Pain Modulation from the Locus Coeruleus in a Model of Hydrocephalus: Searching for Oxidative Stress-Induced Noradrenergic Neuroprotection
Abstract
:1. Introduction
2. Results
2.1. Hydrocephalic Animals Presented Increased Aqueduct and PAG Areas
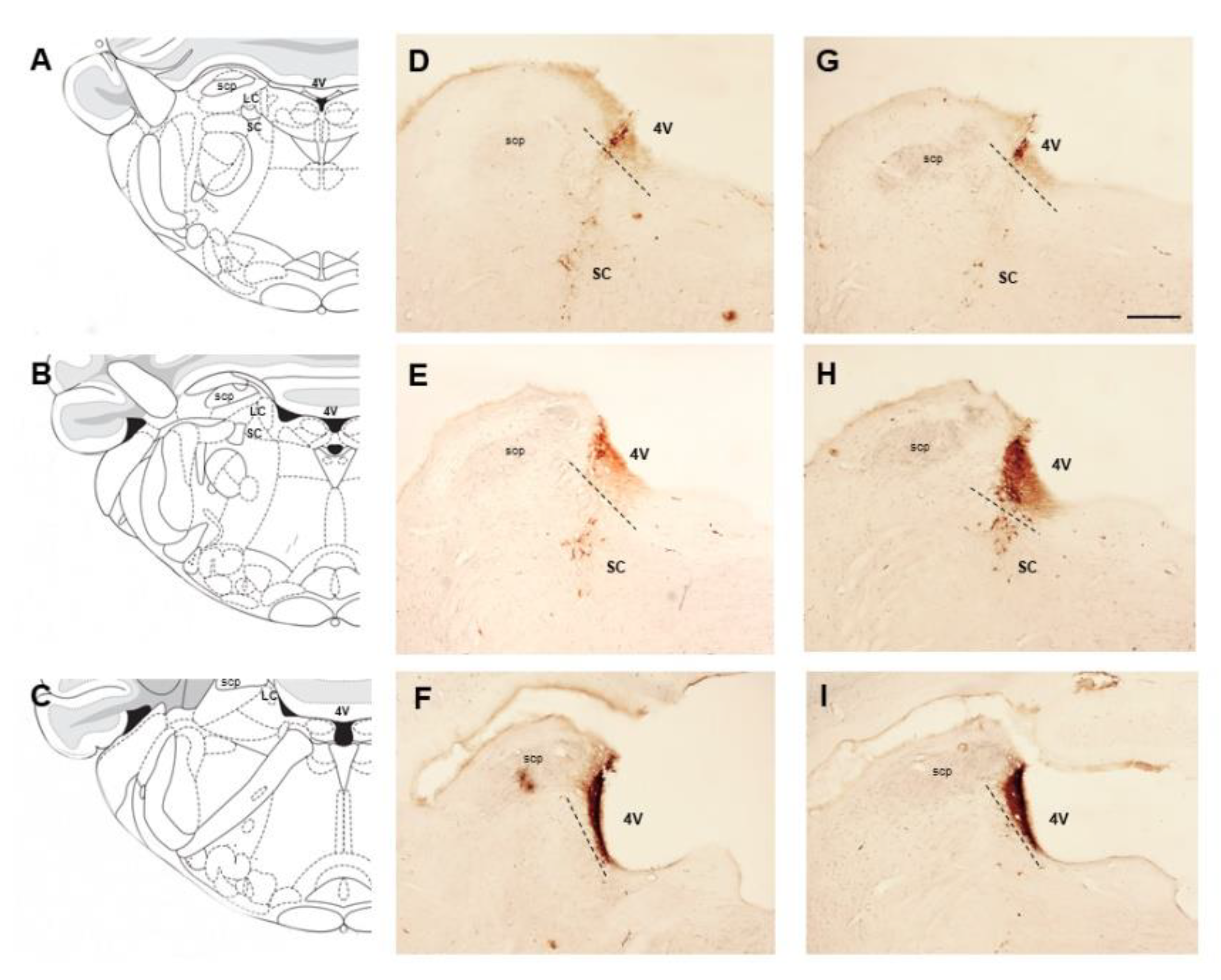
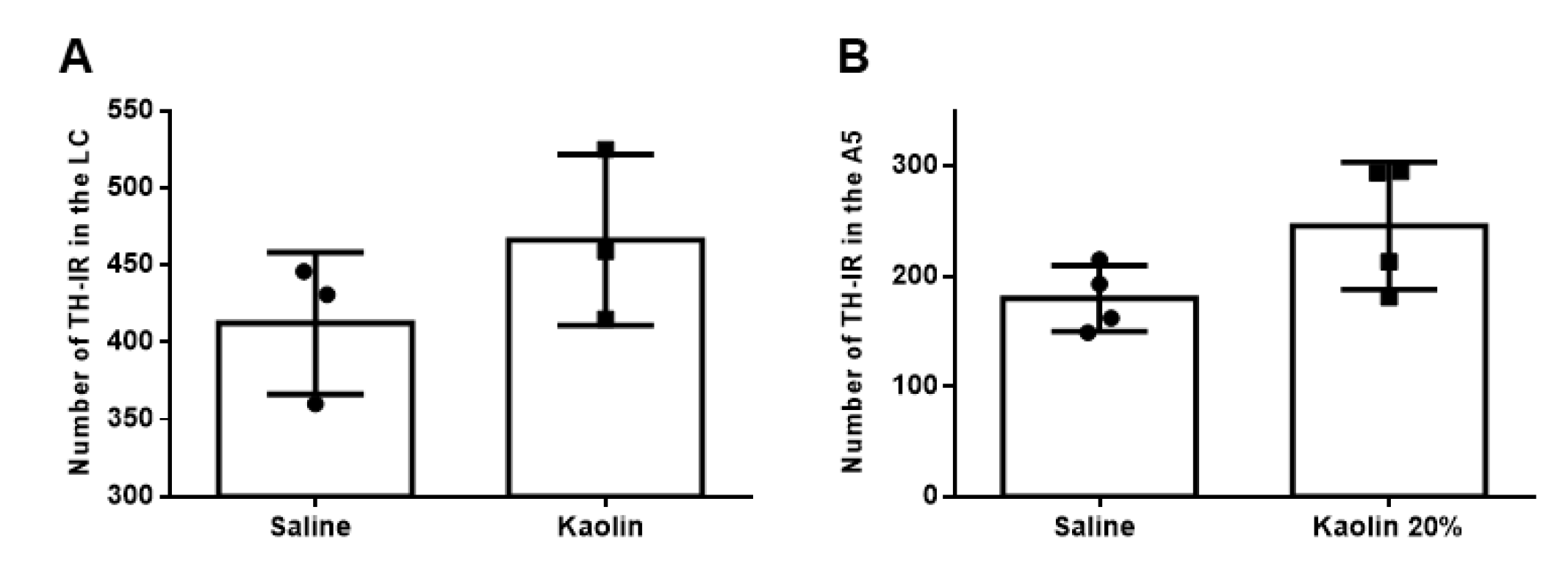
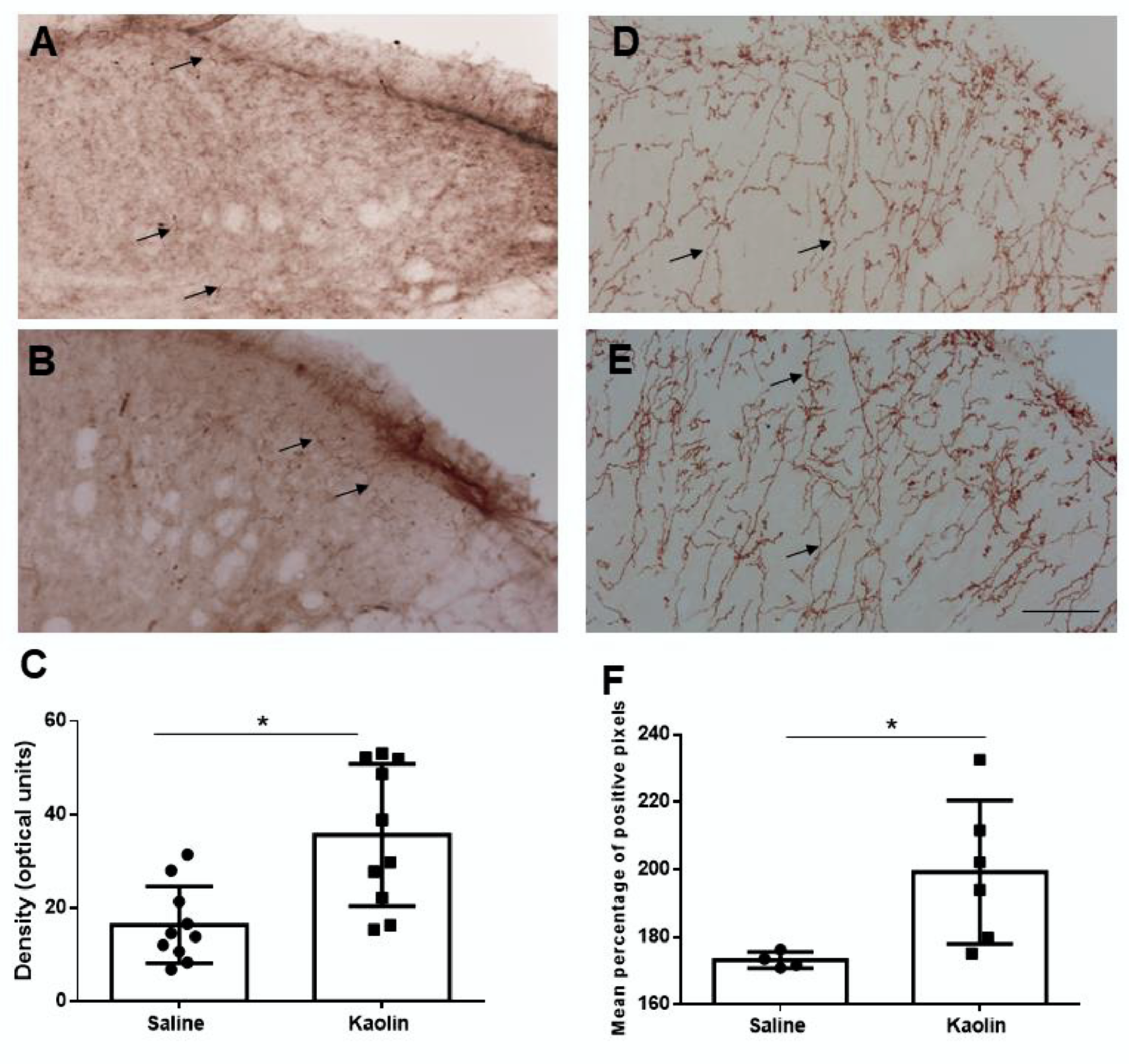
2.2. Hydrocephalic Animals Presented Increases in the Expression of Cathecolamine-Synthetizing Enzymes (Tyrosine Hydroxilase and Dopamine-β-Hydroxylase)
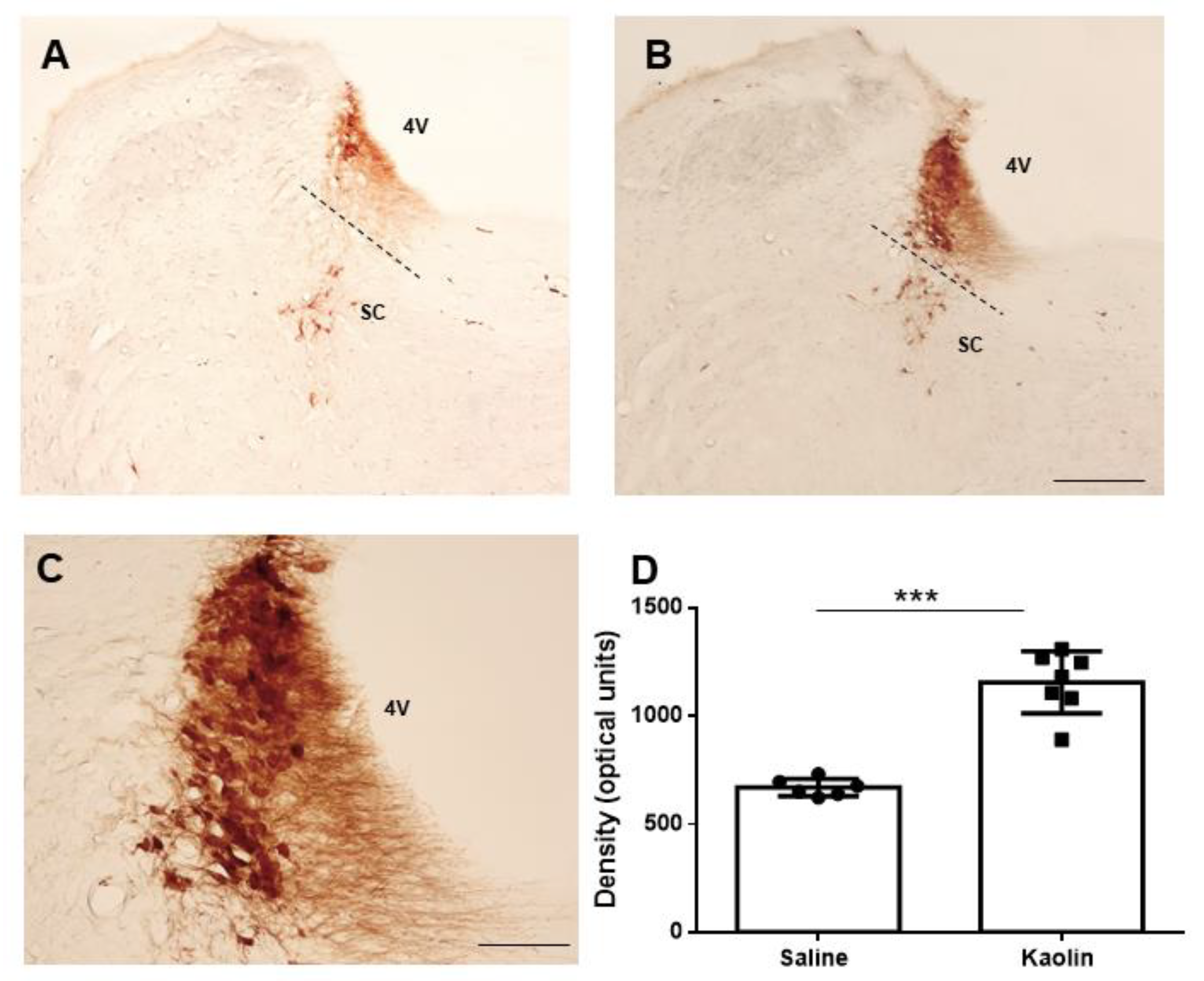
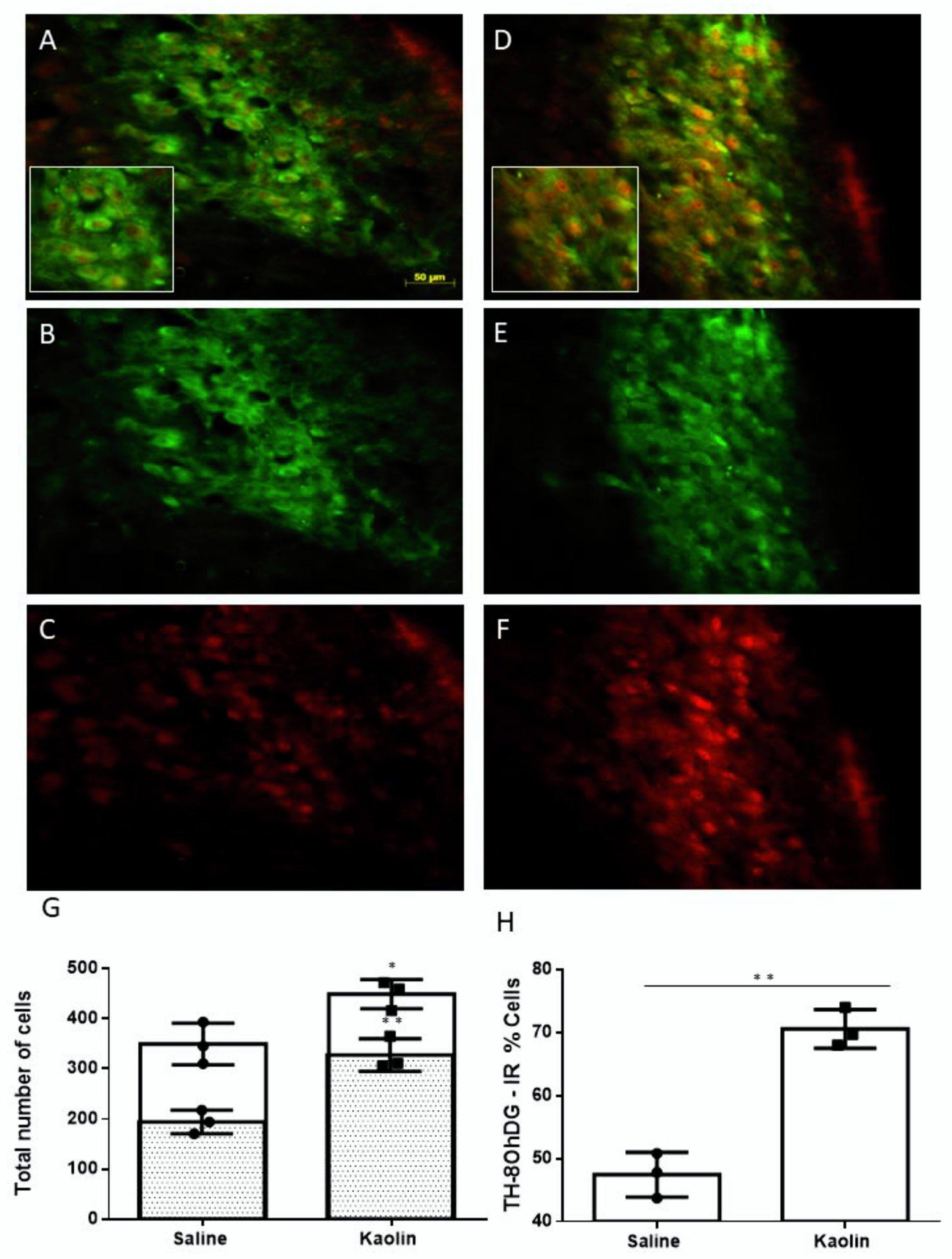
2.3. Hydrocephalic Rats Presented Increases in 8-hydroxyguanosine (8-OHdG) and Higher Co-Localization with TH at the LC
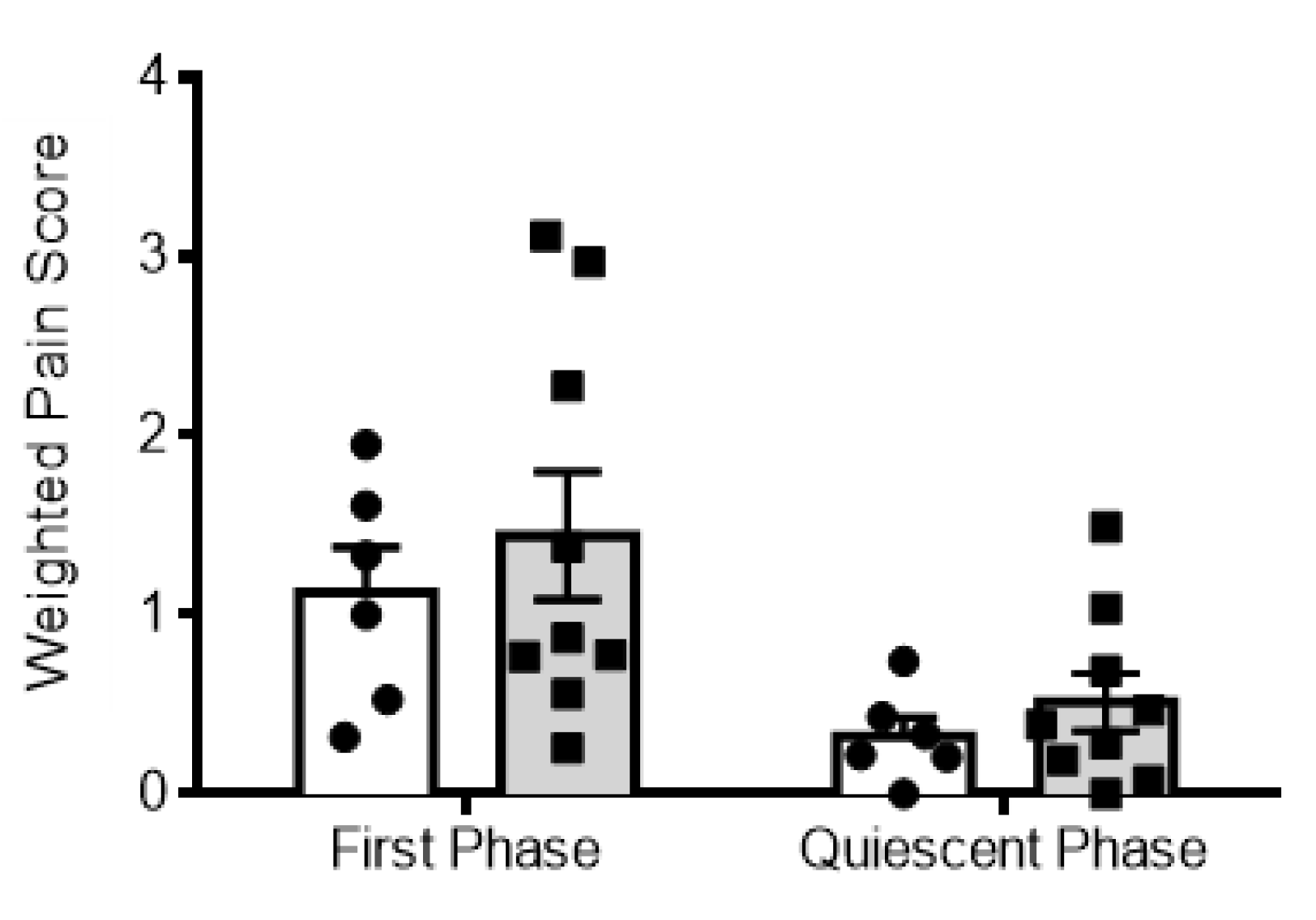
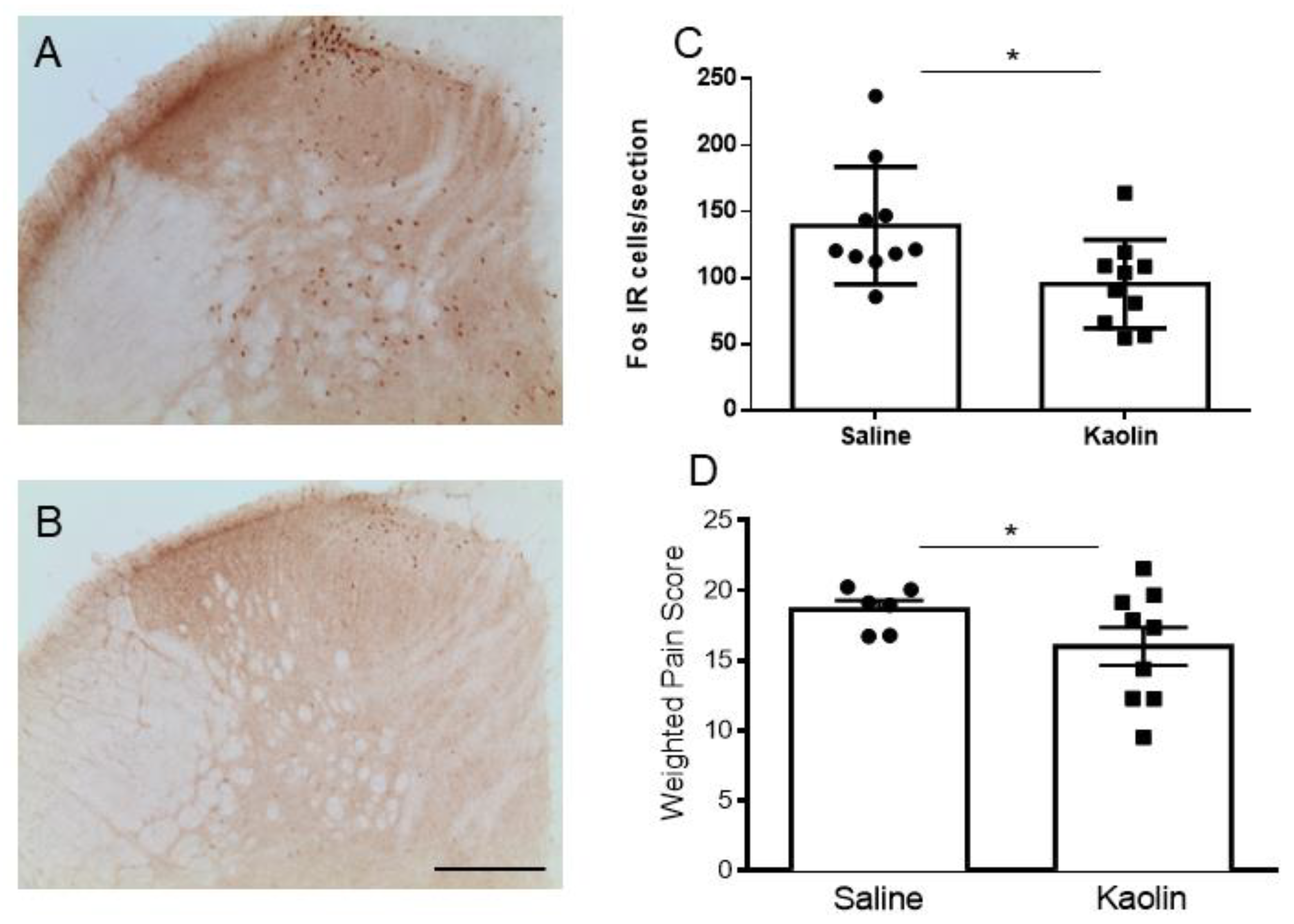
2.4. Hydrocephalic Rats Presented Decreases in Formalin-Evoked Fos Expression in the Spinal Dorsal Horn along with Decreased Nociceptive Behavioral Responses
3. Discussion
4. Materials and Methods
4.1. Surgical Induction of Hydrocephalus
4.2. Nociceptive Behavioral Evaluation (Formalin Test)
4.3. Vascular Perfusion and Material Processing for Immunohistochemical Analysis
4.4. Histological Analysis
4.5. Immunohistochemical Analysis of Fos Expression
4.6. Immunohistochemical Analysis of TH and DBH Expression
4.7. Double Immunodetection of TH and 8-OHdG
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Heinricher, M.; Tavares, I.; Leith, J.; Lumb, B. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.; Tavares, I. Reticular Formation and Pain: The Past and the Future. Front. Neuroanat. 2017, 11, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bigio, M.R. Neuropathology and structural changes in hydrocephalus. Dev. Disabil. Res. Rev. 2010, 16, 16–22. [Google Scholar] [CrossRef]
- Olopade, F.E.; Shokunbi, M.T.; Sirén, A.-L. The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS 2012, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Tan, G.; Zhang, S.; Zhu, H.; Liu, F.; Huang, C.; Zhang, F.; Wang, Z. Minocycline reduces reactive gliosis in the rat model of hydrocephalus. BMC Neurosci. 2012, 13, 148. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, S.; Tan, G.; Zhu, H.; Huang, C.; Zhang, F.; Wang, Z. Reactive gliosis and neuroinflammation in rats with communicating hydrocephalus. Neurosci. 2012, 218, 317–325. [Google Scholar] [CrossRef]
- Juge, L.; Pong, A.C.; Bongers, A.; Sinkus, R.; Bilston, L.E.; Cheng, S. Changes in Rat Brain Tissue Microstructure and Stiffness during the Development of Experimental Obstructive Hydrocephalus. PLoS ONE 2016, 11, e0148652. [Google Scholar] [CrossRef] [Green Version]
- McAllister, J.P., 2nd; Williams, M.A.; Walker, M.L.; Kestle, J.R.; Relkin, N.R.; Anderson, A.M.; Gross, P.H.; Browd, S.R.; Hydrocephalus Symposium Expert, P. An update on research priorities in hydrocephalus: Overview of the third National Institutes of Health-sponsored symposium “Opportunities for Hydrocephalus Research: Pathways to Better Outcomes”. J. Neurosurg. 2015, 123, 1427–1438. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.S.; Shim, I.; Chang, J.W. The behavioral change of locomotor activity in a kaolin-induced hydrocephalus rat model: Evaluation of the effect on the dopaminergic system with progressive ventricle dilatation. Neurosci. Lett. 2009, 462, 198–202. [Google Scholar] [CrossRef]
- Shim, I.; Ha, Y.; Chung, J.Y.; Lee, H.-J.; Yang, K.H.; Chang, J.W. Association of Learning and Memory Impairments with Changes in the Septohippocampal Cholinergic System in Rats with Kaolin-induced Hydrocephalus. Neurosurg. 2003, 53, 416–425. [Google Scholar] [CrossRef]
- Vachha, B.; Adams, R.C.; Rollins, N.K. Limbic Tract Anomalies in Pediatric Myelomeningocele and Chiari II Malformation: Anatomic Correlations with Memory and Learning—Initial Investigation. Radiology 2006, 240, 194–202. [Google Scholar] [CrossRef]
- Kopel, D.; Peeler, C.; Zhu, S. Headache Emergencies. Neurol. Clin. 2021, 39, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgi, F.S.; Biagioni, F.; Galgani, A.; Pavese, N.; Lazzeri, G.; Fornai, F. Locus Coeruleus Modulates Neuroinflammation in Parkinsonism and Dementia. Int. J. Mol. Sci. 2020, 21, 8630. [Google Scholar] [CrossRef]
- Pertovaara, A.; Almeida, A. Chapter 13 Descending inhibitory systems. Handb. Clin. Neurol. 2006, 81, 179–192. [Google Scholar] [CrossRef]
- Tavares, I.; Costa-Pereira, J.T.; Martins, I. Monoaminergic and Opioidergic Modulation of Brainstem Circuits: New Insights into the Clinical Challenges of Pain Treatment? Front. Pain Res. 2021, 2, 29. [Google Scholar] [CrossRef]
- Tavares, L.; Lima, D.; Coimbra, A. The Pontine A5Noradrenergic Cells which Project to the Spinal Cord Dorsal Horn are Reciprocally Connected with the Caudal Ventrolateral Medulla in the Rat. Eur. J. Neurosci. 1997, 9, 2452–2461. [Google Scholar] [CrossRef]
- Hammond, D.; Tyce, G.M.; Yaksh, T.L. Efflux of 5-hydroxytryptamine and noradrenaline into spinal cord superfusates during stimulation of the rat medulla. J. Physiol. 1985, 359, 151–162. [Google Scholar] [CrossRef]
- Cui, M.; Feng, Y.; McAdoo, D.J.; Willis, W.D. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J. Pharmacol. Exp. Ther. 1999, 289, 868–876. [Google Scholar]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [Green Version]
- Pertovaara, A. Noradrenergic pain modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; De Vries, M.G.; Teixeira-Pinto, A.; Fadel, J.; Wilson, S.P.; Westerink, B.H.C.; Tavares, I. Noradrenaline increases pain facilitation from the brain during inflammatory pain. Neuropharmacol. 2013, 71, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Fukumizu, M.; Becker, L.E.; Takashima, S. Periaqueductal microdysplasia in children with congenital aqueductal stenosis. Clin. Neuropathol. 1994, 13, 46–52. [Google Scholar] [PubMed]
- Álvarez-Diduk, R.; Galano, A. Adrenaline and Noradrenaline: Protectors against Oxidative Stress or Molecular Targets? J. Phys. Chem. B 2015, 119, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Jhang, K.A.; Lee, E.O.; Kim, H.-S.; Chong, Y.H. Norepinephrine provides short-term neuroprotection against Aβ1–42 by reducing oxidative stress independent of Nrf2 activation. Neurobiol. Aging 2014, 35, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.D.A.; Geihs, M.A.; Nogueira, T.D.S.; Allodi, S.; Vargas, M.A.; de Barros, C.M. Oxidative stress: Noradrenaline as an integrator of responses in the neuroendocrine and immune systems of the ascidian Phallusia nigra. Dev. Comp. Immunol. 2020, 105, 103573. [Google Scholar] [CrossRef]
- Taylor, B.K.; Westlund, K.N. The noradrenergic locus coeruleus as a chronic pain generator. J. Neurosci. Res. 2017, 95, 1336–1346. [Google Scholar] [CrossRef]
- Guzelcicek, A.; Koyuncu, I.; Gönel, A.; Cigdem, G.; Karadag, M. Relationship between Oxidative Stress, Tau Level and Antioxidant Mechanisms of the KEAP-1/NRF-2/HO-1 in Children with Hydrocephalus. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2021, 20, 282–289. [Google Scholar] [CrossRef]
- Bucci, D.; Busceti, C.L.; Calierno, M.T.; Di Pietro, P.; Madonna, M.; Biagioni, F.; Ryskalin, L.; Limanaqi, F.; Nicoletti, F.; Fornai, F. Systematic Morphometry of Catecholamine Nuclei in the Brainstem. Front. Neuroanat. 2017, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Hoffmeister, J.D.; Kelm-Nelson, C.A.; Ciucci, M.R. Quantification of brainstem norepinephrine relative to vocal impairment and anxiety in the Pink1-/- rat model of Parkinson disease. Behav. Brain Res. 2021, 414, 113514. [Google Scholar] [CrossRef]
- Arikawe, A.P.; Rorato, R.C.; Gomes, N.; Elias, L.L.; Anselmo-Franci, J. Hormonal and neural responses to restraint stress in an animal model of perimenopause in female rats. J. Neuroendocr. 2021, 33, e12976. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Molliver, M. The locus coeruleus in the rat: An immunohistochemical delineation. Neuroscience 1980, 5, 21–40. [Google Scholar] [CrossRef]
- Swanson, L. The locus coeruleus: A cytoarchitectonic, golgi and immunohistochemical study in the albino rat. Brain Res. 1976, 110, 39–56. [Google Scholar] [CrossRef]
- Ehara, K.; Tanaka, C.; Tamaki, N.; Matsumoto, S. Changes in the Hypothalamic and Brain Stem Catecholaminergic Systems in Experimental Hydrocephalus: A Histochemical Observation. Brain Edema XIII 1991, 75–87. [Google Scholar]
- Chovanes, G.I.; McAllister, J.P., 2nd; Lamperti, A.A.; Salotto, A.G.; Truex, R.C., Jr. Monoamine alterations during experimental hydrocephalus in neonatal rats. Neurosurgery 1988, 22, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Eghwrudjakpor, P.O.; Sakamoto, T.; Mori, K. Catecholamine alterations in experimental hydrocephalus. Child’s Nerv. Syst. 1992, 8, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Pong, A.C.; Juge, L.; Bilston, L.E.; Cheng, S. Development of acute hydrocephalus does not change brain tissue mechanical properties in adult rats, but in juvenile rats. PLoS ONE 2017, 12, e0182808. [Google Scholar] [CrossRef] [Green Version]
- Costa-Pereira, J.T.; Serrão, P.; Martins, I.; Tavares, I. Serotoninergic pain modulation from the rostral ventromedial medulla (RVM) in chemotherapy-induced neuropathy: The role of spinal 5-HT3 receptors. Eur. J. Neurosci. 2019, 51, 1756–1769. [Google Scholar] [CrossRef]
- Clark, F.M.; Proudfit, H.K. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991, 547, 279–288. [Google Scholar] [CrossRef]
- García, G.B.; Konjuh, C.; Duffard, R.O.; Evangelista de Duffard, A.M. Dopamine-beta-hydroxylase immunohistochemical study in the locus coeruleus of neonate rats exposed to 2,4-dichlorophenoxyacetic acid through mother’s milk. Drug Chem. Toxicol. 2006, 29, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Waterhouse, B.D.; Mihailoff, G.A. Evidence that dopamine-beta-hydroxylase immunoreactive neurons in the lateral reticular nucleus project to the spinal cord in the rat. Anat. Rec. 2001, 263, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Takahashi, S.; Miyamoto, A.; Oki, J.; Cho, K.; Okuno, A. Developmental changes in the noradrenergic innervations of spinal motoneurons in neonatal rats. Pediatr. Neurol. 1996, 14, 21–27. [Google Scholar] [CrossRef]
- Zhang, J.; Darling, R.D.; Paul, I.A.; Simpson, K.L.; Chen, K.; Shih, J.C.; Lin, R.C. Altered Expression of Tyrosine Hydroxylase in the Locus Coeruleus Noradrenergic System in Citalopram Neonatally Exposed Rats and Monoamine Oxidase A Knock Out Mice. Anat. Rec. 2011, 294, 1685–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, M.; Lima, D.; Tavares, I. Neuronal activation at the spinal cord and medullary pain control centers after joint stimulation: A c-fos study in acute and chronic articular inflammation. Neurosci. 2007, 147, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.A.; Cavazzuti, M. Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog. Neurobiol. 1993, 41, 565–607. [Google Scholar] [CrossRef]
- Wheeler-Aceto, H.; Cowan, A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacol. 1991, 104, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tjølsen, A.; Berge, O.-G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Kalinin, S.; Braun, D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: Noradrenergic signaling system. J. Neurochem. 2016, 139, 154–178. [Google Scholar] [CrossRef] [Green Version]
- Traver, S.; Salthun-Lassalle, B.; Marien, M.; Hirsch, E.; Colpaert, F.; Michel, P.P. The Neurotransmitter Noradrenaline Rescues Septal Cholinergic Neurons in Culture from Degeneration Caused by Low-Level Oxidative Stress. Mol. Pharmacol. 2005, 67, 1882–1891. [Google Scholar] [CrossRef]
- Kelly, S.; He, B.; Perez, S.E.; Ginsberg, S.D.; Mufson, E.J.; Counts, S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ye, K.; Weinshenker, D. Norepinephrine Protects against Amyloid-beta Toxicity via TrkB. J. Alzheimer’s Dis. 2015, 44, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Almeida, A.; Størkson, R.; Lima, D.; Hole, K.; Tjølsen, A. The medullary dorsal reticular nucleus facilitates pain behaviour induced by formalin in the rat. Eur. J. Neurosci. 1999, 11, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 211, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Abbott, F.V.; Franklin, K.B.; Westbrook, F.R. The formalin test: Scoring properties of the first and second phases of the pain response in rats. Pain 1995, 60, 91–102. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier Academic Press: Amsterdam, The Netherlands; London, UK, 2007; ISBN 9780123919496. [Google Scholar]
- Donovick, P.J. A Metachromatic Stain for Neural Tissue. Stain Technol. 1974, 49, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Carvalho, P.; de Vries, M.G.; Teixeira-Pinto, A.; Wilson, S.P.; Westerink, B.H.C.; Tavares, I. Increased Noradrenergic Neurotransmission to a Pain Facilitatory Area of the Brain Is Implicated in Facilitation of Chronic Pain. Anesthesiology 2015, 123, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Wiste, A.K.; Arango, V.; Ellis, S.P.; Mann, J.J.; Underwood, M. Norepinephrine and serotonin imbalance in the locus coeruleus in bipolar disorder. Bipolar Disord. 2008, 10, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, J.T.; Ribeiro, J.; Martins, I.; Tavares, I. Role of Spinal Cord α2-Adrenoreceptors in Noradrenergic Inhibition of Nociceptive Transmission During Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2020, 13, 1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, L.L.; Viola, G.G.; Ferraz, A.C.; Da Cunha, C.; Deonizio, J.M.D.; Netto, C.A.; Achaval, M. A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res. Protoc. 2005, 16, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Biegon, A.; Fieldust, S. Reduced tyrosine hydroxylase immunoreactivity in locus coeruleus of suicide victims. Synapse 1992, 10, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Vathy, I.; He, H.-J.; Iodice, M.; Hnatczuk, O.C.; Rimanóczy, A. Prenatal morphine exposure differentially alters TH-immunoreactivity in the stress-sensitive brain circuitry of adult male and female rats. Brain Res. Bull. 2000, 51, 267–273. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louçano, M.; Oliveira, J.; Martins, I.; Vaz, R.; Tavares, I. Pain Modulation from the Locus Coeruleus in a Model of Hydrocephalus: Searching for Oxidative Stress-Induced Noradrenergic Neuroprotection. Int. J. Mol. Sci. 2022, 23, 3970. https://doi.org/10.3390/ijms23073970
Louçano M, Oliveira J, Martins I, Vaz R, Tavares I. Pain Modulation from the Locus Coeruleus in a Model of Hydrocephalus: Searching for Oxidative Stress-Induced Noradrenergic Neuroprotection. International Journal of Molecular Sciences. 2022; 23(7):3970. https://doi.org/10.3390/ijms23073970
Chicago/Turabian StyleLouçano, Marta, Joana Oliveira, Isabel Martins, Rui Vaz, and Isaura Tavares. 2022. "Pain Modulation from the Locus Coeruleus in a Model of Hydrocephalus: Searching for Oxidative Stress-Induced Noradrenergic Neuroprotection" International Journal of Molecular Sciences 23, no. 7: 3970. https://doi.org/10.3390/ijms23073970




