Ostarine-Induced Myogenic Differentiation in C2C12, L6, and Rat Muscles
Abstract
:1. Introduction
2. Results
2.1. Proliferation and Cell Viability
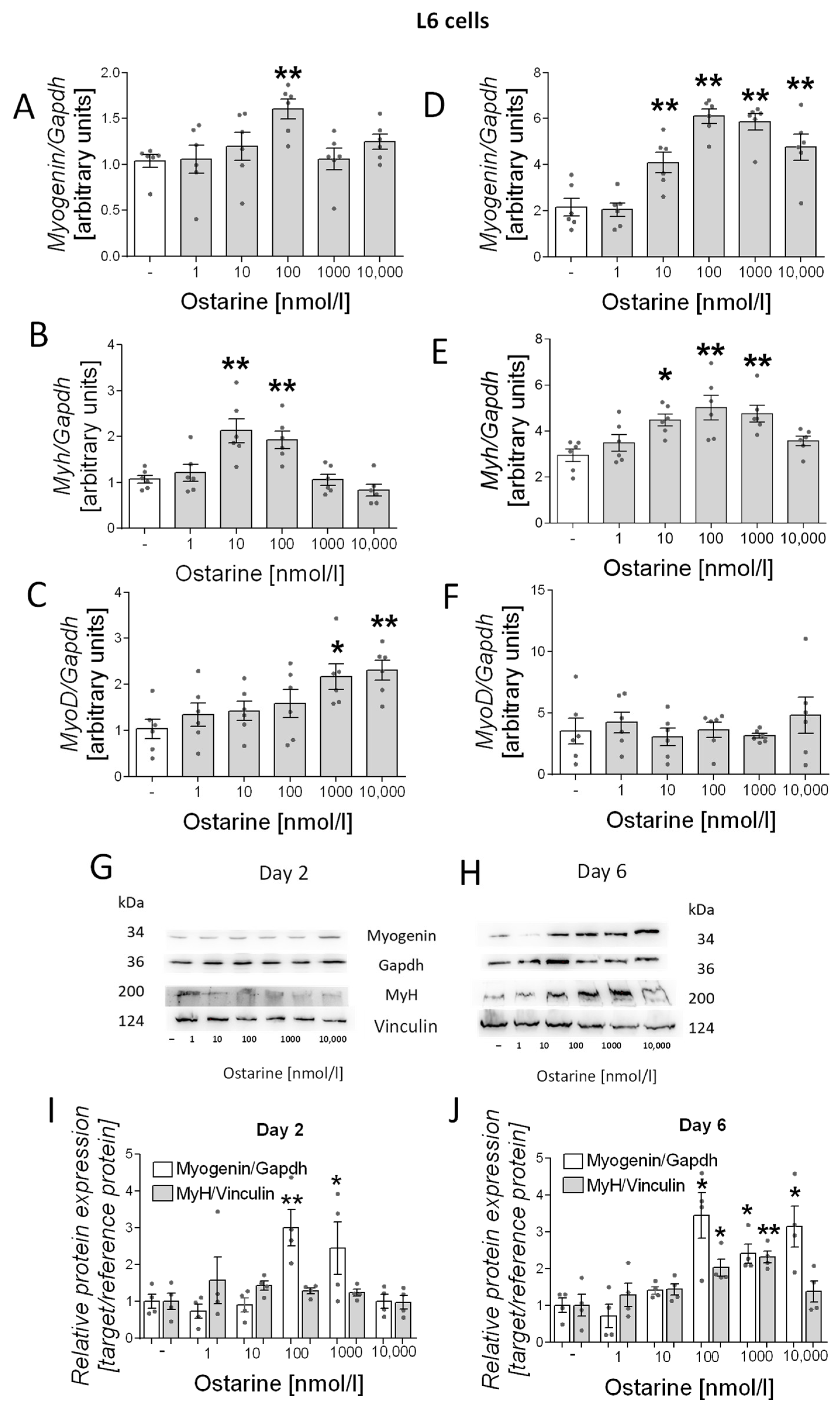
2.2. Differentiation Process in L6 Cells
2.3. Differentiation Process in C2C12 Cells
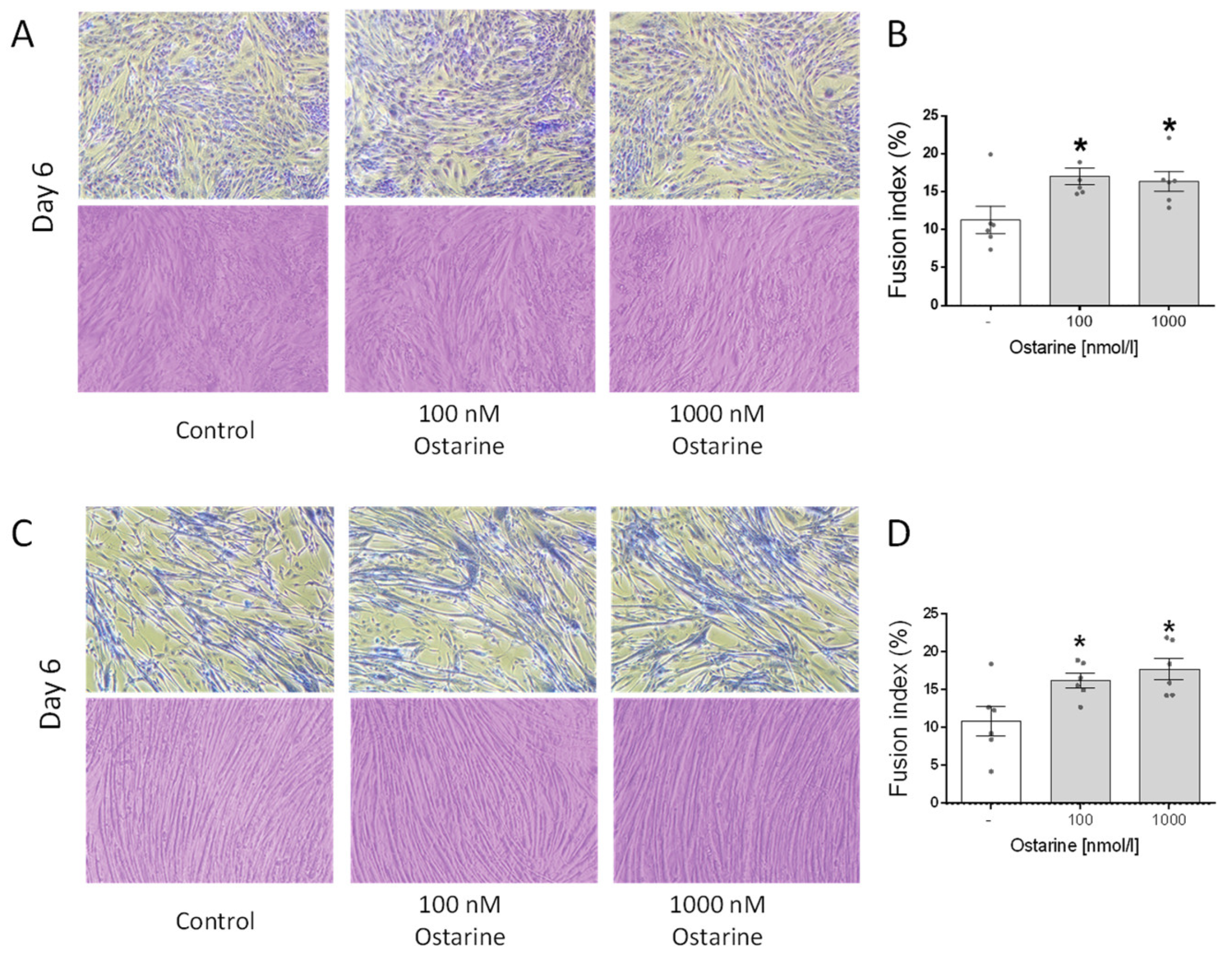
2.4. Effect of Ostarine on Fusion Index in C2C12 and L6 Cells
2.5. Effect of Ostarine Is Mediated by AR in C2C12 and L6 Cells
2.6. Effect of Ostarine on Muscle Differentiation in Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ostarine Injections
4.3. Chemicals
4.4. C2C12 and L6 Cell Culturing
4.5. C2C12 and L6 Cell Differentiation
4.6. Proliferation and Cell Viability
4.7. RNA and Real-Time qPCR
4.8. Western Blot Analysis
4.9. Tissue Staining
4.10. Jenner–Giemsa Staining
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-Induced Skeletal Muscle Atrophy and Fatigue. Med. Sci. Sport. Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving healthy muscle during weight loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha-Hikim, I.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Zheng, W.; Bhasin, S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: Up-regulation by androgen treatment. J. Clin. Endocrinol. Metab. 2004, 89, 5245–5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, V.; Vanderschueren, D.; Antonio, L. Treatment of men with central hypogonadism: Alternatives for testosterone replacement therapy. Int. J. Mol. Sci. 2021, 22, 21. [Google Scholar] [CrossRef]
- Grossmann, M.; Matsumoto, A.M. A perspective on middle-aged and older men with functional hypogonadism: Focus on holistic management. J. Clin. Endocrinol. Metab. 2017, 102, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Barbonetti, A.; D’Andrea, S.; Francavilla, S. Testosterone replacement therapy. Andrology 2020, 8, 1551–1566. [Google Scholar] [CrossRef] [Green Version]
- Berg, W.T.; Miner, M. Hypogonadism and metabolic syndrome: Review and update. Curr. Opin. Endocrinol. Diabetes. Obes. 2020, 27, 404–410. [Google Scholar] [CrossRef]
- Sebo, Z.L.; Rodeheffer, M.S. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol. Metab. 2021, 44, 101141. [Google Scholar] [CrossRef]
- Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Testosterone and Bone Health in Men: A Narrative Review. J. Clin. Med. 2021, 10, 530. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone, mood, behaviour and quality of life. Andrology 2020, 8, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ahn, S.; Moon, D. Evolution of Guidelines for Testosterone Replacement Therapy. J. Clin. Med. 2019, 8, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, Z.J.; Mirabal, J.R.; Mazur, D.J.; Kohn, T.P.; Lipshultz, L.I.; Pastuszak, A.W. Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications. Sex. Med. Rev. 2019, 7, 84–94. [Google Scholar] [CrossRef]
- Simitsidellis, I.; Esnal-Zuffiaure, A.; Kelepouri, O.; O’Flaherty, E.; Gibson, D.A.; Saunders, P.T.K. Selective androgen receptor modulators (SARMs) have specific impacts on the mouse uterus. J. Endocrinol. 2019, 242, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SARMsClinicaltrials. Available online: www.clinicaltrials.gov/ct2/results?recrs=&cond=&term=Selective+androgen+receptor&cntry=&state=&city=&dist= (accessed on 7 March 2022).
- Komrakova, M.; Furtwängler, J.; Hoffmann, D.B.; Lehmann, W.; Schilling, A.F.; Sehmisch, S. The Selective Androgen Receptor Modulator Ostarine Improves Bone Healing in Ovariectomized Rats. Calcif. Tissue Int. 2020, 106, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Leciejewska, N.; Pruszynska-Oszmalek, E.; Bien, J.; Nogowski, L.; Kolodziejski, P.A. Effect of ostarine (Enobosarm/gtx024), a selective androgen receptor modulator, on adipocyte metabolism in wistar rats. J. Physiol. Pharmacol. 2019, 70, 525–533. [Google Scholar] [CrossRef]
- Dubois, V.; Simitsidellis, I.; Laurent, M.R.; Jardi, F.; Saunders, P.T.K.; Vanderschueren, D.; Claessens, F. Enobosarm (GTx-024) modulates adult skeletal muscle mass independently of the androgen receptor in the satellite cell lineage. Endocrinology 2015, 156, 4522–4533. [Google Scholar] [CrossRef] [Green Version]
- Riuzzi, F.; Sorci, G.; Arcuri, C.; Giambanco, I.; Bellezza, I.; Minelli, A.; Donato, R. Cellular and molecular mechanisms of sarcopenia: The S100B perspective. J. Cachexia. Sarcopenia Muscle 2018, 9, 1255–1268. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of selective androgen receptor modulators (SARMs). Mol. Cell. Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Komrakova, M.; Nagel, J.; Hoffmann, D.B.; Lehmann, W.; Schilling, A.F.; Sehmisch, S. Effect of Selective Androgen Receptor Modulator Enobosarm on Bone Healing in a Rat Model for Aged Male Osteoporosis. Calcif. Tissue Int. 2020, 107, 593–602. [Google Scholar] [CrossRef]
- Dalton, J.; Narayanan, R.; Steiner, M. Abstract P5-09-21: Selective androgen receptor modulators (SARMs): Enobosarm as targeted therapy for the treatment of androgen receptor-positive breast cancer. Cancer Res. 2013, 73 Suppl. 24, P5-09–21. [Google Scholar] [CrossRef]
- Christiansen, A.R.; Lipshultz, L.I.; Hotaling, J.M.; Pastuszak, A.W. Selective androgen receptor modulators: The future of androgen therapy? Transl. Androl. Urol. 2020, 9, S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Foradori, C.D.; Weiser, M.J.; Handa, R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008, 29, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 Activation Controls Cell Proliferation and Cell Death Is Subcellular Localization the Answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.; Bulldan, A.; Scheiner-Bobis, G. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gnα11. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 1172–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, K.M.; Liu, J.; Francis, G.D.; Naderi, A. A feedback loop between androgen receptor and erk signaling in estrogen receptor-negative breast cancer. Neoplasia 2011, 13, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Sadar, M.D. Enzalutamide and blocking androgen receptor in advanced prostate cancer: Lessons learnt from the history of drug development of antiandrogens. Res. Reports Urol. 2018, 10, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Liu, J.; Fan, J.; Li, R.; Li, D.; Yin, J.; Cui, S. Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J. Cell. Physiol. 2012, 227, 98–107. [Google Scholar] [CrossRef]
- Wannenes, F.; Caprio, M.; Gatta, L.; Fabbri, A.; Bonini, S.; Moretti, C. Androgen receptor expression during C2C12 skeletal muscle cell line differentiation. Mol. Cell. Endocrinol. 2008, 292, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zajac, J.D.; MacLean, H.E. Androgen regulation of satellite cell function. J. Endocrinol. 2005, 186, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, J.R.; Fletcher, D.K.; McGuigan, M.R. Evidence for a non-genomic action of testosterone in skeletal muscle which may improve athletic performance: Implications for the female athlete. J. Sport. Sci. Med. 2012, 11, 363–370. [Google Scholar]
- Singh, R.; Artaza, J.N.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Bhasin, S. Androgens Stimulate Myogenic Differentiation and Inhibit Adipogenesis in C3H 10T1/2 Pluripotent Cells through an Androgen Receptor-Mediated Pathway. Endocrinology 2003, 144, 5081–5088. [Google Scholar] [CrossRef] [PubMed]
- Muta, Y.; Tanaka, T.; Hamaguchi, Y.; Hamanoue, N.; Motonaga, R.; Tanabe, M.; Nomiyama, T.; Nawata, H.; Yanase, T. Selective androgen receptor modulator, S42 has anabolic and anti-catabolic effects on cultured myotubes. Biochem. Biophys. Reports 2019, 17, 177–181. [Google Scholar] [CrossRef]
- Kitakaze, T.; Oshimo, M.; Kobayashi, Y.; Ryu, M.; Suzuki, Y.A.; Inui, H.; Harada, N.; Yamaji, R. Lactoferrin promotes murine C2C12 myoblast proliferation and differentiation and myotube hypertrophy. Mol. Med. Rep. 2018, 17, 5912–5920. [Google Scholar] [CrossRef]
- Gan, M.; Yang, D.; Fan, Y.; Du, J.; Shen, L.; Li, Q.; Jiang, Y.; Tang, G.; Li, M.; Wang, J.; et al. Bidirectional regulation of genistein on the proliferation and differentiation of C2C12 myoblasts. Xenobiotica 2020, 50, 1352–1358. [Google Scholar] [CrossRef]
- Roch, P.J.; Henkies, D.; Carstens, J.C.; Krischek, C.; Lehmann, W.; Komrakova, M.; Sehmisch, S. Ostarine and Ligandrol Improve Muscle Tissue in an Ovariectomized Rat Model. Front. Endocrinol. 2020, 11, 668. [Google Scholar] [CrossRef]
- Hoffmann, D.B.; Komrakova, M.; Pflug, S.; von Oertzen, M.; Saul, D.; Weiser, L.; Walde, T.A.; Wassmann, M.; Schilling, A.F.; Lehmann, W.; et al. Evaluation of ostarine as a selective androgen receptor modulator in a rat model of postmenopausal osteoporosis. J. Bone Miner. Metab. 2019, 37, 243–255. [Google Scholar] [CrossRef]
- Pavlidou, T.; Rosina, M.; Fuoco, C.; Gerini, G.; Gargioli, C.; Castagnoli, L.; Cesareni, G. Regulation of myoblast differentiation by metabolic perturbations induced by metformin. PLoS ONE 2017, 12, e0182475. [Google Scholar] [CrossRef] [Green Version]
- Lawson, M.A.; Purslow, P.P. Differentiation of myoblasts in serum-free media: Effects of modified media are cell line-specific. Cells Tissues Organs 2000, 167, 130–137. [Google Scholar] [CrossRef]
- Kolodziejski, P.A.; Pruszynska-Oszmalek, E.; Micker, M.; Skrzypski, M.; Wojciechowicz, T.; Szwarckopf, P.; Skieresz-Szewczyk, K.; Nowak, K.W.; Strowski, M.Z. Spexin: A novel regulator of adipogenesis and fat tissue metabolism. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2018, 1863, 1228–1236. [Google Scholar] [CrossRef] [PubMed]





| Target | Forward Primer (5′ > 3′) | Reverse Primer (5′ > 3′) | Product (bp) |
|---|---|---|---|
| Myogenin (Rattus) | CTACAGGCCTTGCTCAGCTC | TGGGAGTTGCATTCACTGG | 101 |
| MyoD (Rattus) | ACTACAGCGGCGACTCAGAC | ACTGTAGTAGGCGGCGTCGT | 122 |
| Myh3 (Rattus) | GCAGAGACCATCAAGCACCT | GTGCAGCTGGGTGTCCTT | 60 |
| GAPDH (Rattus) | CTGCACCACCAACTGCTTAG | TGATGGCATGGACTGTGG | 92 |
| Myogenin (Mus) | CGGTGGAGGATATGTCTGTTG | GGTGTTAGCCTTATGTGAATGG | 215 |
| Myh3 (Mus) | ATGAGCGGCGTGTTAAGGA | ATTGACTTGCGATTCTGCGATA | 227 |
| MyoD (Mus) | AGCACTACAGTGGCGACTCA | GGCCGCTGTAATCCATCAT | 75 |
| GAPDH (Mus) | ATGGTGAAGGTCGGTGTGA | AATCTCCACTTTGCCACTGC | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leciejewska, N.; Kołodziejski, P.A.; Sassek, M.; Nogowski, L.; Małek, E.; Pruszyńska-Oszmałek, E. Ostarine-Induced Myogenic Differentiation in C2C12, L6, and Rat Muscles. Int. J. Mol. Sci. 2022, 23, 4404. https://doi.org/10.3390/ijms23084404
Leciejewska N, Kołodziejski PA, Sassek M, Nogowski L, Małek E, Pruszyńska-Oszmałek E. Ostarine-Induced Myogenic Differentiation in C2C12, L6, and Rat Muscles. International Journal of Molecular Sciences. 2022; 23(8):4404. https://doi.org/10.3390/ijms23084404
Chicago/Turabian StyleLeciejewska, Natalia, Paweł A. Kołodziejski, Maciej Sassek, Leszek Nogowski, Emilian Małek, and Ewa Pruszyńska-Oszmałek. 2022. "Ostarine-Induced Myogenic Differentiation in C2C12, L6, and Rat Muscles" International Journal of Molecular Sciences 23, no. 8: 4404. https://doi.org/10.3390/ijms23084404






