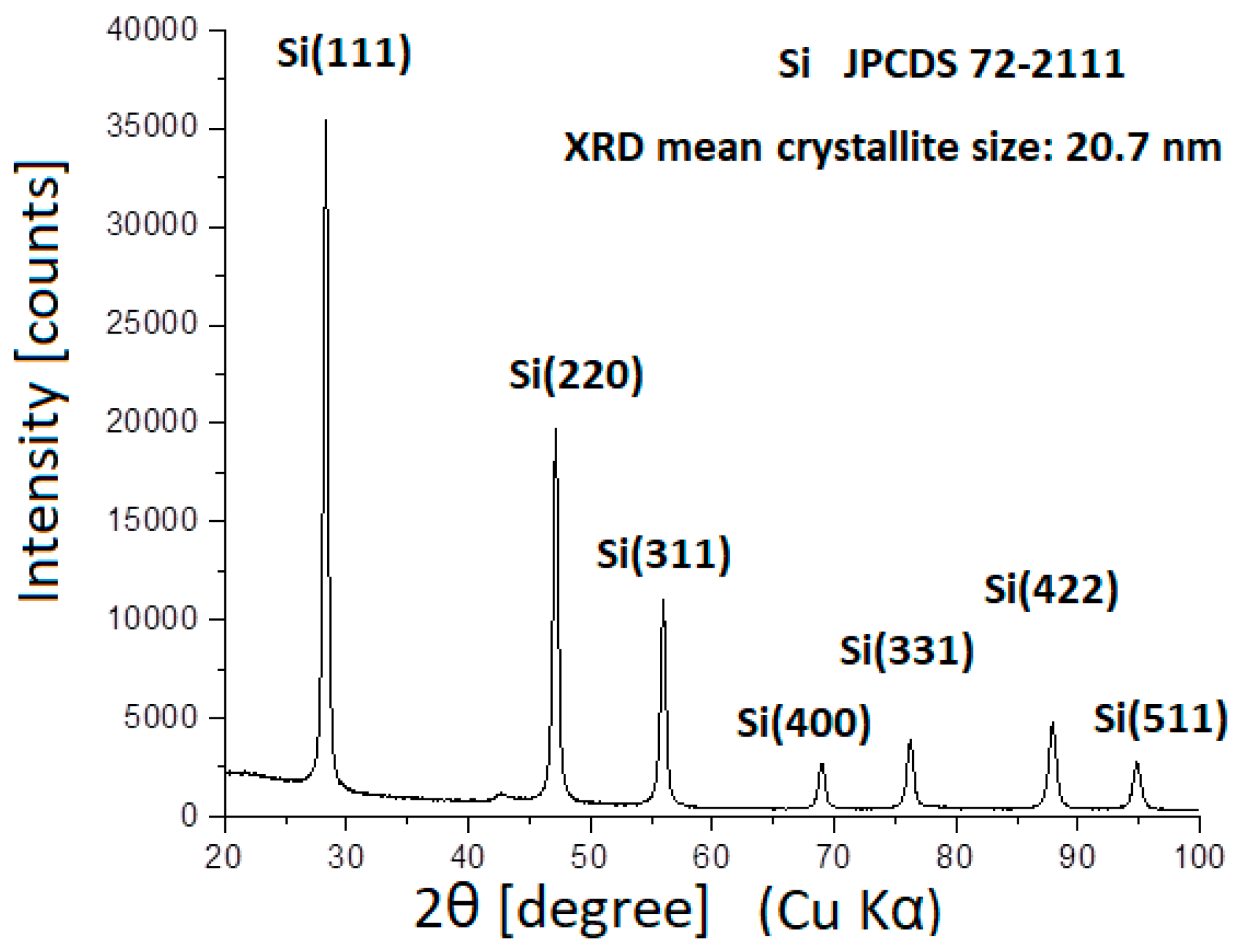
2. Experimental Procedure
3. Results and Discussions
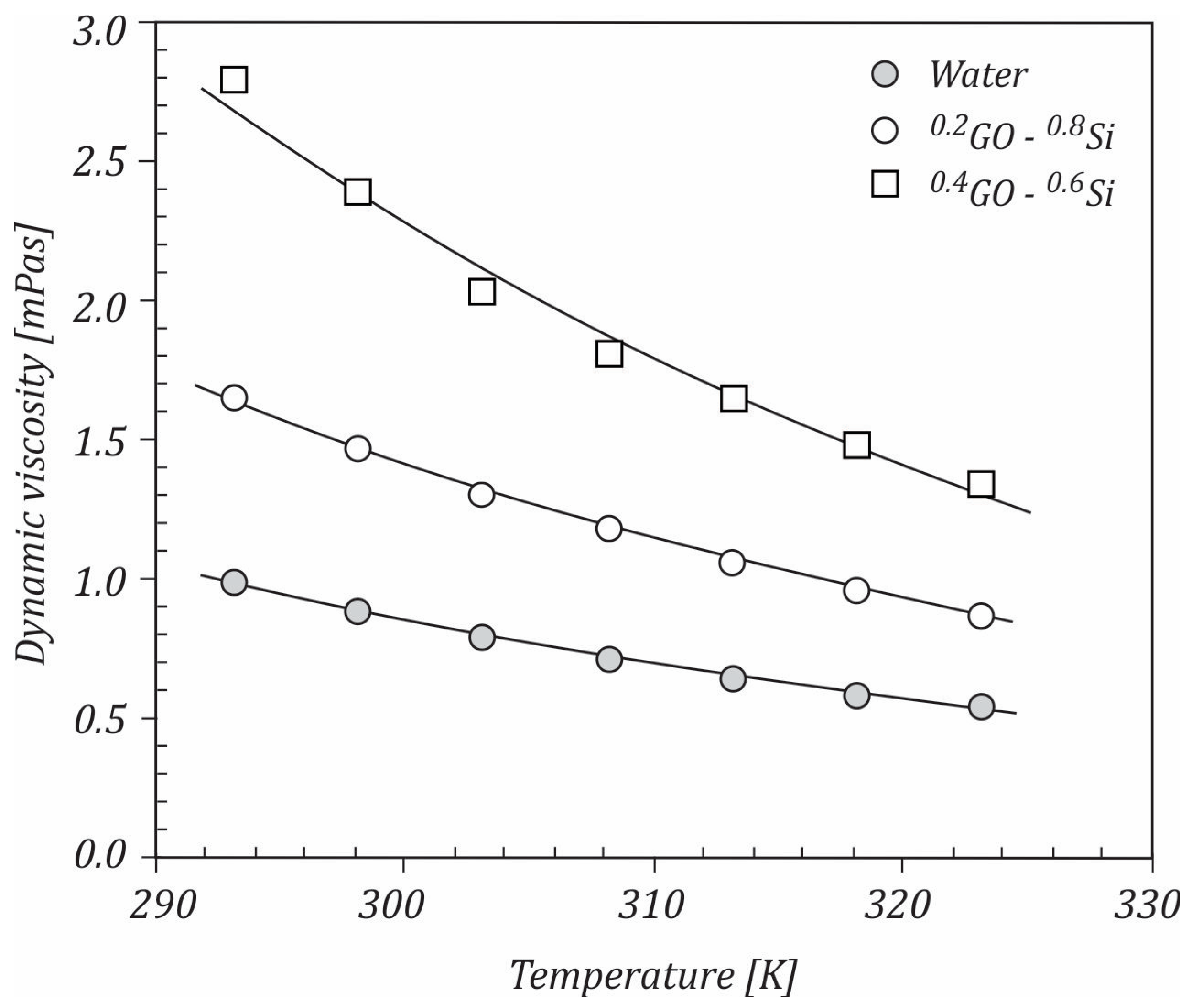
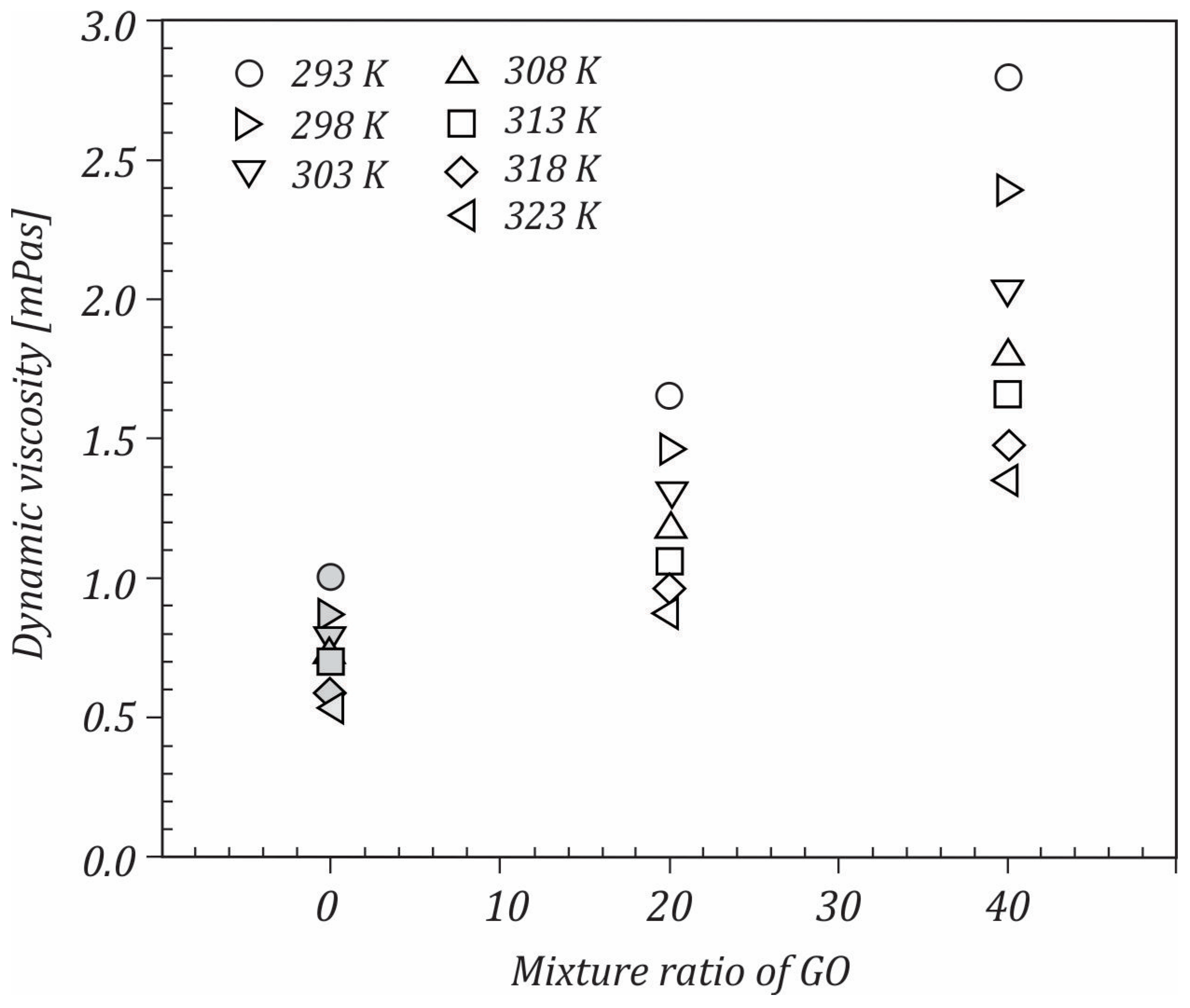
3.1. Dynamic Viscosity
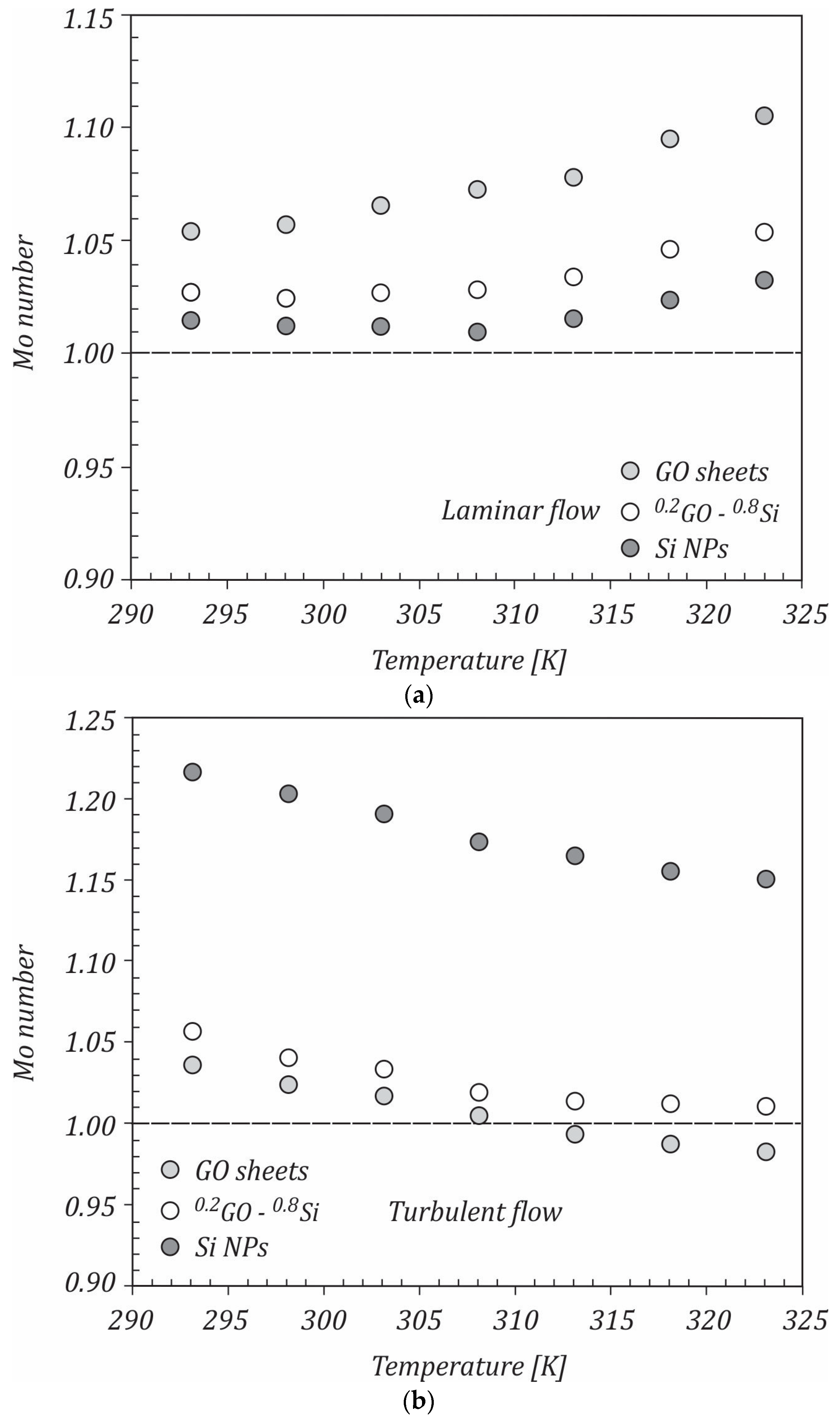
3.2. Criteria of Evaluation of the Performance of the Hybrid Nanofluids Based on Graphene Oxide−Silicon
- -
- density:
- -
- specific heat:
4. Conclusions
- -
- The increase in the graphene oxide content led to dynamic viscosity rising.
- -
- The current results were compared with the research from the available literature.
- -
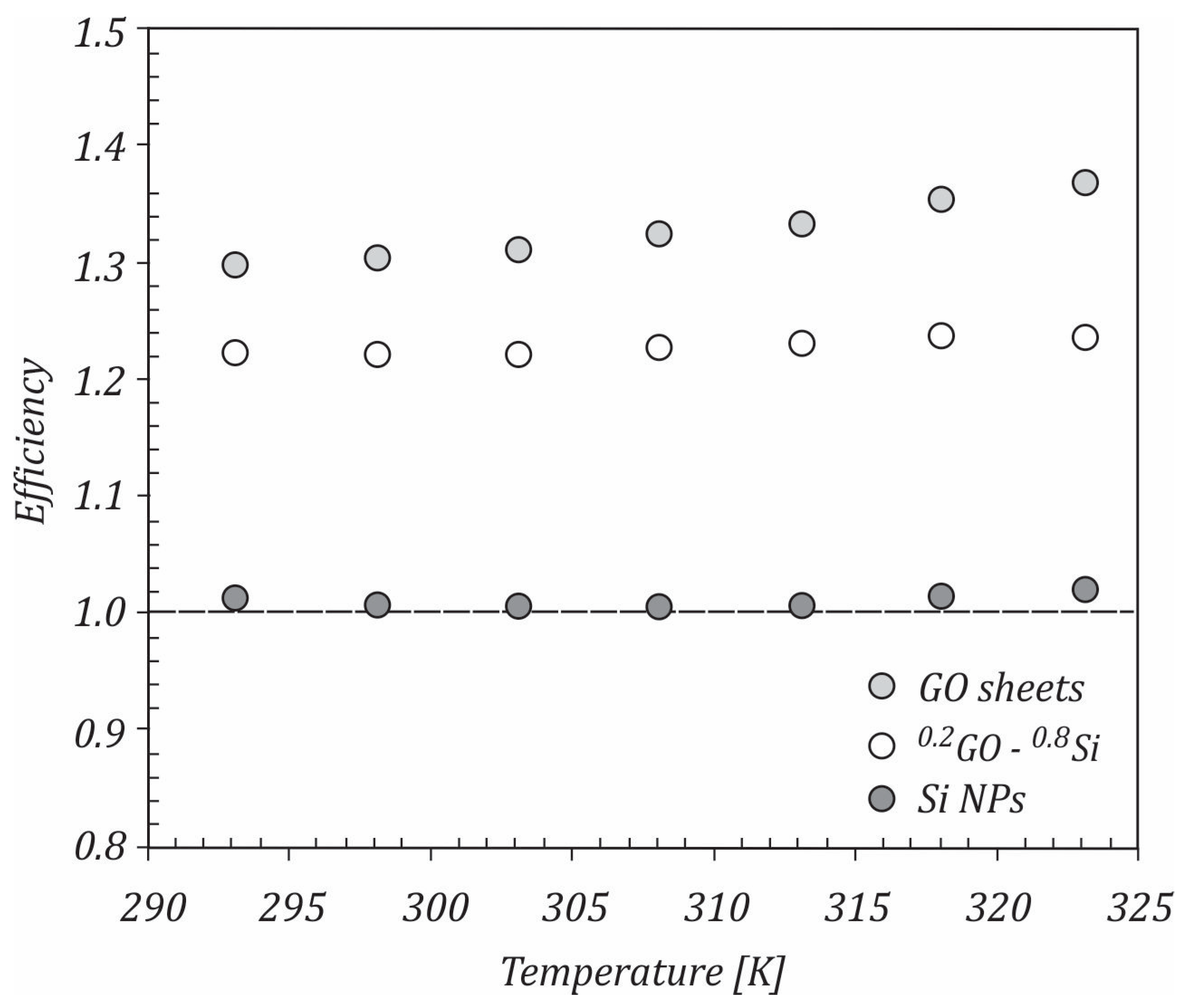
- In laminar and turbulent flows, the studied nanofluids showed an improvement in efficiency.
- -
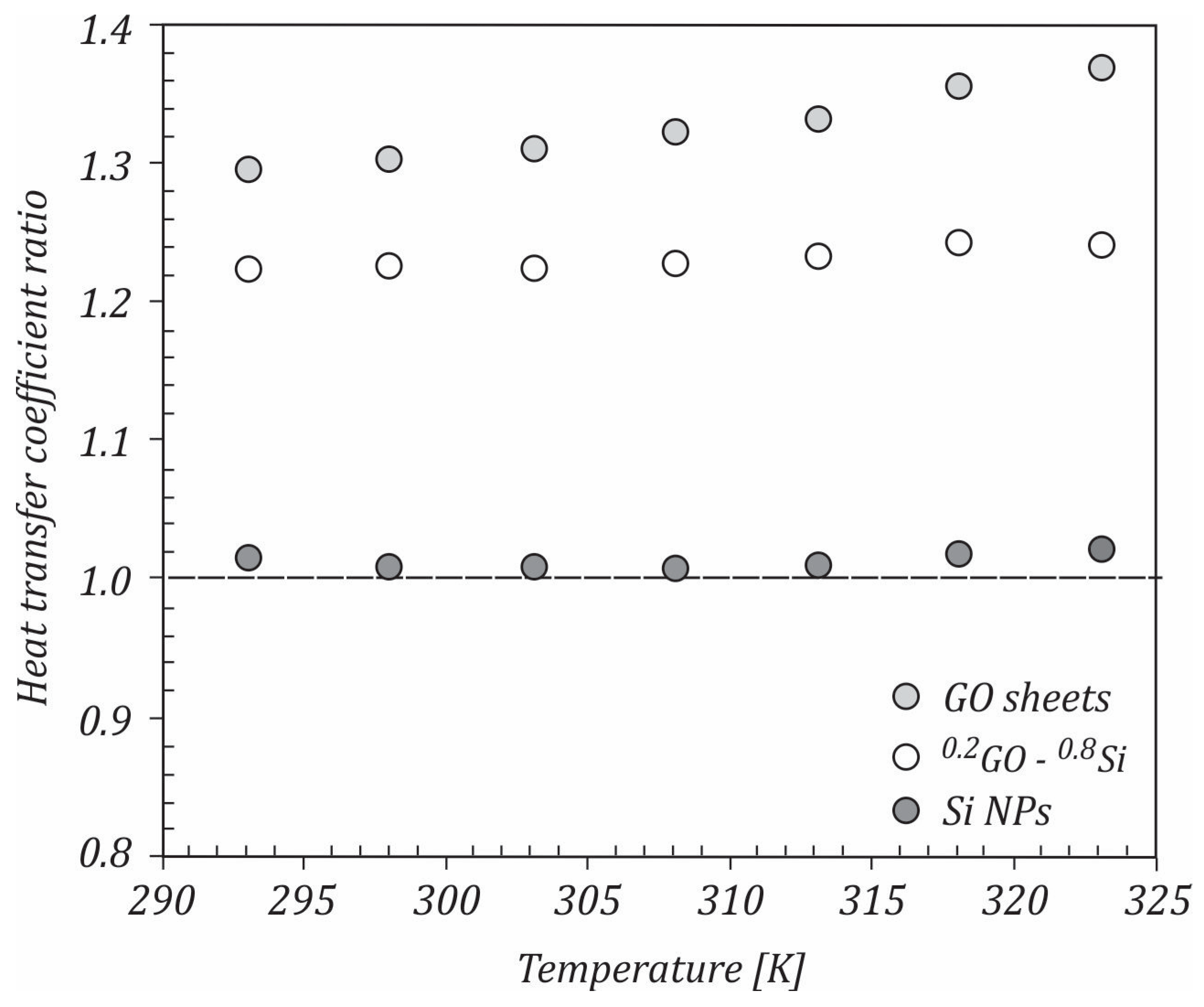
- Heat transfer coefficient ratios in the turbulent flow for all of the studied nanofluids were higher than 1, with the maximum values being achieved for the GO sheet nanofluids.
- -
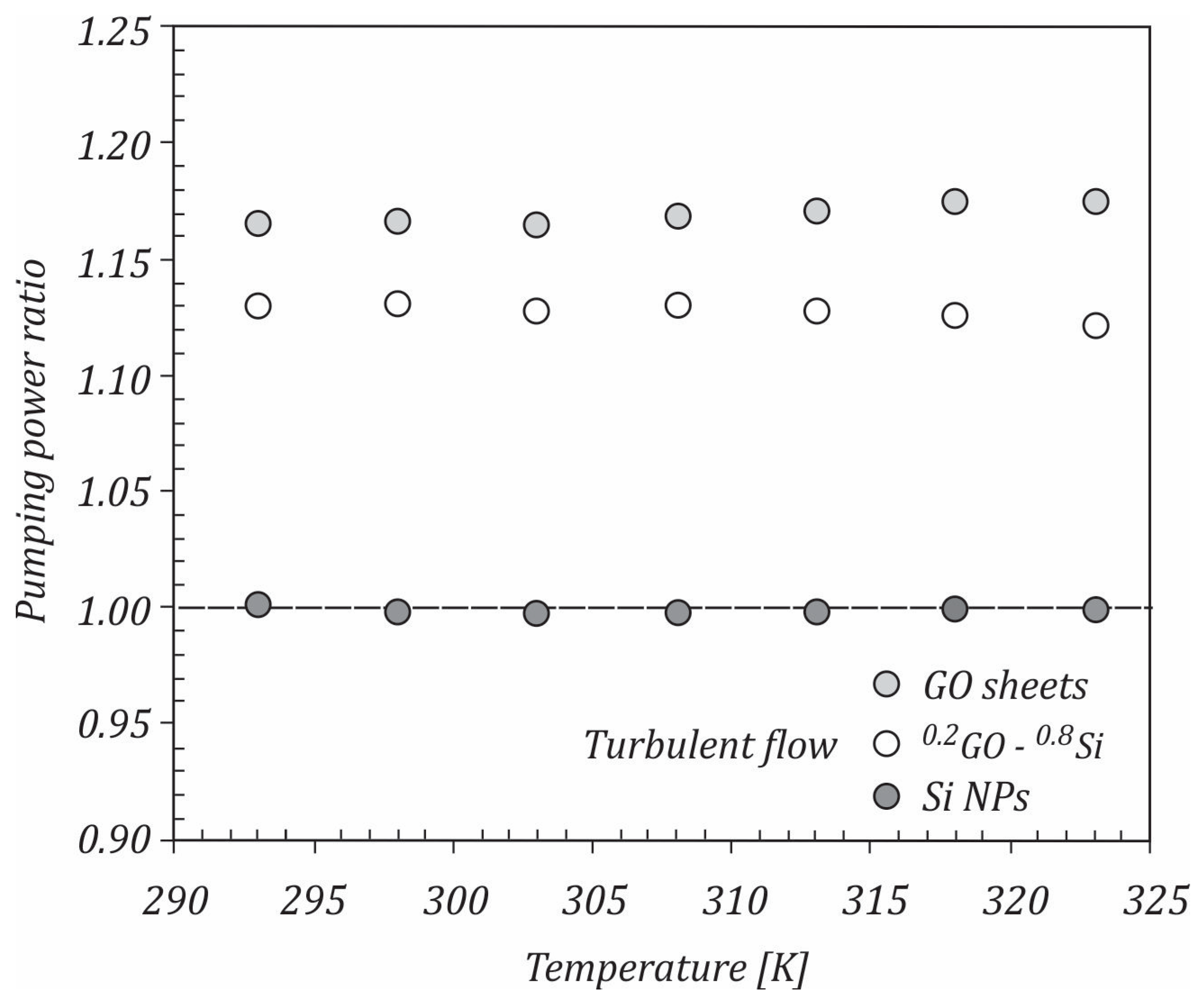
- The pumping power ratio for Si/water nanofluid was close to 1, while for GO/water and 0.2GO-0.8Si/water nanofluids, the pumping power ratios were higher than 1 for all temperatures.
- -
- A correlation for dynamic viscosity was proposed in this study. The equation is valid for temperature in the range of 25 °C to 50 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arshada, A.; Jabbala, M.; Yana, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A.; Fleaca, C.; Dumitrache, F.; Morjan, I. Experimental study on viscosity of water based Fe–Si hybrid nanofluids. J. Mol. Liq. 2021, 321, 114938. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A.; Dumitrache, F.; Fleaca, C.; Morjan, I. Study of the thermal conductivity of hybrid nanofluids: Recent research and experimental study. Powder Technol. 2020, 367, 347–357. [Google Scholar] [CrossRef]
- Hamze, S.; Cabaleiro, D.; Estellé, P. Graphene-based nanofluids: A comprehensive review about rheological behavior and dynamic viscosity. J. Mol. Liq. 2021, 325, 115207. [Google Scholar] [CrossRef]
- Hadadian, M.; Goharshadi, E.K.; Youssefi, A. Electrical conductivity, thermal conductivity, and rheological properties of graphene oxide-based nanofluids. J. Nanopart. Res. 2014, 16, 2788. [Google Scholar] [CrossRef]
- Ijam, A.; Saidur, R.; Ganesan, P.; Golsheikh, A.M. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int. J. Heat Mass Transf. 2015, 87, 92–103. [Google Scholar] [CrossRef]
- Kamatchi, R.; Venkatachalapathy, S.; Srinivas, B.A. Synthesis, stability, transport properties, and surface wettability of reduced graphene oxide/water nanofluids. Int. J. Therm. Sci. 2015, 97, 17–25. [Google Scholar] [CrossRef]
- Sedaghat, F.; Yousef, F. Synthesizes, characterization, measurements and modeling thermal conductivity and viscosity of graphene quantum dots nanofluids. J. Mol. Liq. 2019, 278, 299–308. [Google Scholar] [CrossRef]
- Hosseinghorbani, A.; Mozaffarian, M.; Pazuki, G. Application of graphene oxide IoNanofluid as a superior heat transfer fluid in concentrated solar power plants. Int. Commun. Heat Mass Transf. 2020, 111, 104450. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Estellé, P.; Navas, H.; Desforges, A.; Vigolo, B. Dynamic viscosity and surface tension of stable graphene oxide and reduced graphene oxide aqueous nanofluids. J. Nanofluids 2018, 7, 1081–1088. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M. Exergy analysis of a shell-and-tube heat exchanger using graphene oxide nanofluids. Exp. Therm. Fluid Sci. 2017, 83, 100–106. [Google Scholar] [CrossRef]
- Sarode, H.A.; Barai, D.P.; Bhanvase, B.A.; Ugwekar, R.P.; Saharan, V. Investigation on preparation of graphene oxide-CuO nanocomposite based nanofluids with the aid of ultrasound assisted method for intensified heat transfer properties. Mater. Chem. Phys. 2020, 251, 123102. [Google Scholar] [CrossRef]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Ferro, M.C.; Sousa, A.C.M. Experimental investigation of the thermal transport properties of graphene oxide/Co3O4 hybrid nanofluids. Int. Commun. Heat Mass Transf. 2017, 84, 1–10. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Akhgar, A.; Musivand, S.; Afrand, M. Effects of graphene oxide silicon oxide hybrid nanomaterials on rheological behavior of water at various time durations and temperatures: Synthesis, preparation and stability. Powder Technol. 2018, 335, 375–387. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Meghdadi Isfahani, A.H.; Afrand, M.; Karimipour, A.; Hojaji, M. An experimental study on heat transfer and pressure drop of water/graphene oxide nanofluid in a copper tube under air cross-flow: Applicable as a heat exchanger. Appl. Therm. Eng. 2017, 125, 69–79. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Tahan Latibari, S.; Mehrali, M.; Kazi, S.N.; Oon, C.S.; Metselaar, H.S.C. Experimental investigation of convective heat transfer using graphene nanoplatelet based nanofluids under turbulent flow conditions. Ind. Eng. Chem. Res. 2014, 53, 12455–12465. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Rosen, M.A.; Akhiani, A.R.; Tahan Latibari, S.; Mehrali, M.; Metselaar, H.S.C. Heat transfer and entropy generation for laminar forced convection flow of graphene nanosheets nanofluids in a horizontal tube. Int. Commun. Heat Mass Transf. 2015, 66, 23–31. [Google Scholar] [CrossRef]
- Bai, M.J.; Liu, J.L.; He, J.; Li, W.J.; Wei, J.J.; Chen, L.X.; Miao, J.Y.; Li, C.M. Heat transfer and mechanical friction reduction properties of graphene oxide nanofluids. Diam. Relat. Mater. 2020, 108, 107982. [Google Scholar] [CrossRef]
- Mahyari, A.A.; Karimipour, A.; Afrand, M. Effects of dispersed added Graphene Oxide-Silicon Carbide nanoparticles to present a statistical formulation for the mixture thermal properties. Physica A 2019, 521, 98–112. [Google Scholar] [CrossRef]
- Nguyen, Q.; Rizvandi, R.; Karimipour, A.; Malekahmadi, O.; Bach, Q.-V. A Novel Correlation to Calculate Thermal Conductivity of Aqueous Hybrid Graphene Oxide/Silicon Dioxide Nanofluid: Synthesis, Characterizations, Preparation, and Artificial Neural Network Modeling. Arab. J. Sci. Eng. 2020, 45, 9747–9758. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Thermal conductivity of single-layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hu, S.; Shih, J.F.; Yang, C.Y.; Luo, Y.W.; Jhang, R.H.; Chiang, C.M.; Hung, Y.J. Effective Synthesis of Highly Oxidized Graphene Oxide That Enables Wafer-scale Nanopatterning: Preformed Acidic Oxidizing Medium Approach. Sci. Rep. 2017, 7, 2308. [Google Scholar] [CrossRef] [Green Version]
- Flint, J.H.; Haggerty, J.S. A Model for the Growth of Silicon Particles from Laser-Heated Gases. Aerosol Sci. Technol. 1990, 13, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Lacour, F.; Guillois, O.; Portier, X.; Perez, H.; Herlin, N.; Reynaud, C. Laser pyrolysis synthesis and characterization of luminescent silicon nanocrystals. Physica E 2007, 38, 11–15. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Huang, J. Two Dimensional Soft Material: New Faces of Graphene Oxide. Acc. Chem. Res. 2012, 45, 1356–1364. [Google Scholar] [CrossRef]
- Vărdaru, A.; Huminic, G.; Huminic, A.; Dumitrache, F.; Fleacă, C.; Morjan, I. Synthesis, characterization and thermal conductivity of water based graphene oxide–silicon hybrid nanofluids: An experimental approach. J. Mol. Liq. 2022; submitted for publication. [Google Scholar]
- Zyła, G.; Vallejo, J.P.; Lugo, L. Isobaric heat capacity and density of ethylene glycol based nanofluids containing various nitride nanoparticle types: An experimental study. J. Mol. Liq. 2018, 261, 530–539. [Google Scholar] [CrossRef]
- Simons, R.E. Comparing heat transfer rates of liquid coolants using the Mouromtseff number. Electron. Cool. 2006, 12. [Google Scholar]
- Vajjha, R.S.; Das, D.K. A Review, Analysis on influence of temperature and fraction of nanofluids on thermophysical properties, heat transfer and pumping power. Int. J. Heat Mass Transf. 2012, 55, 4063–4078. [Google Scholar] [CrossRef]
- Cengel, Y.A. Heat Transfer: A Practical Approach, 2nd ed.; Mc Graw-Hill: New York, NY, USA, 2002; p. 441. [Google Scholar]
- Nadooshan, A.A.; Eshgarf, H.; Afrand, M. Measuring the viscosity of Fe3O4-MWCNTs/EG hybrid nanofluid for evaluation of thermal efficiency: Newtonian and non-Newtonian behavior. J. Mol. Liq. 2018, 253, 169–177. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Martín, E.I.; Teruel, M.; Gallardo, J.J.; Aguilar, T.; Gómez-Villarejo, R.; Alcántara, R.; Fernández-Lorenzo, C.; Piñero, J.C.; et al. On the enhancement of heat transfer fluid for concentrating solar power using Cu and Ni nanofluids: An experimental and molecular dynamics study. Nano Energy 2016, 27, 213–224. [Google Scholar] [CrossRef]
- Mansour, B.; Galanis, N.; Nguyen, C.T. Effect of uncertainties in physical properties on forced convection heat transfer with nanofluids. Appl. Therm. Eng. 2007, 27, 240–249. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Heat Transfer Capability of the Hybrid Nanofluids for Heat Transfer Applications. J. Mol. Liq. 2018, 272, 857–870. [Google Scholar] [CrossRef]







| Nanofluid | a | b | |
|---|---|---|---|
| GO sheets | 455.33 | −5.15 | 0.9939 |
| Si NPs | 386.33 | −5.554 | 0.9945 |
| 20GO-80Si | 787.72 | −5.75 | 0.9986 |
| 40G0-60Si | 2952.9 | −6.523 | 0.9874 |
| Nanoparticles/Base Fluid | ||
|---|---|---|
| Graphene oxide sheets (GO) [15] | 1910 | 710 |
| Silicon (Si) | 2329 | 700 |
| Water | 997 | 4181.6 |
| Nanofluid | Weight Concentration [%] | Volume Fraction [-] |
|---|---|---|
| GO sheets | 0.25 | 0.001307 |
| Si NPs | 0.001112 | |
| 20GO-80Si | 0.001073 |
| Nanofluid | GO/Water | 0.2GO-0.8Si/Water | Si/Water | |||
|---|---|---|---|---|---|---|
| Temp. | 293 K | 323 K | 293 K | 323 K | 293 K | 323 K |
| Dittus-Boelter correlation | 0.808 | 0.819 | 0.83 | 0.853 | 1.002 | 1.017 |
| Petukhov correlation | 1.306 | 1.381 | 1.235 | 1.253 | 1.016 | 1.021 |
| Gnielinski correlation | 1.297 | 1.386 | 1.226 | 1.242 | 1.016 | 1.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huminic, G.; Vărdaru, A.; Huminic, A.; Fleacă, C.; Dumitrache, F.; Morjan, I. Water-Based Graphene Oxide–Silicon Hybrid Nanofluids—Experimental and Theoretical Approach. Int. J. Mol. Sci. 2022, 23, 3056. https://doi.org/10.3390/ijms23063056
Huminic G, Vărdaru A, Huminic A, Fleacă C, Dumitrache F, Morjan I. Water-Based Graphene Oxide–Silicon Hybrid Nanofluids—Experimental and Theoretical Approach. International Journal of Molecular Sciences. 2022; 23(6):3056. https://doi.org/10.3390/ijms23063056
Chicago/Turabian StyleHuminic, Gabriela, Alexandru Vărdaru, Angel Huminic, Claudiu Fleacă, Florian Dumitrache, and Ion Morjan. 2022. "Water-Based Graphene Oxide–Silicon Hybrid Nanofluids—Experimental and Theoretical Approach" International Journal of Molecular Sciences 23, no. 6: 3056. https://doi.org/10.3390/ijms23063056







