Sex-Specific Alterations in Dopamine Metabolism in the Brain after Methamphetamine Self-Administration
Abstract
:1. Introduction
2. Results
2.1. Sex Differences in METH Intake
2.2. Levels of Dopamine and Its Metabolites
2.2.1. Prefrontal Cortex (PFC)
2.2.2. Nucleus Accumbens (NAc)
2.2.3. Dorsal Striatum (dSTR)
2.2.4. Hippocampus (HIP)
3. Discussion
4. Materials and Methods
4.1. Animals and SA Procedures
4.2. Tissue Collection
4.3. Measurement of Dopamine and Metabolites
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APA. DSM–5, Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: New York, NY, USA, 2013. [Google Scholar]
- Proebstl, L.; Kamp, F.; Koller, G.; Soyka, M. Cognitive deficits in methamphetamine users: How strong is the evidence? Pharmacopsychiatry 2018, 51, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Duflou, J.; Kaye, S. Prevalence and nature of cardiovascular disease in methamphetamine-related death: A national study. Drug Alcohol Depend. 2017, 179, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Kaye, S.; Duflou, J. Rates, characteristics and circumstances of methamphetamine-related death in Australia: A national 7-year study. Addiction 2017, 112, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Lappin, J.; Kaye, S.; Duflou, J. Clinical characteristics of fatal methamphetamine-related stroke: A national study. J. Forensic Sci. 2018, 63, 735–739. [Google Scholar] [CrossRef]
- Darke, S.; Kaye, S.; Duflou, J.; Lappin, J. Completed suicide among methamphetamine users: A national study. Suicide Life Threat Behav. 2019, 49, 328–337. [Google Scholar] [CrossRef] [Green Version]
- National Survey on Drug Use and Health (NSDUH), 2019. Substance Abuse and Mental Health Services Administration. Results from the 2018 National Survey on Drug Use and Health. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2019. Available online: https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables (accessed on 17 March 2022).
- Bonk, R.; Miller, R.J.; Lanter, J.; Niblo, C.; Kemp, J.; Shelton, J. Accidental overdose deaths in oklahoma, 2002–2017: Opioid and methamphetamine trends. J. Anal. Toxicol. 2020, 44, 672–678. [Google Scholar] [CrossRef]
- Canadian Centre on Substance Use and Addiction. Methamphetamine (Canadian Drug Summary), Methamphetamine (Canadian Drug Summary); Canadian Centre on Substance Use and Addiction: Ottawa, ON, CA, 2020. [Google Scholar]
- Jones, C.M.; Compton, W.M.; Mustaquim, D. Patterns and characteristics of methamphetamine use among adults—United States, 2015–2018. CDC Morb. Mortal. Wkly. Rep. 2020, 69, 317–323. [Google Scholar] [CrossRef]
- Jones, C.M.; Olsen, E.O.; O’Donnell, J.; Mustaquim, D. Resurgent methamphetamine use at treatment admission in the United States, 2008–2017. Am. J. Public Health 2020, 110, 509–516. [Google Scholar] [CrossRef]
- Paknahad, S.; Akhgari, M.; Ghadipasha, M. An alarming rise in the prevalence of deaths with methamphetamine involved in Tehran, Iran 2011–2018. Forensic Sci. Med. Pathol. 2021, 17, 208–215. [Google Scholar] [CrossRef]
- UNODC. Drug Market. Trends: Cocaine, Amphetamine-Type Stimulants; United Nations Publication: Vienna, Austria, 2021; ISBN 978-92-1-148361-1. [Google Scholar]
- Zhao, S.X.; Deluna, A.; Kelsey, K.; Wang, C.; Swaminathan, A.; Staniec, A.; Crawford, M.H. Socioeconomic burden of rising methamphetamine-associated heart failure hospitalizations in California from 2008 to 2018. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007638. [Google Scholar] [CrossRef]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [CrossRef] [PubMed]
- Cloak, C.C.; Alicata, D.; Chang, L.; Andrews-Shigaki, B.; Ernst, T. Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug Alcohol Depend. 2011, 119, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salo, R.; Buonocore, M.H.; Leamon, M.; Natsuaki, Y.; Waters, C.; Moore, C.D.; Galloway, G.P.; Nordahl, T.E. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: A proton MRS study. Drug Alcohol Depend. 2011, 113, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, J.J., 3rd; Kalechstein, A.D.; De La Garza, R., 2nd; Newton, T.F. Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependent participants. Am. J. Addict. 2008, 17, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.J., 3rd; Hawkins, R.Y.; De La Garza, R., 2nd; Kalechstein, A.D.; Newton, T.F. Relationship between gender and psychotic symptoms in cocaine-dependent and methamphetamine-dependent participants. Gend Med. 2010, 7, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Polcin, D.L.; Buscemi, R.; Nayak, M.; Korcha, R.; Galloway, G. Gender differences in psychiatric symptoms among methamphetamine dependent residents in sober living houses. Addict. Disord. Treat. 2012, 11, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Zhang, J.; Ren, W.; Xie, Y.; Tao, J.; Zhang, X.; He, J. Anxiety level and correlates in methamphetamine-dependent patients during acute withdrawal. Medicine 2017, 96, e6434. [Google Scholar] [CrossRef]
- Lisa, P.; Felicia, K.; Laura, H.; Daniela, K.; Marlies, R.; Stefanie, N.; Maik, S.J.; Anne, S.; Maximilian, S.; Kirsi, M.; et al. Associations between methamphetamine use, psychiatric comorbidities and treatment outcome in two inpatient rehabilitation centers. Psychiatry Res. 2019, 280, 112505. [Google Scholar] [CrossRef]
- Roth, M.E.; Carroll, M.E. Sex differences in the acquisition of IV methamphetamine self- administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology 2004, 172, 443–449. [Google Scholar] [CrossRef]
- Reichel, C.M.; Chan, C.H.; Ghee, S.M.; See, R.E. Sex differences in escalation of methamphetamine self-administration: Cognitive and motivational consequences in rats. Psychopharmacology 2012, 223, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Ruda-Kucerova, J.; Amchova, P.; Babinska, Z.; Dusek, L.; Micale, V.; Sulcova, A. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front. Psychiatry 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordie, R.; McFadden, L.M. Optogenetic inhibition of the medial prefrontal cortex reduces methamphetamine-primed reinstatement in male and female rats. Behav. Pharmacol. 2019, 30, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Daiwile, A.P.; Subramaniam, J.; Ladenheim, B.; McCoy, M.T.; Brannock, C.; Schroeder, J.; Cadet, J.L. Sex differences in escalated methamphetamine self-administration and altered gene expression associated with incubation of methamphetamine seeking. Int. J. Neuropsychopharmacol. 2019, 22, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Job, M.O.; Chojnacki, M.R.; Daiwile, A.P.; Cadet, J.L. Chemogenetic inhibition of dopamine d1-expressing neurons in the dorsal striatum does not alter methamphetamine intake in either male or female Long Evans rats. Neurosci. Lett. 2020, 729, 134987. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.; McFadden, L.M. The neurochemical consequences of methamphetamine self-administration in male and female rats. Drug Alcohol Depend. 2017, 178, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Hankosky, E.R.; Westbrook, S.R.; Haake, R.M.; Willing, J.; Raetzman, L.T.; Juraska, J.M.; Gulley, J.M. Age- and sex-dependent effects of methamphetamine on cognitive flexibility and 5-HT2C receptor localization in the orbitofrontal cortex of Sprague-Dawley rats. Behav. Brain 2018, 349, 16–24. [Google Scholar] [CrossRef]
- McFadden, L.M.; Cordie, R.; Livermont, T.; Johansen, A. Behavioral and serotonergic changes in the frontal cortex following methamphetamine self-administration. Int. J. Neuropsychopharmacol. 2018, 21, 758–763. [Google Scholar] [CrossRef]
- Pittenger, S.T.; Chou, S.; Murawski, N.J.; Barrett, S.T.; Loh, O.; Duque, J.F.; Li, M.; Bevins, R.A. Female rats display higher methamphetamine-primed reinstatement and c-Fos immunoreactivity than male rats. Pharmacol. Biochem. Behav. 2021, 201, 173089. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Cadet, J.L. Sex- and brain region-specific changes in gene expression in male and female rats as consequences of methamphetamine self-administration and abstinence. Neuroscience 2021, 452, 265–279. [Google Scholar] [CrossRef]
- Jaffard, R.; Meunier, M. Role of the hippocampal formation in learning and memory. Hippocampus 1993, 3, 203–217. [Google Scholar] [CrossRef]
- Walsh, J.J.; Han, M.H. The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context. Neuroscience 2014, 282, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keiflin, R.; Janak, P.H. Dopamine prediction errors in reward learning and addiction: From theory to neural circuitry. Neuron 2015, 88, 247–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funahashi, S. Prefrontal contribution to decision-making under free-choice conditions. Front. Neurosci. 2017, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Witten, I.B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 2019, 20, 482–494. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Keleta, Y.B.; Martinez, J.L. Brain circuits of methamphetamine place reinforcement learning: The role of the hippocampus-VTA loop. Brain Behav. 2012, 2, 128–141. [Google Scholar] [CrossRef]
- Cadet, J.L.; Bisagno, V.; Milroy, C.M. Neuropathology of substance use disorders. Acta Neuropathol. 2014, 127, 91–107. [Google Scholar] [CrossRef]
- Tanabe, J.; Regner, M.; Sakai, J.; Martinez, D.; Gowin, J. Neuroimaging reward, craving, learning, and cognitive control in substance use disorders: Review and implications for treatment. Br. J. Radiol. 2019, 92, 20180942. [Google Scholar] [CrossRef]
- Hirata, H.; Asanuma, M.; Cadet, J.L. Melatonin attenuates methamphetamine-induced toxic effects on dopamine and serotonin terminals in mouse brain. Synapse 1998, 30, 150–155. [Google Scholar] [CrossRef]
- Deng, X.; Ladenheim, B.; Jayanthi, S.; Cadet, J.L. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol. Psychiatry 2007, 61, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Krasnova, I.N.; Ladenheim, B.; Cai, N.S.; McCoy, M.T.; Atianjoh, F.E. Methamphetamine preconditioning: Differential protective effects on monoaminergic systems in the rat brain. Neurotox. Res. 2009, 15, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnova, I.N.; Justinova, Z.; Ladenheim, B.; Jayanthi, S.; McCoy, M.T.; Barnes, C.; Warner, J.E.; Goldberg, S.R.; Cadet, J.L. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE 2010, 5, e8790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnova, I.N.; Chiflikyan, M.; Justinova, Z.; McCoy, M.T.; Ladenheim, B.; Jayanthi, S.; Quintero, C.; Brannock, C.; Barnes, C.; Adair, J.E.; et al. CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis. 2013, 58, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Dluzen, D.E.; McDermott, J.L.; Bourque, M.; Di Paolo, T.; Darvesh, A.S.; Buletko, A.B.; Laping, N.J. Markers associated with sex differences in methamphetamine-induced striatal dopamine neurotoxicity. Curr. Neuropharmacol. 2011, 9, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Dluzen, D.E.; McDermott, J.L.; Darvesh, A.S. Relationships among gender, age, time, and temperature in methamphetamine-induced striatal dopaminergic neurotoxicity. Neuroscience 2010, 167, 985–993. [Google Scholar] [CrossRef]
- Bourque, M.; Liu, B.; Dluzen, D.E.; Di Paolo, T. Sex differences in methamphetamine toxicity in mice: Effect on brain dopamine signaling pathways. Psychoneuroendocrinology 2012, 36, 955–969. [Google Scholar] [CrossRef]
- Jasinska, A.J.; Chen, B.T.; Bonci, A.; Stein, E.A. Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: Implications for drug addiction therapies. Addict. Biol. 2015, 20, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.B.; Robinson, T.E.; Lorenz, K.A. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur. J. Pharmacol. 1982, 80, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Cornish, J.L.; Duffy, P.; Kalivas, P.W. A role for nucleus accumbens glutamate transmission in the relapse to cocaine seeking behavior. Neuroscience 1999, 93, 1359–1367. [Google Scholar] [CrossRef]
- Fuchs, R.A.; Evans, K.A.; Parker, M.C.; See, R.E. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 2004, 176, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Bossert, J.M.; Poles, G.C.; Wihbey, K.A.; Koya, E.; Shaham, Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J. Neurosci. 2017, 27, 12655–12663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadet, J.L. Sex in the nucleus accumbens: ΔFosB, addiction, and affective states. Biol. Psychiatry 2021, 90, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Lipton, D.M.; Gonzales, B.J.; Citri, A. Dorsal striatal circuits for habits, compulsions and addictions. Front. Syst. Neurosci. 2019, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Kutlu, M.G.; Gould, T.J. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn. Mem. 2016, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Foltin, R.W. Self-administration of methamphetamine aerosol by male and female baboons. Pharmacol. Biochem. Behav. 2018, 168, 17–24. [Google Scholar] [CrossRef]
- Zlebnik, N.E.; Holtz, N.A.; Lepak, V.C.; Saykao, A.T.; Zhang, Y.; Carroll, M.E. Age-specific treatment effects of orexin/hypocretin-receptor antagonism on methamphetamine-seeking behavior. Drug Alcohol Depend. 2021, 224, 108719. [Google Scholar] [CrossRef]
- Mattson, M.E. Emergency Department Visits Involving Methamphetamine: 2007 to 2011. In The CBHSQ Report; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2013. [Google Scholar]
- Jones, R.; Woods, C.; Barker, R.; Usher, K. Patterns and features of methamphetamine-related presentations to emergency departments in QLD from 2005 to 2017. Int. J. Ment. Health Nurs. 2019, 28, 833–844. [Google Scholar] [CrossRef]
- Richards, J.R.; Placone, T.W.; Wang, C.G.; van der Linden, M.C.; Derlet, R.W.; Laurin, E.G. Methamphetamine, Amphetamine, and MDMA Use and Emergency Department Recidivism. J. Emerg. Med. 2020, 59, 320–328. [Google Scholar] [CrossRef]
- McFaull, S.R.; Champagne, A.; Thompson, W.; Bang, F. Injuries and poisonings associated with methamphetamine use: Sentinel surveillance, the electronic Canadian Hospitals Injury Reporting and Prevention Program (eCHIRPP), 2011–2019. Health Promot Chronic Dis. Prev. Can. 2020, 40, 126–129. [Google Scholar] [CrossRef]
- Fehr, C.; Yakushev, I.; Hohmann, N.; Buchholz, H.G.; Landvogt, C.; Deckers, H.; Eberhardt, A.; Kläger, M.; Smolka, M.N.; Scheurich, A.; et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am. J. Psychiatry 2008, 165, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossong, M.G.; van Berckel, B.N.; Boellaard, R.; Zuurman, L.; Schuit, R.C.; Windhorst, A.D.; van Gerven, J.M.; Ramsey, N.F.; Lammertsma, A.A.; Kahn, R.S. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 2009, 34, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.; Swanson, J.M.; Telang, F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch. Neurol. 2007, 64, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wiers, C.E.; Shokri-Kojori, E.; Tomasi, D.; Wang, G.J.; Baler, R. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology 2017, 122, 175–188. [Google Scholar] [CrossRef]
- Jan, R.K.; Kydd, R.R.; Russell, B.R. Functional and structural brain changes associated with methamphetamine abuse. Brain Sci. 2012, 2, 434–482. [Google Scholar] [CrossRef]
- Kish, S.J.; Boileau, I.; Callaghan, R.C.; Tong, J. Brain dopamine neurone ‘damage’: Methamphetamine users vs. Parkinson’s disease—A critical assessment of the evidence. Eur. J. Neurosci. 2017, 45, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Shukla, M.; Vincent, B. Methamphetamine abuse disturbs the dopaminergic system to impair hippocampal-based learning and memory: An overview of animal and human investigations. Neurosci. Biobehav. Rev. 2021, 131, 541–559. [Google Scholar] [CrossRef]
- Ares-Santos, S.; Granado, N.; Moratalla, R. The role of dopamine receptors in the neurotoxicity of methamphetamine. J. Intern. Med. 2013, 273, 437–453. [Google Scholar] [CrossRef] [Green Version]
- Shin, E.J.; Dang, D.K.; Tran, T.V.; Tran, H.Q.; Jeong, J.H.; Nah, S.Y.; Jang, C.G.; Yamada, K.; Nabeshima, T.; Kim, H.C. Current understanding of methamphetamine-associated dopaminergic neurodegeneration and psychotoxic behaviors. Arch. Pharm. Res. 2017, 40, 403–428. [Google Scholar] [CrossRef]
- Hirata, H.; Ladenheim, B.; Carlson, E.; Epstein, C.; Cadet, J.L. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: Attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996, 714, 95–103. [Google Scholar] [CrossRef]
- Szczepanik, J.C.; de Almeida, G.R.L.; Cunha, M.P.; Dafre, A.L. Repeated methylglyoxal treatment depletes dopamine in the prefrontal cortex and causes memory impairment and depressive-like behavior in mice. Neurochem. Res. 2020, 45, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lin, V.; Wu, F.; Hser, Y.I. Gender comparisons among asian american and pacific islander patients in drug dependency treatment. Subst. Use Misuse 2016, 51, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Kopin, I.J. Linking Stress, Catecholamine autotoxicity, and allostatic load with neurodegenerative diseases: A focused review in memory of Richard Kvetnansky. Cell. Mol. Neurobiol. 2018, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Kopin, I.J.; Sharabi, Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014, 144, 268–282. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.L.; Krasnova, I.N.; Jayanthi, S.; Lyles, J. Neurotoxicity of substituted amphetamines: Molecular and cellular mechanisms. Neurotox. Res. 2007, 11, 183–202. [Google Scholar] [CrossRef]
- Almalki, A.H.; Das, S.C.; Alshehri, F.S.; Althobaiti, Y.S.; Sari, Y. Effects of sequential ethanol exposure and repeated high-dose methamphetamine on striatal and hippocampal dopamine, serotonin and glutamate tissue content in Wistar rats. Neurosci. Lett. 2018, 665, 61–66. [Google Scholar] [CrossRef]
- Schwendt, M.; Rocha, A.; See, R.E.; Pacchioni, A.M.; McGinty, J.F.; Kalivas, P.W. Extended methamphetamine self-administration in rats’ results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J. Pharmacol. Exp. Ther. 2009, 331, 555–562. [Google Scholar] [CrossRef]
- Casida, J.E.; Ford, B.; Jinsmaa, Y.; Sullivan, P.; Cooney, A.; Goldstein, D.S. Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem. Res. Toxicol. 2014, 27, 1359–1361. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Miller, G.W.; Sharabi, Y.; Kopin, I.J. A vesicular sequestration to oxidative deamination shift in myocardial sympathetic nerves in Parkinson’s disease. J. Neurochem. 2014, 131, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Landrock, K.K.; Sullivan, P.; Martini-Stoica, H.; Goldstein, D.S.; Graham, B.H.; Yamamoto, S.; Bellen, H.J.; Gibbs, R.A.; Chen, R.; D’Amelio, M.; et al. Pleiotropic neuropathological and biochemical alterations associated with Myo5a mutation in a rat model. Brain Res. 2018, 1679, 155–170. [Google Scholar] [CrossRef]
- Wey, M.C.; Fernandez, E.; Martinez, P.A.; Sullivan, P.; Goldstein, D.S.; Strong, R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: Implications for Parkinson’s disease. PLoS ONE 2012, 7, e31522. [Google Scholar] [CrossRef] [PubMed]
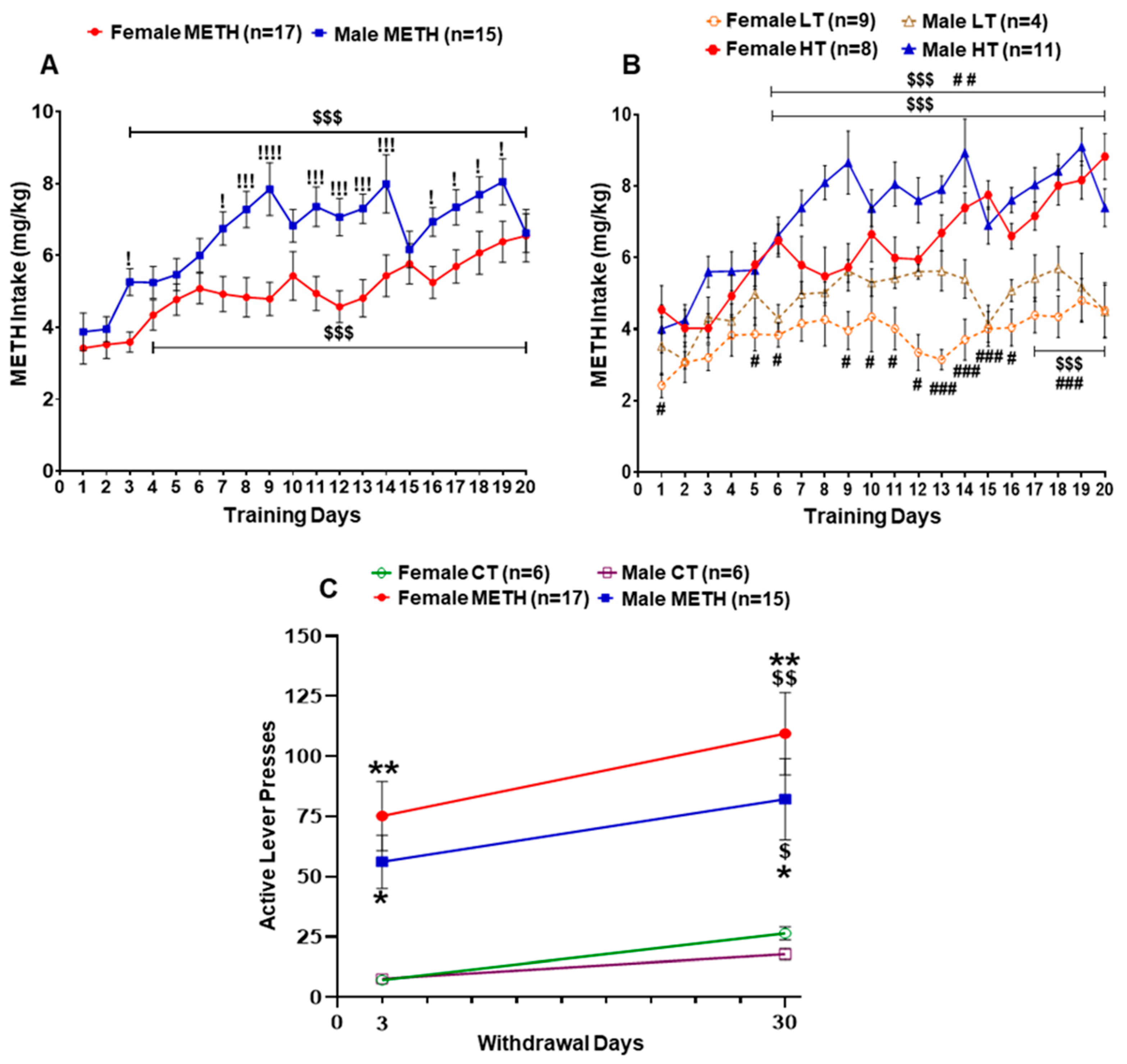

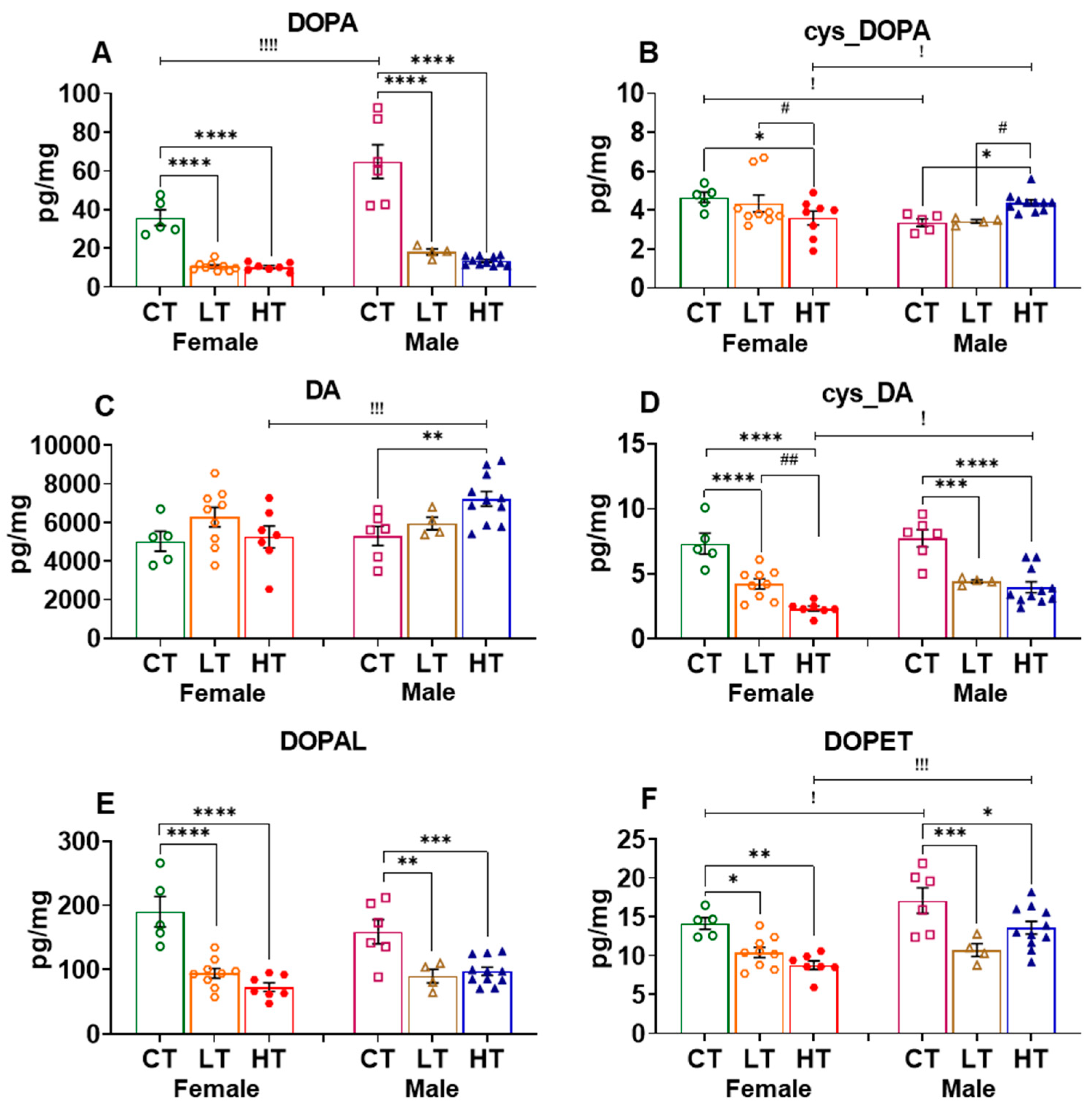
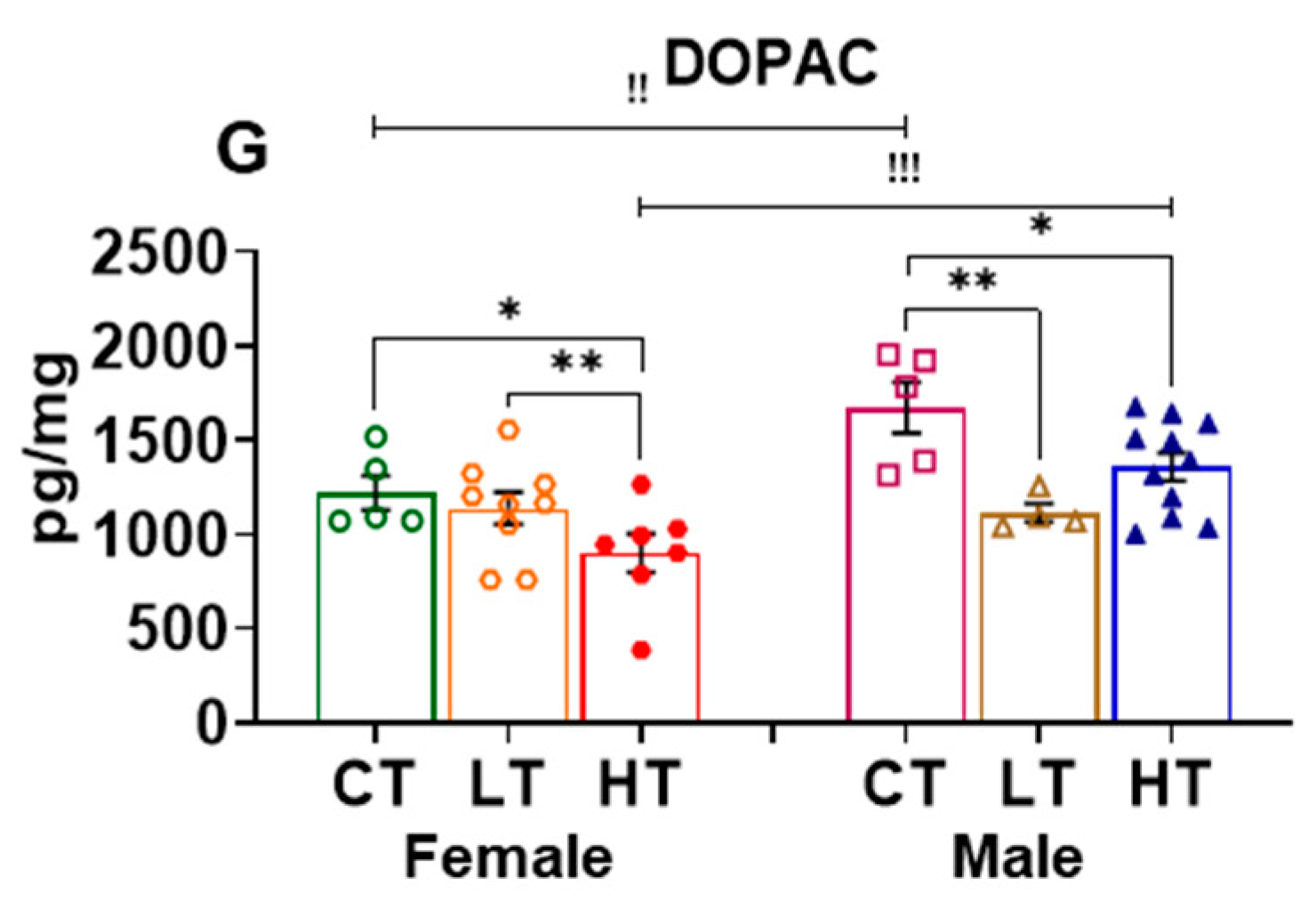

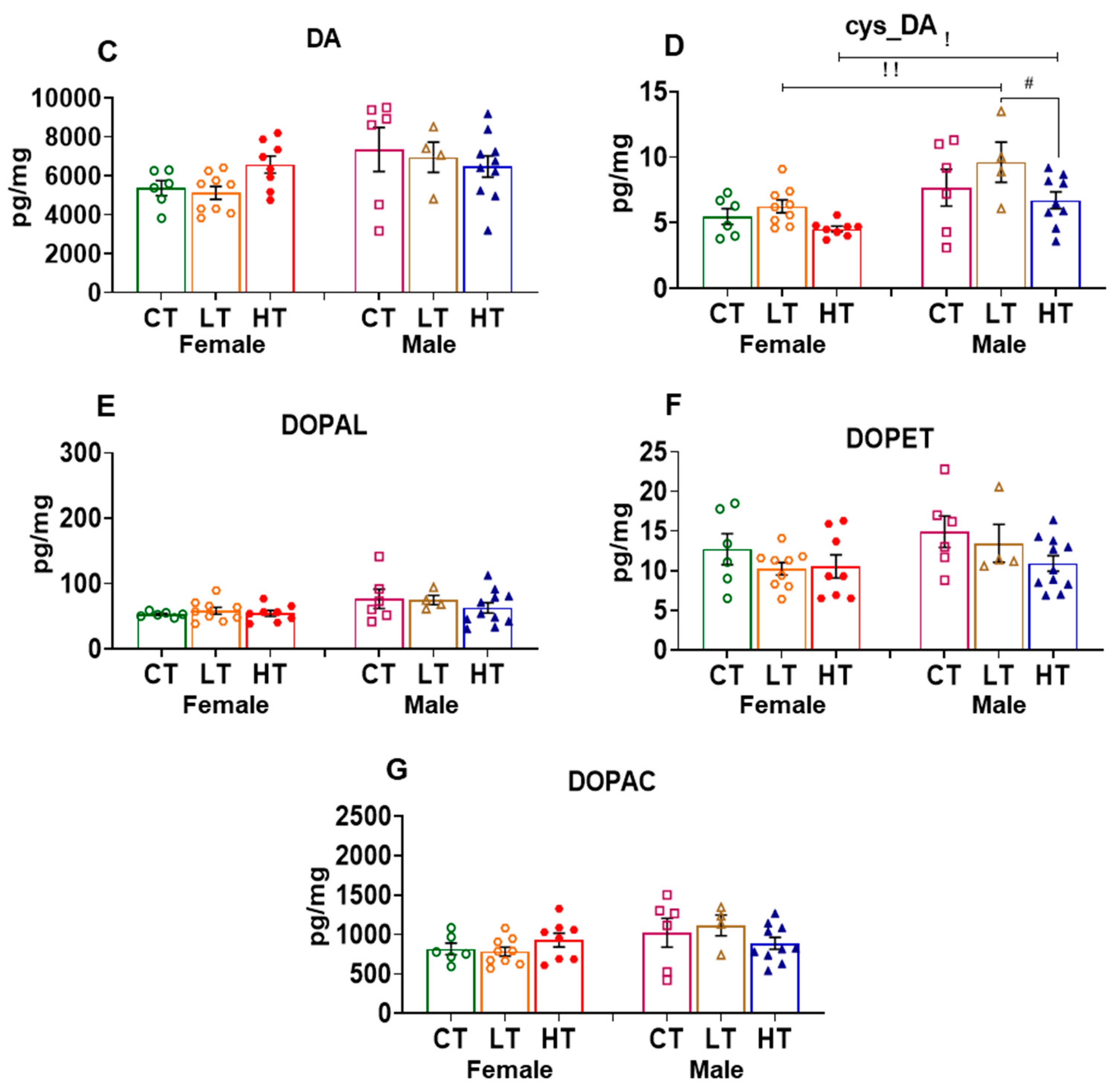

| Effect of Sex | Female vs. Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFC | NAc | dSTR | HIP | |||||||||
| CT | LT | HT | CT | LT | HT | CT | LT | HT | CT | LT | HT | |
| DOPA | ↓ | ↓↓↓ | NS | ↓↓↓ | NS | ↓↓↓ | ↓↓ | ↓↓↓ | ↓↓↓ | ↓ | ↓↓↓ | |
| cys_DOPA | NS | NS | NS | ↑ | NS | ↓ | NS | NS | NS | NS | ↑↑ | NS |
| DA | ↑↑↑ | NS | NS | NS | NS | ↓↓↓ | NS | NS | NS | ↓↓ | NS | NS |
| cys_DA | ND | ND | ND | NS | NS | ↓ | NS | ↓↓ | ↓ | ND | ND | ND |
| DOPAL | ↑ | NS | NS | NS | NS | NS | NS | NS | NS | ↓↓ | NS | ↑ |
| DOPET | NS | NS | NS | ↑ | NS | ↓↓↓ | NS | NS | NS | NS | NS | NS |
| DOPAC | ↑↑ | NS | NS | ↓↓ | NS | ↓↓↓ | NS | NS | NS | ↓↓↓ | ↓ | NS |
| Effect of METH SA | PFC | NAc | dSTR | HIP | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |||||||||||||||||
| CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | CT vs. LT | CT vs. HT | HT vs. LT | |
| DOPA | NS | NS | NS | ↑ | NS | ↑↑↑ | ↓↓↓ | ↓↓↓ | NS | ↓↓↓ | ↓↓↓ | NS | NS | NS | NS | ↓ | ↓↓ | NS | NS | NS | NS | ↓ | ↑ | ↑↑↑ |
| cys_DOPA | NS | NS | NS | NS | NS | NS | NS | ↓ | ↓ | NS | ↑ | ↑ | NS | NS | NS | NS | NS | NS | NS | NS | ↓ | NS | NS | NS |
| DA | ↓↓↓ | ↓↓↓ | NS | NS | NS | NS | NS | NS | NS | NS | ↑ | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ↓↓ | ↓ |
| cys_DA | ND | ND | ND | ND | ND | ND | ↓↓↓ | ↓↓↓ | ↓↓ | ↓↓↓ | ↓↓↓ | NS | NS | NS | NS | NS | NS | ↓ | ND | ND | ND | ND | ND | ND |
| DOPAL | ↓↓ | ↓↓↓ | NS | NS | NS | NS | ↓↓↓ | ↓↓↓ | NS | ↓↓↓ | ↓↓↓ | NS | NS | NS | NS | NS | NS | NS | NS | ↑ | NS | ↓ | ↓↓ | NS |
| DOPET | NS | ↑↑↑ | ↑↑ | ↑ | ↑↑↑ | ↑ | ↓ | ↓↓ | NS | ↓↓↓ | ↓ | NS | NS | NS | NS | NS | NS | NS | ↑ | ↑↑ | NS | NS | NS | NS |
| DOPAC | ↓↓ | ↓↓ | NS | NS | NS | NS | ↓ | ↓↓ | ↓↓ | ↓ | NS | NS | NS | NS | NS | NS | ↑ | ↑↑↑ | NS | NS | NS | ↓ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daiwile, A.P.; Sullivan, P.; Jayanthi, S.; Goldstein, D.S.; Cadet, J.L. Sex-Specific Alterations in Dopamine Metabolism in the Brain after Methamphetamine Self-Administration. Int. J. Mol. Sci. 2022, 23, 4353. https://doi.org/10.3390/ijms23084353
Daiwile AP, Sullivan P, Jayanthi S, Goldstein DS, Cadet JL. Sex-Specific Alterations in Dopamine Metabolism in the Brain after Methamphetamine Self-Administration. International Journal of Molecular Sciences. 2022; 23(8):4353. https://doi.org/10.3390/ijms23084353
Chicago/Turabian StyleDaiwile, Atul P., Patricia Sullivan, Subramaniam Jayanthi, David S. Goldstein, and Jean Lud Cadet. 2022. "Sex-Specific Alterations in Dopamine Metabolism in the Brain after Methamphetamine Self-Administration" International Journal of Molecular Sciences 23, no. 8: 4353. https://doi.org/10.3390/ijms23084353







