CD38–Cyclic ADP-Ribose Signal System in Physiology, Biochemistry, and Pathophysiology
Abstract
:1. Introduction
2. cADPR as an Intracellular Ca2+-Releasing Messenger
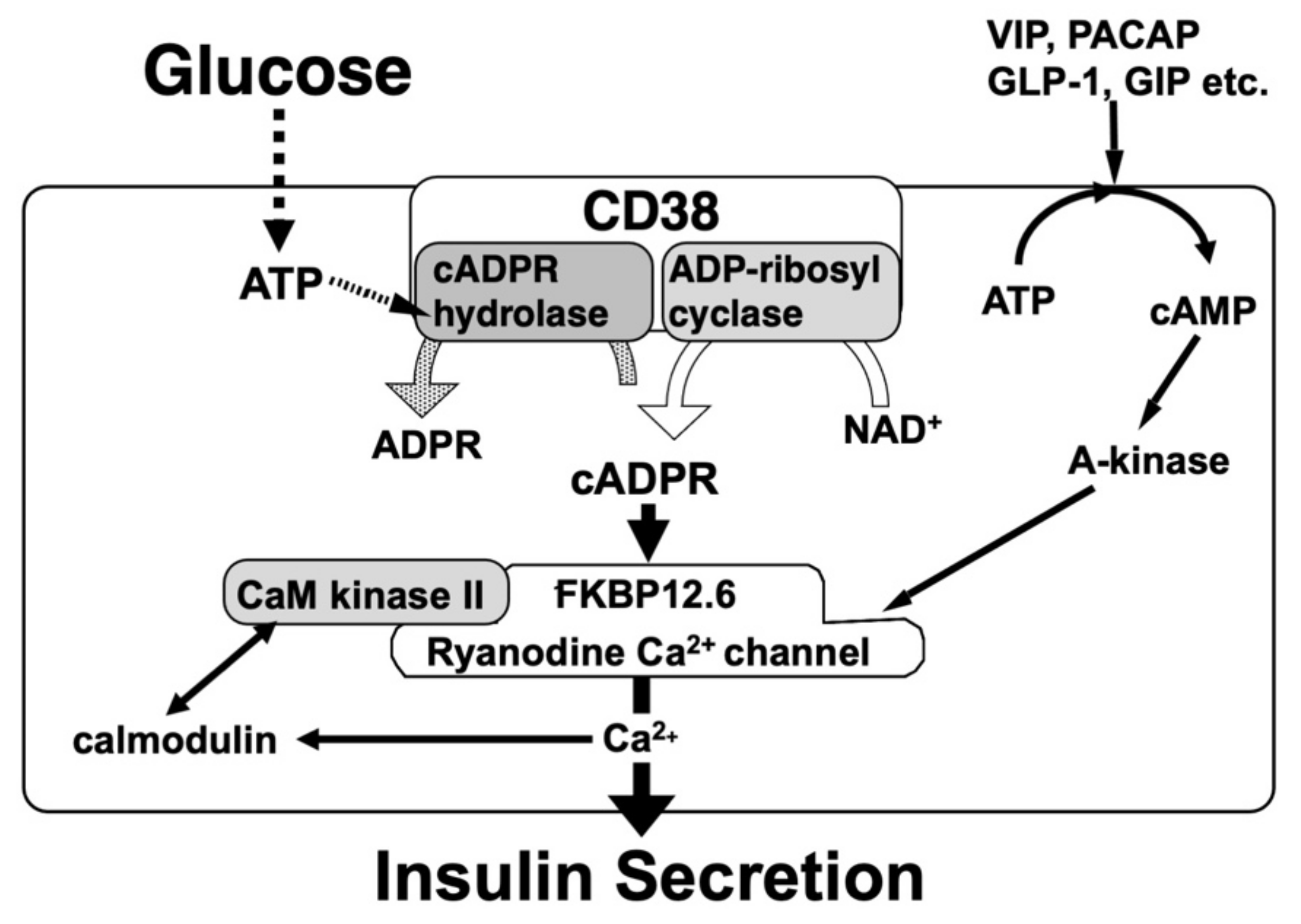
3. cADPR in Insulin Secretion from Pancreatic β-Cells
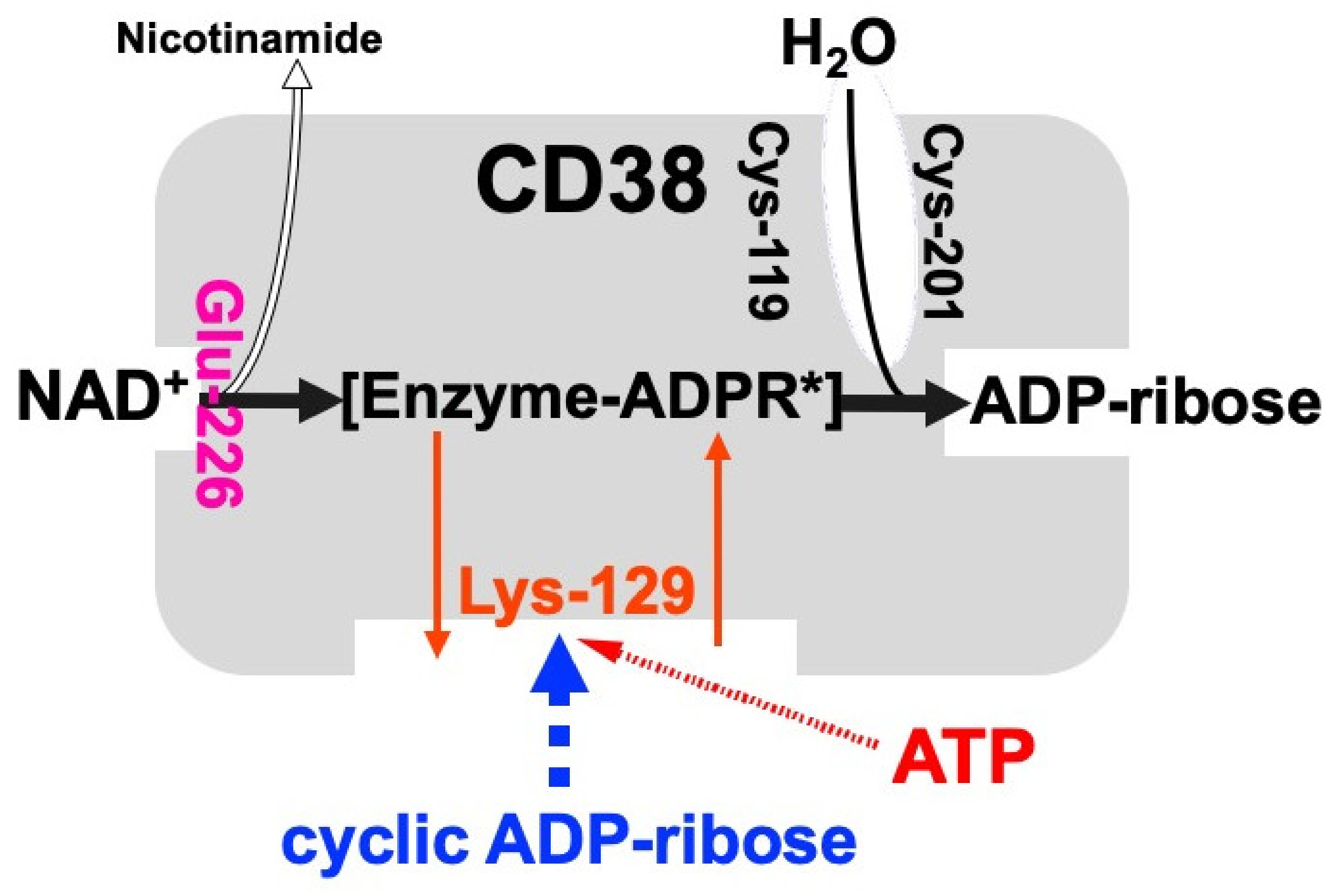
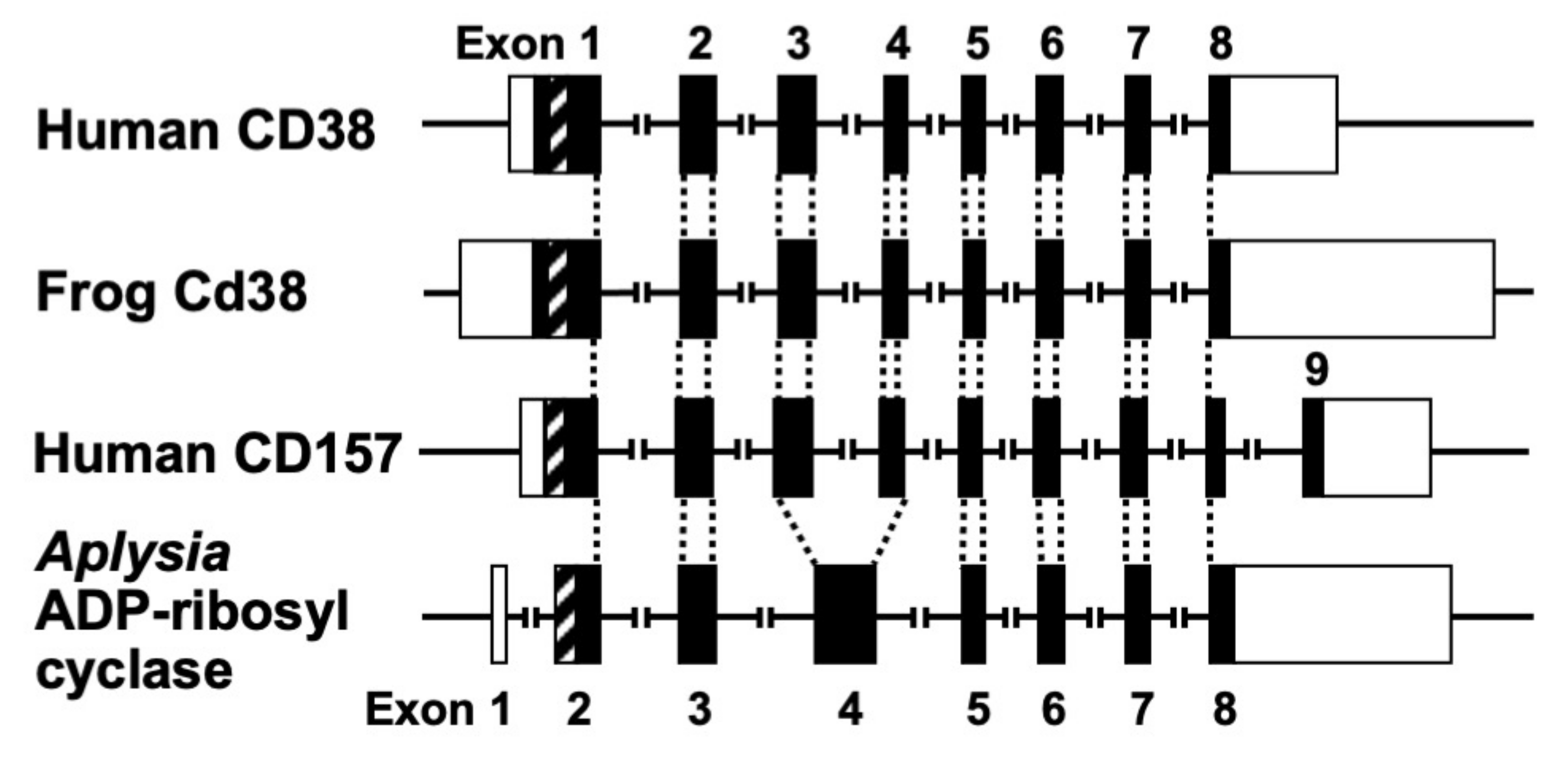
4. CD38 as a Major Enzyme for the Synthesis of cADPR
5. Physiological Significance of the CD38-cADPR Signal System in Mammalian Cells
6. Pathological Significance of the CD38-cADPR Signal System
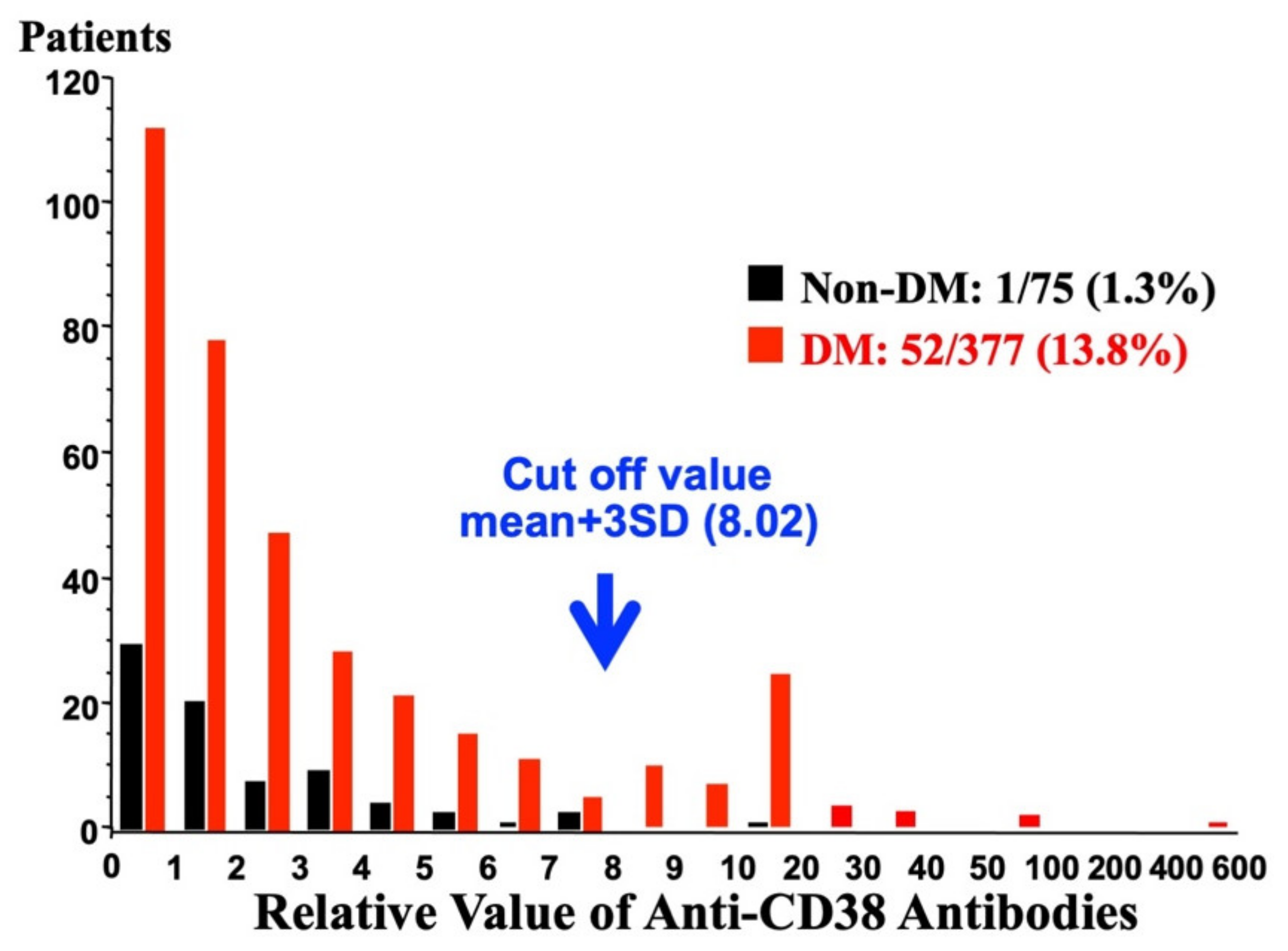
6.1. Pathological Significance of the CD38-cADPR System in Diabetes
6.2. Pathological Significance of the CD38-cADPR System Other Than Diabetes
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A-kinase | Cyclic AMP-dependent protein kinase |
| BST-1 | Bone marrow stromal cell antigen 1 |
| cADPR | Cyclic ADP-ribose |
| cAMP | Cyclic AMP |
| CaM | Calmodulin |
| CaM kinase | Ca2+/calmodulin-dependent protein kinase |
| CD38 | Cluster of differentiation 38 |
| CD157 | Cluster of differentiation 157 |
| CICR | Ca2+-induced Ca2+ release |
| EPAC2 | Exchange protein activated by cAMP2 |
| FKBP | FK506 binding protein |
| GIP | Gastric inhibitory polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| IH | Intermittent hypoxia |
| IP3 | Inositol 1,4,5-trisphosphate |
| NAADP | Nicotinic acid adenine dinucleotide phosphate |
| RyR | Ryanodine receptor Ca2+ channel |
| SAS | Sleep apnea syndrome |
References
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility of calcium signalling. Nat. Rev. Mol Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Guse, A.H.; Lee, H.C. NAADP: A universal Ca2+ trigger. Sci. Signal. 2008, 1, re10. [Google Scholar] [CrossRef] [PubMed]
- Clapper, D.L.; Walseth, T.F.; Dargie, P.J.; Lee, H.C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987, 262, 9561–9568. [Google Scholar] [CrossRef]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Strumwasser, F. Purification and characterization of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991, 2, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.C.; Aarhus, R. ADP-ribosyl cyclase: An enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991, 2, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Glick, D.L.; Hellmich, M.R.; Beushausen, S.; Tempst, P.; Bayley, H.; Strumwasser, F. Primary structure of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991, 2, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Nata, K.; Sugimoto, T.; Tohgo, A.; Takamura, T.; Noguchi, N.; Matsuoka, A.; Numakunai, T.; Shikama, K.; Yonekura, H.; Takasawa, S.; et al. The structure of the Aplysia kurodai gene encoding ADP-ribosyl cyclase, a second-messenger enzyme. Gene 1995, 158, 213–218. [Google Scholar] [CrossRef]
- States, D.J.; Walseth, T.F.; Lee, H.C. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human leukocyte antigen CD38. Trends. Biochem. Sci. 1992, 17, 495. [Google Scholar] [CrossRef]
- Howard, M.; Grimaldi, J.C.; Bazan, J.F.; Lund, F.E.; Santos-Argumedo, L.; Parkhouse, R.M.; Walseth, T.F.; Lee, H.C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993, 262, 1056–1059. [Google Scholar] [CrossRef]
- Takasawa, S.; Tohgo, A.; Noguchi, N.; Koguma, T.; Nata, K.; Sugimoto, T.; Yonekura, H.; Okamoto, H. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J. Biol. Chem. 1993, 268, 26052–26054. [Google Scholar] [CrossRef]
- Summerhill, R.J.; Jackson, D.G.; Galione, A. Human leukocyte antigen CD38 catalyzes the production of cyclic ADP-ribose. FEBS Lett. 1993, 335, 231–233. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Kimura, N.; Sato, K.; Ohsugi, Y.; Takasawa, S.; Okamoto, H.; Ishikawa, J.; Kaisho, T.; Ishihara, K.; Hirano, T. ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett. 1994, 356, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Chini, E.N.; Chini, C.C.; Kato, I.; Takasawa, S.; Okamoto, H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem. J. 2002, 362, 125–130. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Harrison, D.E.; Ashcroft, S.J.H. Glucose induced closure of single potassium channels in isolated rat pancreatic β-cells. Nature 1984, 312, 446–448. [Google Scholar] [CrossRef]
- Takasawa, S.; Nata, K.; Yonekura, H.; Okamoto, H. Cyclic ADP-ribose in insulin secretion from pancreatic β cells. Science 1993, 259, 370–373. [Google Scholar] [CrossRef]
- Okamoto, H.; Takasawa, S.; Tohgo, H. New aspects of the physiological significance of NAD, poly ADP-ribose and cyclic ADP-ribose. Biochimie 1995, 77, 356–363. [Google Scholar] [CrossRef]
- Okamoto, H.; Takasawa, S.; Nata, K. The CD38-cyclic ADP-ribose signalling system in insulin secretion: Molecular basis and clinical implications. Diabetologia 1997, 40, 1485–1491. [Google Scholar] [CrossRef] [Green Version]
- Koguma, T.; Takasawa, S.; Tohgo, A.; Karasawa, T.; Furuya, Y.; Yonekura, H.; Okamoto, H. Cloning and characterization of cDNA encoding rat ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase (homologue to human CD38) from islets of Langerhans. Biochim. Biophys. Acta 1994, 1223, 160–162. [Google Scholar] [CrossRef]
- Tohgo, A.; Takasawa, S.; Noguchi, N.; Koguma, T.; Nata, K.; Sugimoto, T.; Furuya, Y.; Yonekura, H.; Okamoto, H. Essential cysteine residues for cyclic ADP-ribose synthesis and hydrolysis by CD38. J. Biol. Chem. 1994, 269, 28555–28557. [Google Scholar] [CrossRef]
- Nakagawara, K.; Mori, M.; Takasawa, S.; Nata, K.; Takamura, T.; Berlova, A.; Tohgo, A.; Karasawa, T.; Yonekura, H.; Takeuchi, T.; et al. Assignment of CD38, the gene encoding human leukocyte antigen CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase), to chromosome 4p15. Cytogenet. Cell Genet. 1995, 69, 38–39. [Google Scholar] [CrossRef]
- Kato, I.; Takasawa, S.; Akabane, A.; Tanaka, O.; Abe, H.; Takamura, T.; Suzuki, Y.; Nata, K.; Yonekura, H.; Yoshimoto, T.; et al. Regulatory role of CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) in insulin secretion by glucose in pancreatic β cells. Enhanced insulin secretion in CD38-expressing transgenic mice. J. Biol. Chem. 1995, 270, 30045–30050. [Google Scholar] [CrossRef] [Green Version]
- Takasawa, S.; Ishida, A.; Nata, K.; Nakagawa, K.; Noguchi, N.; Tohgo, A.; Kato, I.; Yonekura, H.; Fujisawa, H.; Okamoto, H. Requirement of calmodulin-dependent protein kinase II in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization. J. Biol. Chem. 1995, 270, 30257–30259. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, N.; Takasawa, S.; Nata, K.; Tohgo, A.; Kato, I.; Ikehata, F.; Yonekura, H.; Okamoto, H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J. Biol. Chem. 1997, 272, 3133–3136. [Google Scholar] [CrossRef] [Green Version]
- Tohgo, A.; Munakata, H.; Takasawa, S.; Nata, K.; Akiyama, T.; Hayashi, N.; Okamoto, H. Lysine 129 of CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) participates in the binding of ATP to inhibit the cyclic ADP-ribose hydrolase. J. Biol. Chem. 1997, 272, 3879–3882. [Google Scholar] [CrossRef] [Green Version]
- Nata, K.; Takamura, T.; Karasawa, T.; Kumagai, T.; Hashioka, W.; Tohgo, A.; Yonekura, H.; Takasawa, S.; Nakamura, S.; Okamoto, H. Human gene encoding CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase): Organization, nucleotide sequence and alternative splicing. Gene 1997, 186, 285–292. [Google Scholar] [CrossRef]
- Takasawa, S.; Akiyama, T.; Nata, K.; Kuroki, M.; Tohgo, A.; Noguchi, N.; Kobayashi, S.; Kato, I.; Katada, T.; Okamoto, H. Cyclic ADP-ribose and inositol 1,4,5-trisphosphate as alternate second messengers for intracellular Ca2+ mobilization in normal and diabetic β-cells. J. Biol. Chem. 1998, 273, 2497–2500. [Google Scholar] [CrossRef] [Green Version]
- Yagui, K.; Shimada, F.; Miura, M.; Hashimoto, N.; Suzuki, Y.; Tokuyama, Y.; Nata, K.; Tohgo, A.; Ikehata, F.; Takasawa, S.; et al. A missense mutation in the CD38 gene, a novel factor for insulin secretion: Association with Type II diabetes mellitus in Japanese subjects and evidence of abnormal function when expressed in vitro. Diabetologia 1998, 41, 1024–1028. [Google Scholar] [CrossRef] [Green Version]
- Ikehata, F.; Satoh, J.; Nata, K.; Tohgo, A.; Nakazawa, T.; Kato, I.; Kobayashi, S.; Akiyama, T.; Takasawa, S.; Toyota, T.; et al. Autoantibodies against CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) that impair glucose-induced insulin secretion in noninsulin- dependent diabetes patients. J. Clin. Investig. 1998, 102, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Kato, I.; Yamamoto, Y.; Fujimura, M.; Noguchi, N.; Takasawa, S.; Okamoto, H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 1999, 274, 1869–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, H.; Takasawa, S.; Nata, K.; Kato, I.; Tohgo, A.; Noguchi, N. Physiological and pathological significance of the CD38-cyclic ADP-ribose signaling system. Chem. Immunol. 2000, 75, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, S.; Okamoto, H. Pancreatic β-cell death, regeneration and insulin secretion: Roles of poly(ADP-ribose) polymerase and cyclic ADP-ribose. Int. J. Diabetes Res. 2002, 3, 79–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, H.; Takasawa, S. Recent advances in the Okamoto model: The CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in β-cells. Diabetes 2002, 51 (Suppl. S3), S462–S473. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Larsson, O.; Berggren, P.O.; Takasawa, S.; Nata, K.; Yonekura, H.; Okamoto, H.; Galione, A. Cyclic ADP-ribose in β cells. Science 1993, 262, 584–586. [Google Scholar] [CrossRef] [Green Version]
- Rutter, G.A.; Theler, J.-M.; Wollheim, C.B. Ca2+ stores in insulin-secreting cells: Lack of the effect of cADP ribose. Cell Calcium 1994, 16, 71–80. [Google Scholar] [CrossRef]
- Webb, D.-L.; Islam, M.S.; Efanov, A.M.; Brown, G.; Köhler, M.; Larsson, O.; Berggren, P.-O. Insulin exocytosis and glucose-mediated increase in cytoplasmic free Ca2+ concentration in the pancreatic β-cell are independent of cyclic ADP-ribose. J. Biol. Chem. 1996, 271, 19074–19079. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Berggren, P.O. Cyclic ADP-ribose and the pancreatic beta cell: Where do we stand? Diabetologia 1997, 40, 1480–1484. [Google Scholar] [CrossRef] [Green Version]
- Malaisse, W.J.; Kanda, Y.; Inageda, K.; Scruel, O.; Sener, A.; Katada, T. Cyclic ADP-ribose measurements in rat pancreatic islets. Biochem. Biophys. Res. Commun. 1997, 231, 546–548. [Google Scholar] [CrossRef]
- Scruel, O.; Wada, T.; Kontani, K.; Sener, A.; Katada, T.; Malaisse, W.J. Effects of D-glucose and starvation upon the cyclic ADP-ribose content of rat pancreatic islets. Biochem. Mol. Biol. Int. 1998, 45, 783–790. [Google Scholar] [CrossRef] [Green Version]
- An, N.H.; Han, M.K.; Um, C.; Park, B.H.; Park, B.J.; Kim, H.K.; Kim, U.H. Significance of ecto-cyclase activity of CD38 in insulin secretion of mouse pancreatic islet cells. Biochem. Biophys. Res. Commun. 2001, 282, 781–786. [Google Scholar] [CrossRef]
- Varadi, A.; Rutter, G.A. Dynamic imaging of endoplasmic reticulum Ca2+ concentration in insulin-secreting MIN6 cells using recombinant targeted cameleons: Roles of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2 and ryanodine receptors. Diabetes 2002, 51, S190–S201. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, K.J.; Pinton, P.; Varadi, A.; Tacchetti, C.; Ainscow, E.K.; Pozzan, T.; Rizzuto, R.; Rutter, G.A. Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J. Cell Biol. 2001, 155, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Takasawa, S.; Noguchi, N.; Nata, K.; Yamauchi, A.; Takahashi, I.; Yoshikawa, T.; Sugawara, A.; Yonekura, H.; Okamoto, H. Identification of a major enzyme for the synthesis and hydrolysis of cyclic ADP-ribose in amphibian cells and evolutional conservation of the enzyme from human to invertebrate. Mol. Cell. Biochem. 2012, 366, 69–80. [Google Scholar] [CrossRef]
- Zocchi, E.; Franco, L.; Guida, L.; Benatti, U.; Bagellesi, A.; Malavasi, F.; Lee, H.C.; De Flora, A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem. Biophys. Res. Commun. 1993, 196, 1459–1465. [Google Scholar] [CrossRef]
- Munshi, C.; Thiel, D.J.; Mathews, I.I.; Aarhus, R.; Walseth, T.F.; Lee, H.C. Characterization of the active site of ADP-ribosyl cyclase. J. Biol. Chem. 1999, 274, 30770–30777. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H.; Takasawa, S. CD38. In Encyclopedia of Molecular Medicine; Creighton, T.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. 601–604. [Google Scholar]
- Ferrero, E.; Lo Buono, N.; Morone, S.; Parrotta, R.; Mancini, C.; Brusco, A.; Giancomino, A.; Augeri, S.; Rosal-Vela, A.; García-Rodríguez, S.; et al. Human canonical CD157/Bst1 is an alternatively spliced isoform masking a previously unidentified primate-specific exon included in a novel transcript. Sci. Rep. 2017, 7, 15923. [Google Scholar] [CrossRef] [Green Version]
- Kaisho, T.; Ishikawa, J.; Oritani, K.; Inazawa, J.; Muraoka, O.; Ochi, T.; Hirano, T. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc. Natl. Acad. Sci. USA 1994, 91, 5325–5329. [Google Scholar] [CrossRef] [Green Version]
- Muraoka, O.; Tanaka, H.; Itoh, M.; Ishihara, K.; Hirano, T. Genomic structure of human BST-1. Immunol. Lett. 1996, 54, 1–4. [Google Scholar] [CrossRef]
- Dong, C.; Willerford, D.; Alt, F.W.; Cooper, M.D. Genomic organization and chromosomal localization of the mouse BP3 gene, a member of the CD38/ADP-ribosy cyclase family. Immunogenetics 1996, 45, 35–43. [Google Scholar] [CrossRef]
- Furuya, Y.; Takasawa, S.; Yonekura, H.; Tanaka, T.; Takehara, J.; Okamoto, H. Cloning of a cDNA encoding rat bone marrow stromal cell antigen 1 (BST-1) from the islets of Langerhans. Gene 1995, 165, 329–330. [Google Scholar] [CrossRef]
- Kajimoto, Y.; Miyagawa, J.; Ishihara, K.; Okuyama, Y.; Fujitani, Y.; Itoh, M.; Yoshida, H.; Kaisho, T.; Matsuoka, T.; Watada, H.; et al. Pancreatic islets cells express BST-1, a CD38-like surface molecule having ADP-ribosyl cyclase activity. Biochem. Biophys. Res. Commun. 1996, 219, 941–946. [Google Scholar] [CrossRef]
- Itoh, M.; Ishihara, K.; Hiroi, T.; Lee, B.O.; Maeda, H.; Iijima, H.; Yanagita, M.; Kiyono, H.; Hirano, H. Deletion of bone marrow stromal cell antigen-1 (CD157) gene impaired systemic thymus independent-2 antigen-induced IgG3 and mucosal TD antigen-elicited IgA responses. J. Immunol. 1998, 161, 3974–3983. [Google Scholar] [PubMed]
- Jackson, D.G.; Bell, J.I. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J. Immunol. 1990, 144, 2811–2815. [Google Scholar] [PubMed]
- Harada, N.; Santos-Arugumedo, L.; Chang, R.; Grimaldi, J.C.; Lund, F.E.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; Heath, A.W.; Parkhouse, R.M. Expression cloning of a cDNA encoding a novel murine B cell activation marker. Homology to human CD38. J. Immunol. 1993, 151, 3111–3118. [Google Scholar]
- Itoh, M.; Ishihara, K.; Tomizawa, H.; Tanaka, H.; Kobune, Y.; Ishikawa, J.; Kaisho, T.; Hirano, T. Molecular cloning of murine BST-1 having homology with CD38 and Aplysia ADP-ribosyl cyclase. Biochem. Biophys. Res. Commun. 1994, 203, 1309–1317. [Google Scholar] [CrossRef]
- Churamani, D.; Boulware, M.J.; Geach, T.J.; Martin, A.C.R.; Moy, G.W.; Su, Y.-H.; Vacquier, V.D.; Marchant, J.S.; Dale, L.; Patel, S. Molecular characterization of a novel intracellular ADP-ribosyl cyclase. PLoS ONE 2007, 2, e797. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, S.P.; Muller-Steffner, M.; Osman, A.; Moutin, M.-J.; Kusser, K.; Roberts, A.; Woodland, D.L.; Randall, T.D.; Kellenberger, E.; LoVerde, P.T.; et al. Production of calcium-mobilizing metabolites by a novel member of the ADP-ribosyl cyclase family expressed in Schistosoma mansoni. Biochemistry 2005, 44, 11082–11097. [Google Scholar] [CrossRef]
- Kelu, J.J.; Web, S.E.; Galione, A.; Miller, A.L. Characterization of ADP-ribosyl cyclase 1-like (ARC1-like) activity and NAADP signaling during slow muscle cell development in zebrafish embryos. Dev. Biol. 2019, 445, 211–225. [Google Scholar] [CrossRef]
- Noguchi, N.; Yoshikawa, T.; Ikeda, T.; Takahashi, I.; Shervani, N.J.; Uruno, A.; Yamauchi, A.; Nata, K.; Takasawa, S.; Okamoto, H.; et al. FKBP12.6 disruption impairs glucose-induced insulin secretion. Biochem. Biophys. Res. Commun. 2008, 371, 735–740. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Ashcroft, S.J.H. (Eds.) Insulin: Molecular Biology to Pathology; IRL Press: Oxford, UK, 1992; pp. 97–150. [Google Scholar]
- Galione, A. Cyclic ADP-ribose: A new way to control calcium. Science 1993, 259, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, S.; Kuroki, M.; Nata, K.; Noguchi, N.; Ikeda, T.; Yamauchi, A.; Ota, H.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takahashi, I.; et al. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem. Biophys. Res. Commun. 2010, 397, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pirsch, J.D.; Miller, J.; Deierhoi, M.H.; Vincenti, F.; Filo, R.S. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation: FK506 Kidney Transplant Study Group. Transplantation 1997, 63, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Park, K.H.; Yim, C.Y.; Takasawa, S.; Okamoto, H.; Im, M.J.; Kim, U.H. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes 2008, 57, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H.; Takasawa, S.; Yamamoto, Y. From insulin synthesis to secretion: Alternative splicing of type 2 ryanodine receptor gene is essential for insulin secretion in pancreatic β cells. Int. J. Biochem. Cell Biol. 2017, 91, 176–183. [Google Scholar] [CrossRef]
- Makino, M.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Takasawa, S. Tissue-specific alternative splicing of type 2 ryanodine receptor gene affects insulin biosynthesis in pancreatic β cells. Diabetologia 2020, 63 (Suppl. S1), 385. Available online: https://www.easd.org/virtualmeeting/home.html#!resources/tissue-specific-alternative-splicing-of-type-2-ryanodine-receptor-gene-affects-insulin-biosynthesis-in-pancreatic-beta-cells-f6bbb60b-8e31-40c9-ab74-c0702c5f27f6 (accessed on 22 September 2020).
- Mount, S.M. A catalog of splice junction sequences. Nucleic Acids Res. 1982, 10, 459–472. [Google Scholar] [CrossRef]
- Nakai, K.; Sakamoto, H. Construction of a novel database containing aberrant splicing mutations of mammalian genes. Gene 1994, 141, 171–177. [Google Scholar] [CrossRef]
- Sakai, N.; Santamarina-Fojo, S.; Yamashita, S.; Matsuzawa, Y.; Brewer, H.B., Jr. Exon 10 skipping caused by intron 10 splice donor site mutation in cholesterol ester transfer protein gene results in abnormal downstream splice site selection. J. Lipid Res. 1996, 37, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, C.; Giannini, S.; Marchetti, P.; Lupi, R.; Antonelli, A.; Malavasi, F.; Takasawa, S.; Okamoto, H.; Ferrannini, E. Autoantibodies to CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) in Caucasian patients with diabetes: Effects on insulin release from human islets. Diabetes 1999, 48, 2309–2315. [Google Scholar] [CrossRef]
- Mallone, R.; Ortolan, E.; Baj, G.; Funaro, A.; Giunti, S.; Lillaz, E.; Saccucci, F.; Cassader, M.; Cavallo-Perin, P.; Malavasi, F. Autoantibody response to CD38 in Caucasian patients with type 1 and type 2 diabetes: Immunological and genetic characterization. Diabetes 2001, 50, 752–762. [Google Scholar] [CrossRef]
- Antonelli, A.; Baj, G.; Marchetti, P.; Fallahi, P.; Surico, N.; Pupilli, C.; Malavasi, F.; Ferrannini, E. Human anto-CD38 autoantibodies raise intracellular calcium and stimulate insulin release in human pancreatic islets. Diabetes 2001, 50, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.Y.; Tokimasa, T.; Takasawa, S.; Furuya, Y.; Nohmi, M.; Okamoto, H.; Kuba, K. Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron 1994, 12, 1073–1079. [Google Scholar] [CrossRef]
- Karasawa, T.; Takasawa, S.; Yamakawa, K.; Yonekura, H.; Okamoto, H.; Nakamura, S. NAD+-glycohydrolase from Streptococcus pyogenes shows cyclic ADP-ribose forming activity. FEMS Microbiol. Lett. 1995, 130, 201–204. [Google Scholar] [CrossRef]
- Ebihara, S.; Sasaki, T.; Hida, W.; Kikuchi, Y.; Ohshiro, T.; Shimura, S.; Takasawa, S.; Okamoto, H.; Nishiyama, A.; Akaike, N.; et al. Role of cyclic ADP-ribose in ATP-activated potassium currents in alveolar macrophages. J. Biol. Chem. 1997, 272, 16023–16029. [Google Scholar] [CrossRef] [Green Version]
- Higashida, H.; Yokoyama, S.; Hashii, M.; Taketo, M.; Higashida, M.; Takayasu, T.; Ohshima, T.; Takasawa, S.; Okamoto, H.; Noda, M. Muscarinic receptor-mediated dual regulation of ADP-ribosyl cyclase in NG108-15 neuronal cell membranes. J. Biol. Chem. 1997, 272, 31272–31277. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kuzma, J.; Maréchal, E.; Graeff, R.; Lee, H.C.; Foster, R.; Chua, N.-H. Abscisic acid signalling through cyclic ADP-ribose in plats. Science 1997, 278, 2126–2130. [Google Scholar] [CrossRef]
- Leckie, C.P.; McAinsh, M.R.; Allen, G.J.; Sanders, D.; Hetherington, A.M. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 15837–15842. [Google Scholar] [CrossRef] [Green Version]
- Yano, M.; Ono, K.; Ohkusa, T.; Suetsugu, M.; Kohno, M.; Hisaoka, T.; Kobayashi, S.; Hisamatsu, Y.; Yamamoto, T.; Kohno, M.; et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circilation 2000, 102, 2131–2136. [Google Scholar] [CrossRef] [Green Version]
- Fukushi, Y.; Kato, I.; Takasawa, S.; Sasaki, T.; Ong, B.H.; Sato, M.; Ohsaga, A.; Sato, K.; Shirato, K.; Okamoto, H.; et al. Identification of cyclic ADP-ribose-dependent mechanisms in pancreatic muscarinic Ca2+ signaling using CD38 knockout mice. J. Biol. Chem. 2001, 276, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.-B.; Senbonmatsu, T.; Cheng, D.-S.; Wang, Y.-X.; Copello, J.A.; Ji, G.-J.; Collier, M.L.; Deng, Y.-X.; Jeyakumar, L.H.; Magnuson, M.A.; et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 2002, 416, 334–337. [Google Scholar] [CrossRef]
- Takahashi, J.; Kagaya, Y.; Kato, I.; Ohta, J.; Isoyama, S.; Miura, M.; Sugai, Y.; Hirose, M.; Wakayama, Y.; Ninomiya, M.; et al. Deficit of CD38/cyclic ADP-ribose is differentially compensated in hearts by gender. Biochem. Biophys. Res. Commun. 2003, 312, 434–440. [Google Scholar] [CrossRef]
- Mitsui-Saito, M.; Kato, I.; Takasawa, S.; Okamoto, H.; Yanagisawa, T. CD38 gene disruption inhibits the contraction induced by α-adrenoceptor stimulation in mouse aorta. J. Vet. Med. Sci. 2003, 65, 1325–1330. [Google Scholar] [CrossRef] [Green Version]
- Sasamori, K.; Sasaki, T.; Takasawa, S.; Tamada, T.; Nara, M.; Irokawa, T.; Shimura, S.; Shirato, K.; Hattori, T. Cyclic ADP-ribose, a putative Ca2+-mobilizing second messenger, operates in submucosal gland acinar cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L69–L78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellner, S.K.; Parker, L. Endothelin-1, superoxide and adeninediphosphate ribose cyclase in shark vascular smooth muscle. J. Exp. Biol. 2005, 208, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Dodd, A.N.; Gardner, M.J.; Hotta, C.T.; Hubbard, K.E.; Delchau, N.; Love, J.; Assie, J.M.; Robertson, F.C.; Jakobsen, M.K.; Gonçalves, J.; et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 2007, 318, 1789–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, H.; Tamaki, S.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Morioka, T.; Takasawa, S.; Kimura, H. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci. 2012, 90, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Ota, H.; Kawaguchi, R.; Takasawa, S. Intermittent hypoxia upregulates the Renin and Cd38 mRNAs in renin-producing cells via the downregulation of miR-203. Int. J. Mol. Sci. 2021, 22, 10127. [Google Scholar] [CrossRef]
- Munesue, T.; Yokoyama, S.; Nakamura, K.; Anitha, A.; Yamada, K.; Hayashi, K.; Asaka, T.; Liu, H.X.; Jin, D.; Koizumi, K.; et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci. Res. 2010, 67, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Enami, N.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Takasawa, S.; Takahashi, Y. The CD38 genotype (rs1800561 (4693C>T): R140W) is associated with an increased risk of admission to the neonatal intensive care unit. Early Hum. Dev. 2015, 91, 467–470. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takasawa, S. CD38–Cyclic ADP-Ribose Signal System in Physiology, Biochemistry, and Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4306. https://doi.org/10.3390/ijms23084306
Takasawa S. CD38–Cyclic ADP-Ribose Signal System in Physiology, Biochemistry, and Pathophysiology. International Journal of Molecular Sciences. 2022; 23(8):4306. https://doi.org/10.3390/ijms23084306
Chicago/Turabian StyleTakasawa, Shin. 2022. "CD38–Cyclic ADP-Ribose Signal System in Physiology, Biochemistry, and Pathophysiology" International Journal of Molecular Sciences 23, no. 8: 4306. https://doi.org/10.3390/ijms23084306




