Hydrothermal Synthesis of β-Nb2ZnO6 Nanoparticles for Photocatalytic Degradation of Methyl Orange and Cytotoxicity Study
Abstract
:1. Introduction
2. Results and Discussion
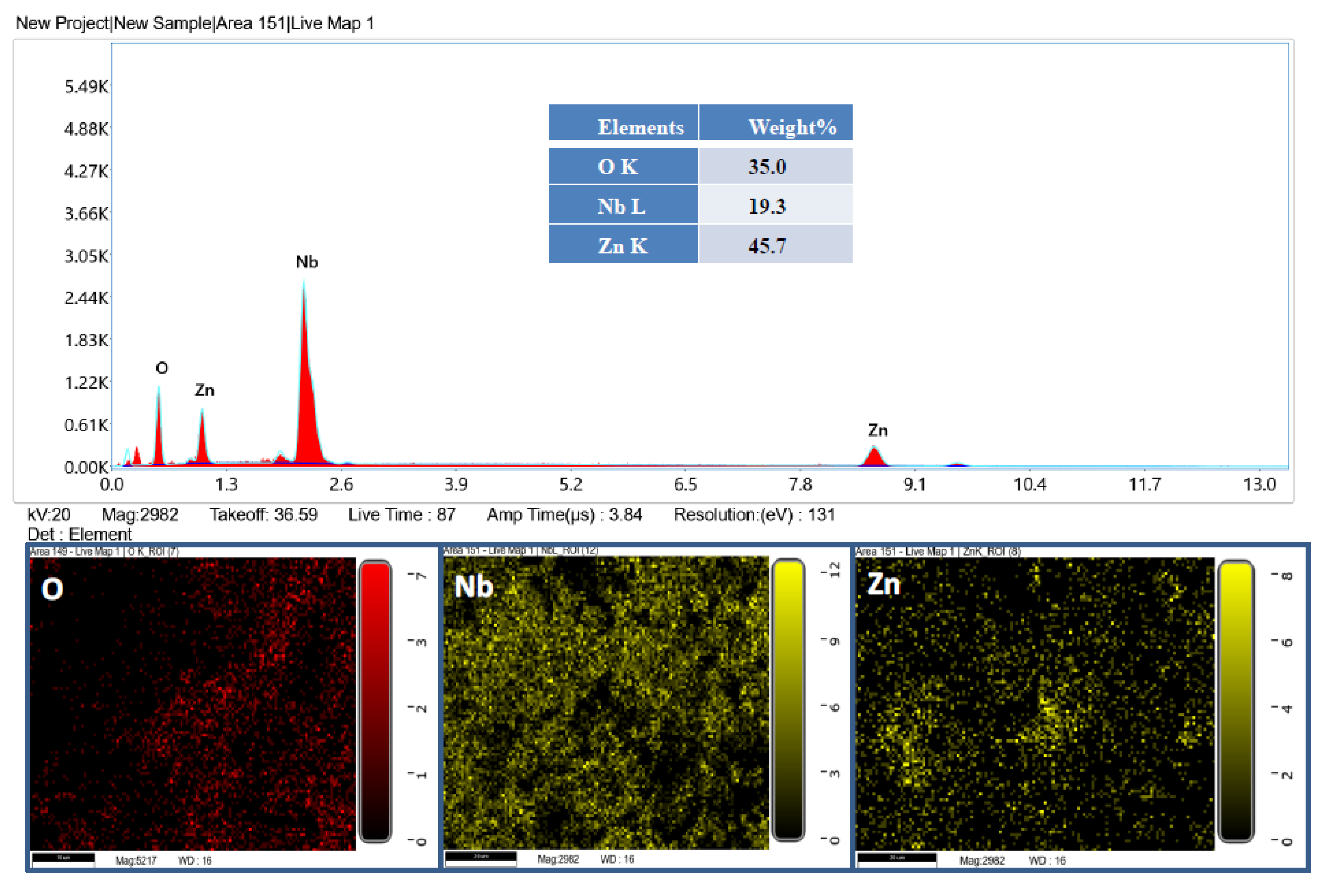
2.1. Characterization of β-ZnNb2O6 Nanoparticles
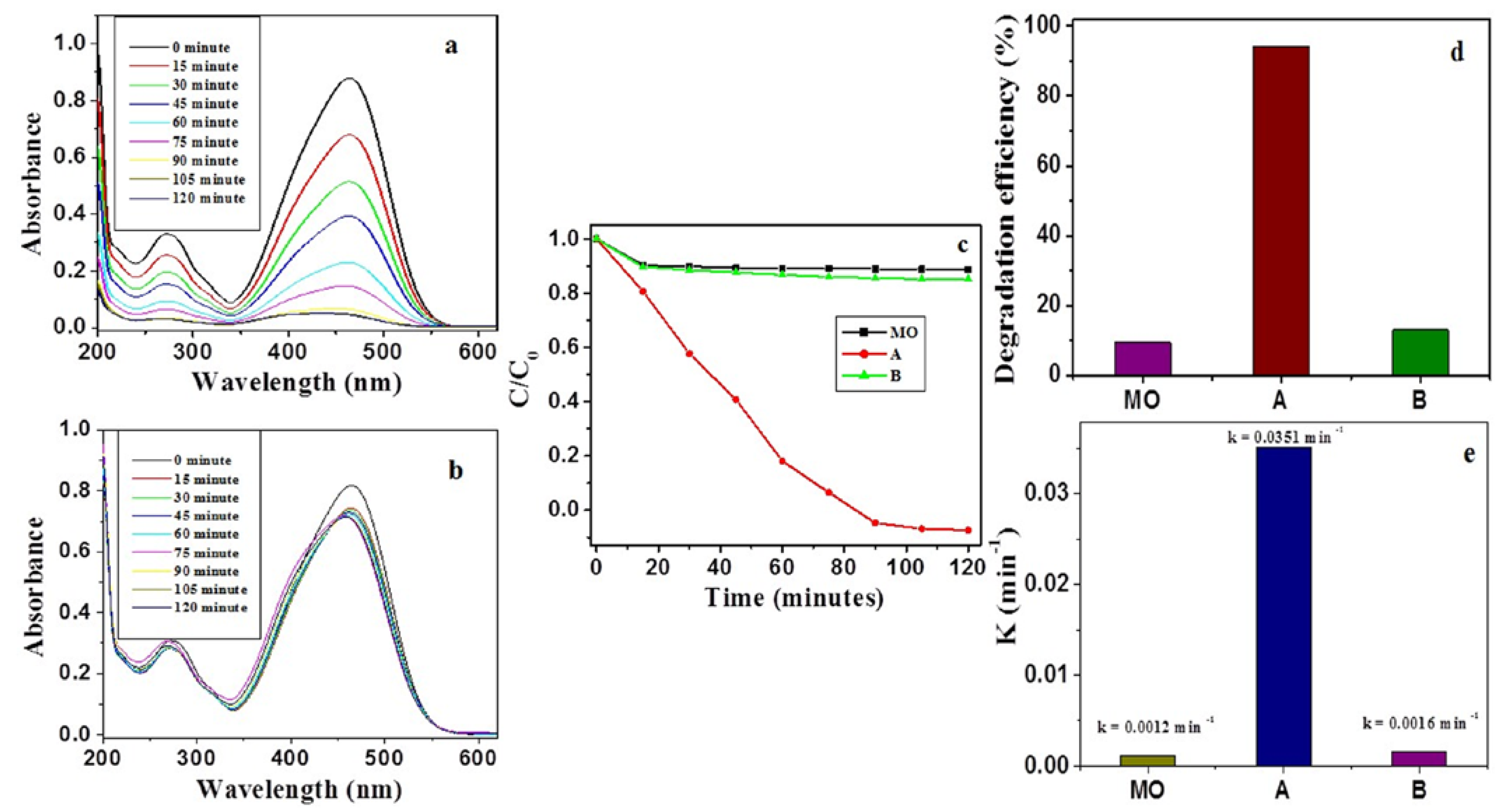
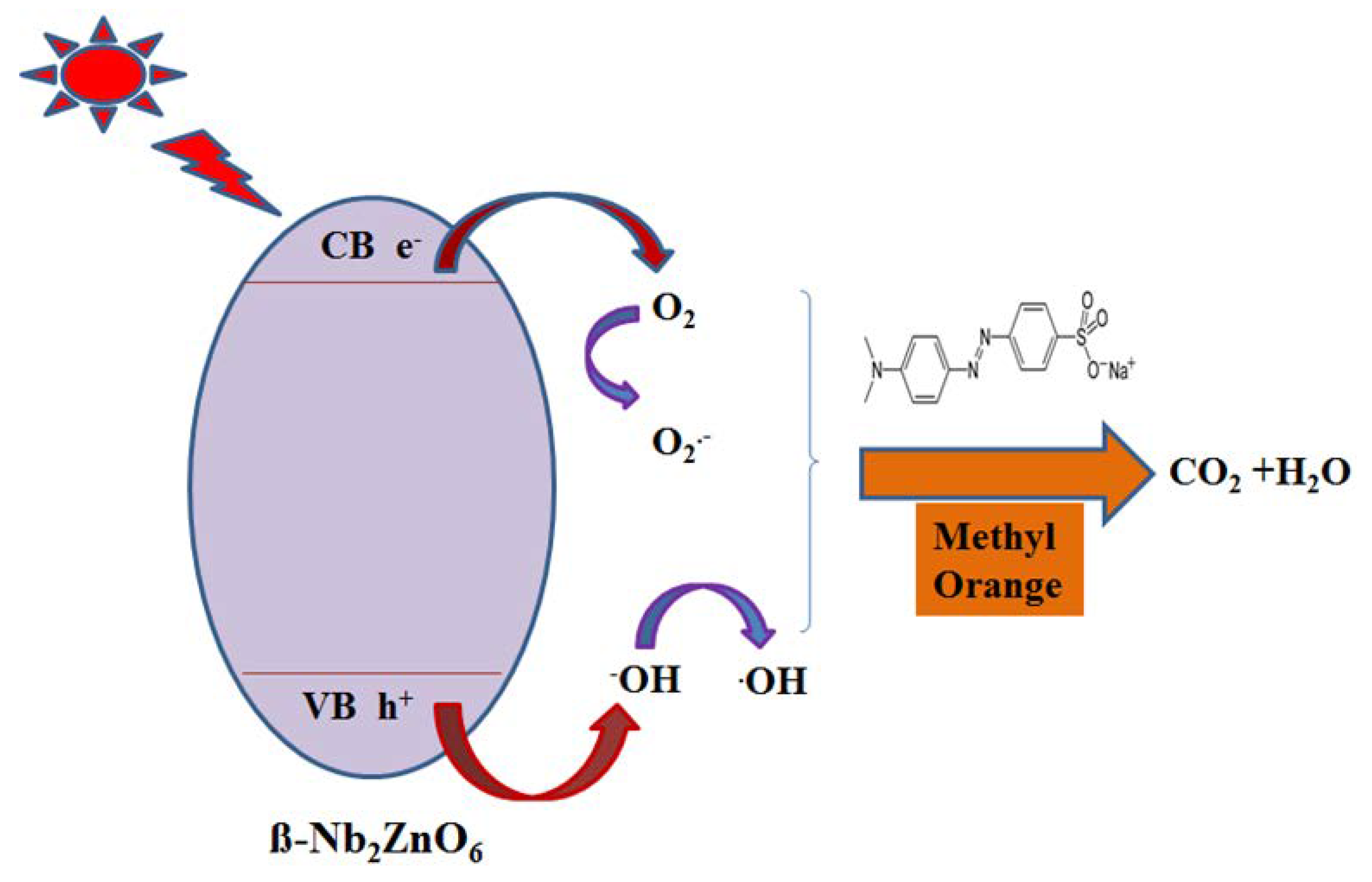
2.2. Photocatalytic Activity of β-Nb2ZnO6 Nanoparticles
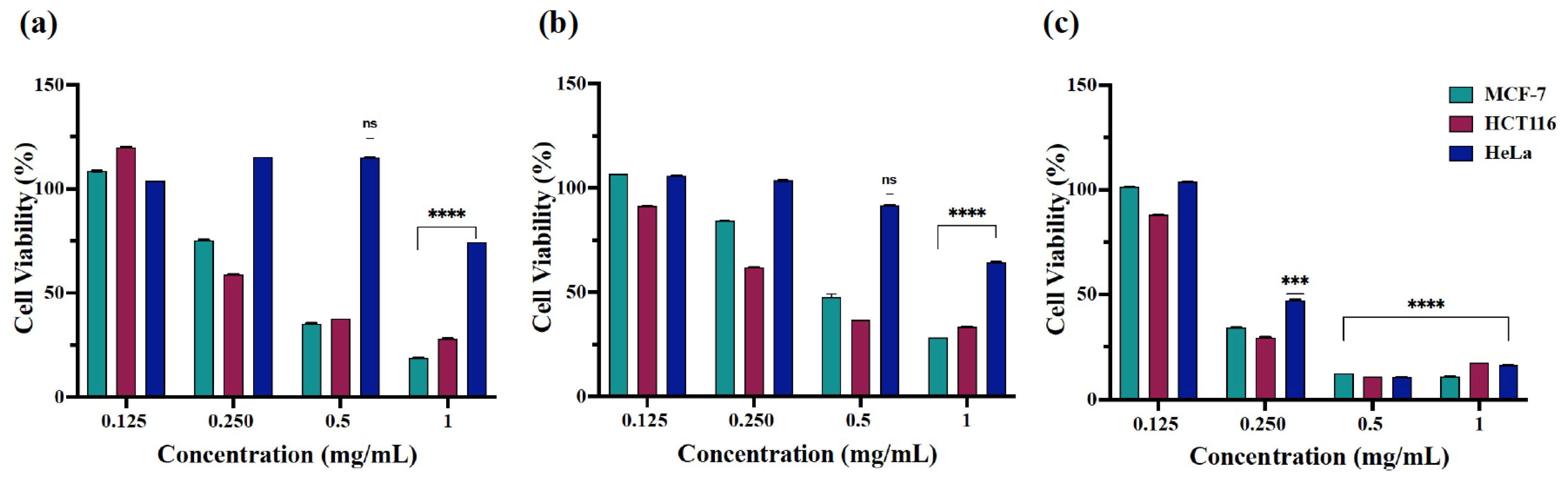
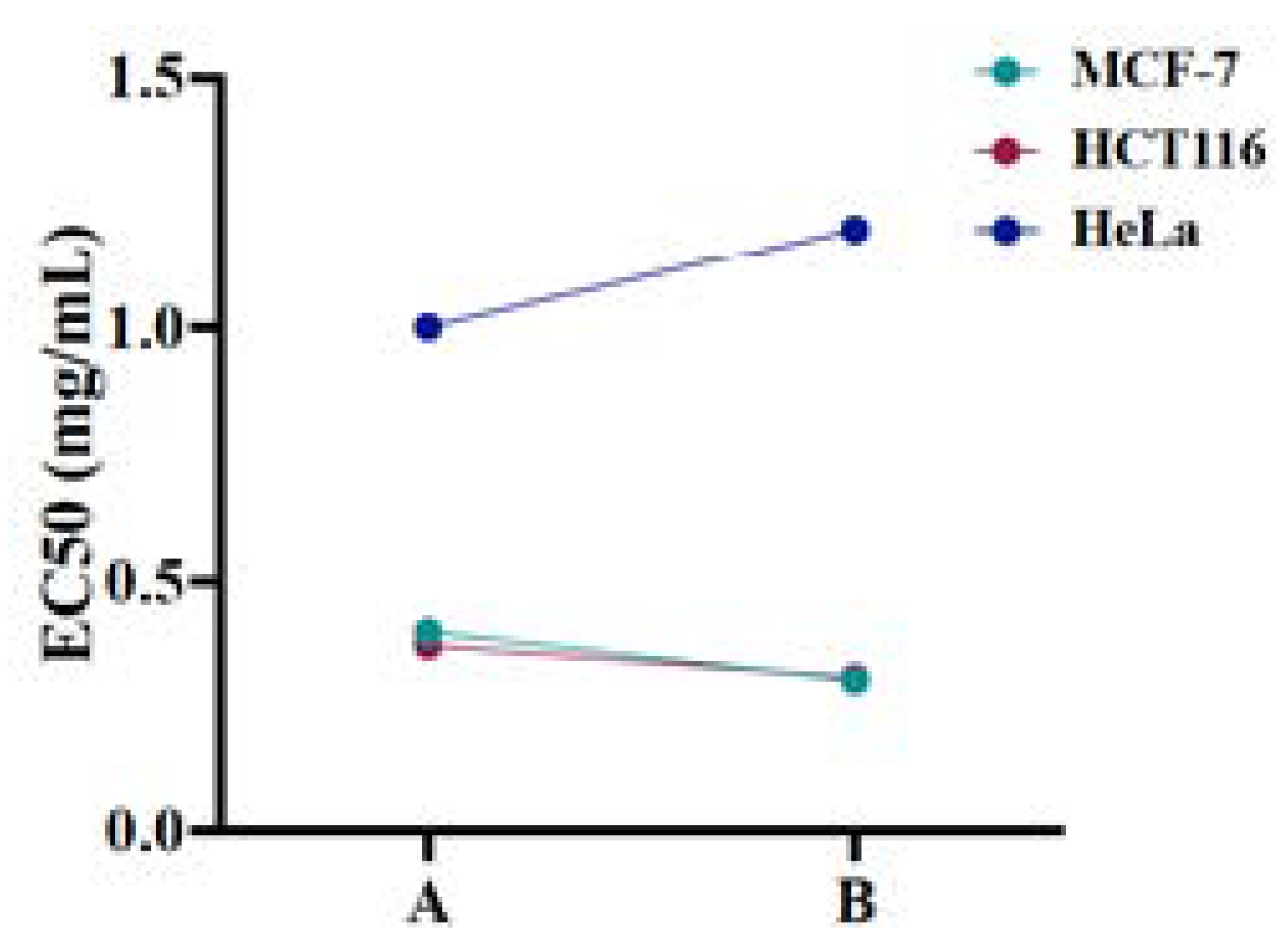
2.3. Cytotoxicity of β-Nb2ZnO6 Nanoparticles
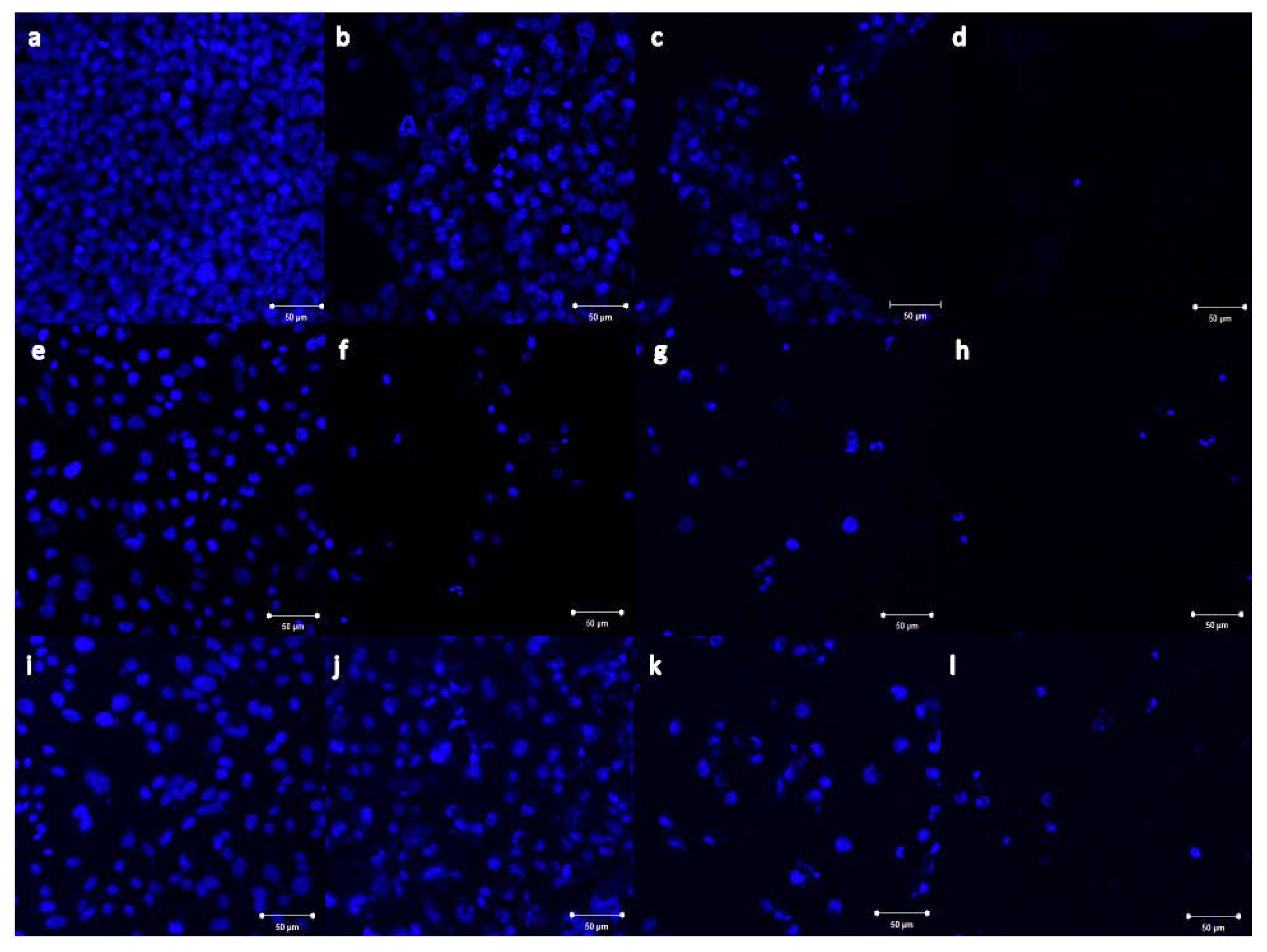
2.4. Imaging of Nb2ZnO6 Nanoparticles Treated Cells
3. Materials and Methods
3.1. Synthesis of β-Nb2ZnO6
3.2. Photocatalytic Activity
3.3. Cell Culture and Cytotoxicity of β-Nb2ZnO6 Nanoparticles
3.4. Imaging by Confocal Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chałupniak, A.; Morales-Narváez, E.; Merkoçi, A. Micro and nanomotors in diagnostics. Adv. Drug Deliv. Rev. 2015, 95, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Kerssemakers, J.; Ionov, L.; Queitsch, U.; Luna, S.; Hess, H.; Diez, S. 3D Nanometer Tracking of Motile Microtubules on Reflective Surfaces. Small 2009, 5, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, H.; Tanaka, H.; Rice, K.M.; Kolli, M.B.; Nalabotu, S.K.; Kohama, K.; Famouri, P.; Blough, E.R. Transport of single cells using an actin bundle–myosin bionanomotor transport system. Nanotechnology 2011, 22, 245101. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Zharov, V.P.; Brazel, C.S.; Nikles, D.E.; Johnson, D.T.; Everts, M. Combination of viral biology and nanotechnology: New applications in nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 200–206. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Qureshi, F. Review: Nanoparticles in delivery of cardiovascular drugs. Pak. J. Pharm. Sci. 2007, 20, 340–348. [Google Scholar] [PubMed]
- Nawaz, M. Nanotechnology-Based Approaches in Pediatric Parasitic Infections. J. Pediatr. Infect. Dis. 2017, 12, 264–270. [Google Scholar] [CrossRef]
- Khandelwal, H.; Singh, G.; Agrawal, K.; Prakash, S.; Agarwal, R. Characterization of hydroxyapatite coating by pulse laser deposition technique on stainless steel 316 L by varying laser energy. Appl. Surf. Sci. 2013, 265, 30–35. [Google Scholar] [CrossRef]
- Qureshi, F.; Nawaz, M.; Rehman, S.; Almofty, S.A.; Shahzad, S.; Nissapatorn, V.; Taha, M. Synthesis and characterization of cadmium-bismuth microspheres for the catalytic and photocatalytic degradation of organic pollutants, with antibacterial, antioxidant and cytotoxicity assay. J. Photochem. Photobiol. B Biol. 2020, 202, 111723. [Google Scholar] [CrossRef]
- Shao, H.; Yu, C.; Xu, X.; Ji, W.; Rui, Z.; Xiaojing, W. Influence of TiO2 nanocrystallization on microstructure, interface bonding, surface energy and blood compatibility of surface TiO2 films. Appl. Surf. Sci. 2010, 257, 1649–1654. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Chen, Y.; Gu, H. Improving endothelialization on 316L stainless steel through wettability controllable coating by sol–gel technology. Appl. Surf. Sci. 2013, 268, 73–78. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zhu, L.; Chen, S.; Zhang, Y.; Tang, Y.; Zhu, Y.; Li, Y. Synthesis of nano titania particles embedded in mesoporous SBA-15: Characterization and photocatalytic activity. J. Hazard. Mater. 2006, 137, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Yu, J.; Zhao, J. Enhanced photocatalytic activity of mesoporous and ordinary TiO2 thin films by sulfuric acid treatment. Appl. Catal. B-Environ. 2002, 36, 31–43. [Google Scholar] [CrossRef]
- Khaleel, A.; Nawaz, M.; Al-Hadrami, S.; Greish, Y.; Saeed, T. The effect of metal ion dopants (V3+, Cr3+, Fe3+, Mn2+, Ce3+) and their concentration on the morphology and the texture of doped γ-alumina. Microporous Mesoporous Mater. 2012, 168, 7–14. [Google Scholar] [CrossRef]
- Khaleel, A.; Nawaz, M.; Hindawi, B. Sol-gel derived Cr(III) and Cu(II)/γ-Al2O3 doped solids: Effect of the dopant precursor nature on the structural, textural and morphological properties. Mater. Res. Bull. 2013, 48, 1709–1715. [Google Scholar] [CrossRef]
- Khaleel, A.; Nawaz, M. Enhanced catalytic complete oxidation of 1,2-dichloroethane over mesoporous transition-metal doped gamma-Al2O3. J. Environ. Sci. 2015, 29, 199–209. [Google Scholar] [CrossRef]
- Khaleel, A.; Nawaz, M. The effect of composition and gel treatment conditions on the textural properties, reducibility and catalytic activity of sol-gel prepared Fe-(III)-Cr(III) bulk mixed oxides. Colloid Surf. A-Physicochem. Eng. Asp. 2016, 488, 52–57. [Google Scholar] [CrossRef]
- Nawaz, M.; Almofty, S.A.; Qureshi, F. Preparation, formation mechanism, photocatalytic, cytotoxicity and antioxidant activity of sodium niobate nanocubes. PLoS ONE 2018, 13, e0204061. [Google Scholar] [CrossRef]
- Nawaz, M.; Akhtar, S.; Qureshi, F.; Almofty, S.A.; Nissapatron, V. Preparation of indium-cadmium sulfide nanoparticles with diverse morphologies: Photocatalytic and cytotoxicity study. J. Mol. Struct. 2021, 1253, 132288. [Google Scholar] [CrossRef]
- Lin, W. Introduction: Nanoparticles in Medicine. Chem. Rev. 2015, 115, 10407–10409. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.-P. Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Stanley, S.A.; Sauer, J.; Kane, R.S.; Dordick, J.S.; Friedman, J.M. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 2015, 21, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboob, T.; Nawaz, M.; Tian-Chye, T.; Samudi, C.; Wiart, C.; Nissapatorn, V. Preparation of Poly (dl-Lactide-co-Glycolide) Nanoparticles Encapsulated with Periglaucine A and Betulinic Acid for In Vitro Anti-Acanthamoeba and Cytotoxicity Activities. Pathogens 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-Y.; Young, D.; Martel, J.; Young, J.D. A story told by a single nanoparticle in the body fluid: Demonstration of dissolution-reprecipitation of nanocrystals in a biological system. Nanomedicine 2015, 10, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Yasur, J.; Rani, P.U. Lepidopteran insect susceptibility to silver nanoparticles and measurement of changes in their growth, development and physiology. Chemosphere 2015, 124, 92–102. [Google Scholar] [CrossRef]
- Tun, L.; Libo, W.; Ye, J.; Qiuwen, L.; Caijin, H. Hexagonal boron nitride nanoplates as emerging biological nanovectors and their potential applications in biomedicine. J. Mater. Chem. B 2016, 4, 6103–6110. [Google Scholar]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020, 65, 21RM02. [Google Scholar] [CrossRef]
- Nawaz, M. Morphology-controlled preparation of Bi2S3-ZnS chloroplast-like structures, formation mechanism and photocatalytic activity for hydrogen production. J. Photochem. Photobiol. A Chem. 2017, 332, 326–330. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical Applications of ReactiveOxygen Species Generation by Metal Nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Pradeep PremKumar, K.; Duraipandy, N.; Kiran, M.S.; Rajendrana, N. Antibacterial effects, biocompatibility and electrochemical behavior of zinc incorporated niobium oxide coating on 316L SS for biomedical applications. Appl. Surf. Sci. 2018, 427, 1166–1181. [Google Scholar]
- Liang, Z.; Yan, C.-F.; Rtimi, S.; Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B Environ. 2019, 241, 256–269. [Google Scholar] [CrossRef]
- Chaojun, Z.; Weihua, F.; Haoqing, W.; Najun, L.; Dongyun, C.; Qingfeng, X.; Hua, L.; Jinghui, H.; Jianmei, L. p-n Heterojunction of BiOI/ZnO nanorod arrays for piezo-photocatalytic degradation of bisphenol A in water. J. Hazard. Mater. 2020, 399, 123109. [Google Scholar]
- Xiaoyi, S.; Youjiang, S.; Hongmei, S.; Yan, L.; Yuchun, Z. Synthesis and photocatalytic degradation ability evaluation for rhodamine B of ZnO@SiO2 composite with flower-like structure. Water Sci. Technol. 2020, 80, 1986–1995. [Google Scholar]
- Zhen, L.; Dan, J.; Zhenghua, W. ZnO/CdSe-diethylenetriamine nanocomposite as astep-scheme photocatalyst for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 529, 147071. [Google Scholar]
- Bingxuan, N.; Dapeng, W.; Jinshui, W.; Le, W.; Wenlong, Z. Salt-sealing-pyrolysis derived Ag/ZnO@C hollow structures towards efficient photo-oxidation of organic dye and water-born bacteria. Appl. Surf. Sci. 2020, 528, 146965. [Google Scholar]
- Yan, Y.; Binghua, Y.; Yangqing, H.; Baoyue, C.; Youliang, R.; Qiangqiang, S. Piezo-enhanced photodegradation of organic pollutants on Ag3PO4/ZnO nanowires using visible light and ultrasonic. Appl. Surf. Sci. 2020, 528, 146819. [Google Scholar]
- Nawaz, M.; Fangzhi, M.; Leilei, X.; Hao, T.; Jianguo, G. F–Bi4TaO8Cl flower-like hierarchical structures: Controlled preparation, formation mechanism and visible photocatalytic hydrogen production. RSC Adv. 2017, 7, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Fangzhi, M.; Leilei, X.; Jianguo, G. Effect of solvents and reaction parameters on the morphology of Ta2O5 and photocatalytic activity. J. Mol. Liq. 2018, 269, 211–216. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Zhang, X.; Qin, X.; Dai, Y.; Jin, H.; Wei, J.; Whangbo, M.-H. Composite Semiconductor H2WO4⋅H2O/AgCl as an Efficient and Stable Photocatalyst under Visible Light. Chem. Eur. J. 2008, 14, 10543–10546. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; An, T.; Li, G.; Wang, W.; Zhao, H.; Wong, P.K. Synergistic photocatalytic inactivation mechanisms of bacteria by graphene sheets grafted plasmonic AgAgX (X = Cl, Br, I) composite photocatalyst under visible light irradiation. Water Res. 2016, 99, 149–161. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almofty, S.A.; Nawaz, M.; Qureshi, F.; Al-Mutairi, R. Hydrothermal Synthesis of β-Nb2ZnO6 Nanoparticles for Photocatalytic Degradation of Methyl Orange and Cytotoxicity Study. Int. J. Mol. Sci. 2022, 23, 4777. https://doi.org/10.3390/ijms23094777
Almofty SA, Nawaz M, Qureshi F, Al-Mutairi R. Hydrothermal Synthesis of β-Nb2ZnO6 Nanoparticles for Photocatalytic Degradation of Methyl Orange and Cytotoxicity Study. International Journal of Molecular Sciences. 2022; 23(9):4777. https://doi.org/10.3390/ijms23094777
Chicago/Turabian StyleAlmofty, Sarah Ameen, Muhammad Nawaz, Faiza Qureshi, and Rayanah Al-Mutairi. 2022. "Hydrothermal Synthesis of β-Nb2ZnO6 Nanoparticles for Photocatalytic Degradation of Methyl Orange and Cytotoxicity Study" International Journal of Molecular Sciences 23, no. 9: 4777. https://doi.org/10.3390/ijms23094777





