Octanoic Acid—An Insecticidal Metabolite of Conidiobolus coronatus (Entomopthorales) That Affects Two Majors Antifungal Protection Systems in Galleria mellonella (Lepidoptera): Cuticular Lipids and Hemocytes
Abstract
:1. Introduction
2. Results
2.1. The Insecticidal Activity of Fungal Filtrates against G. mellonella Larvae and Adults
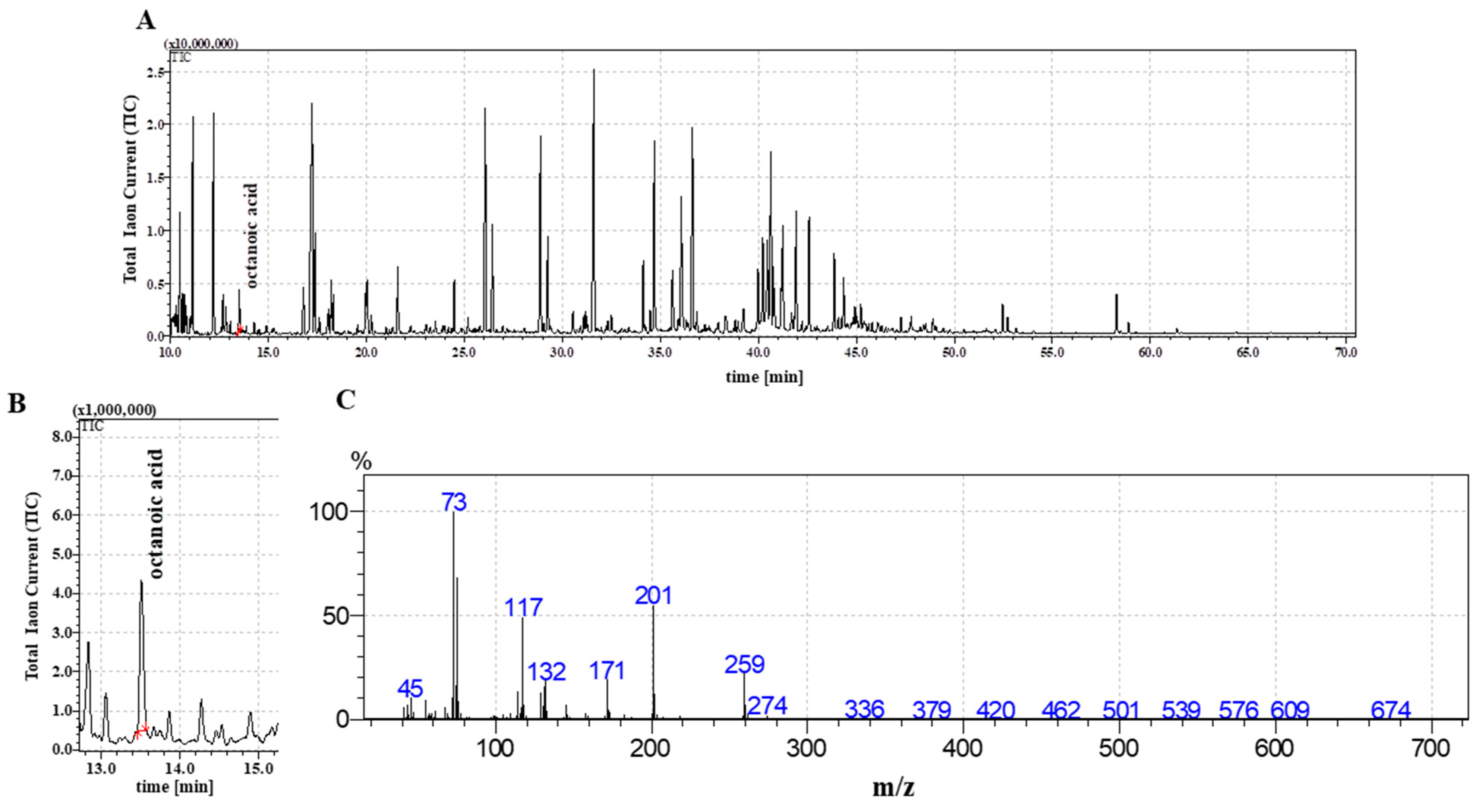
2.2. The Detection of Octanoic Acid in FR3
2.3. The Insecticidal Activity of Octanoic Acid
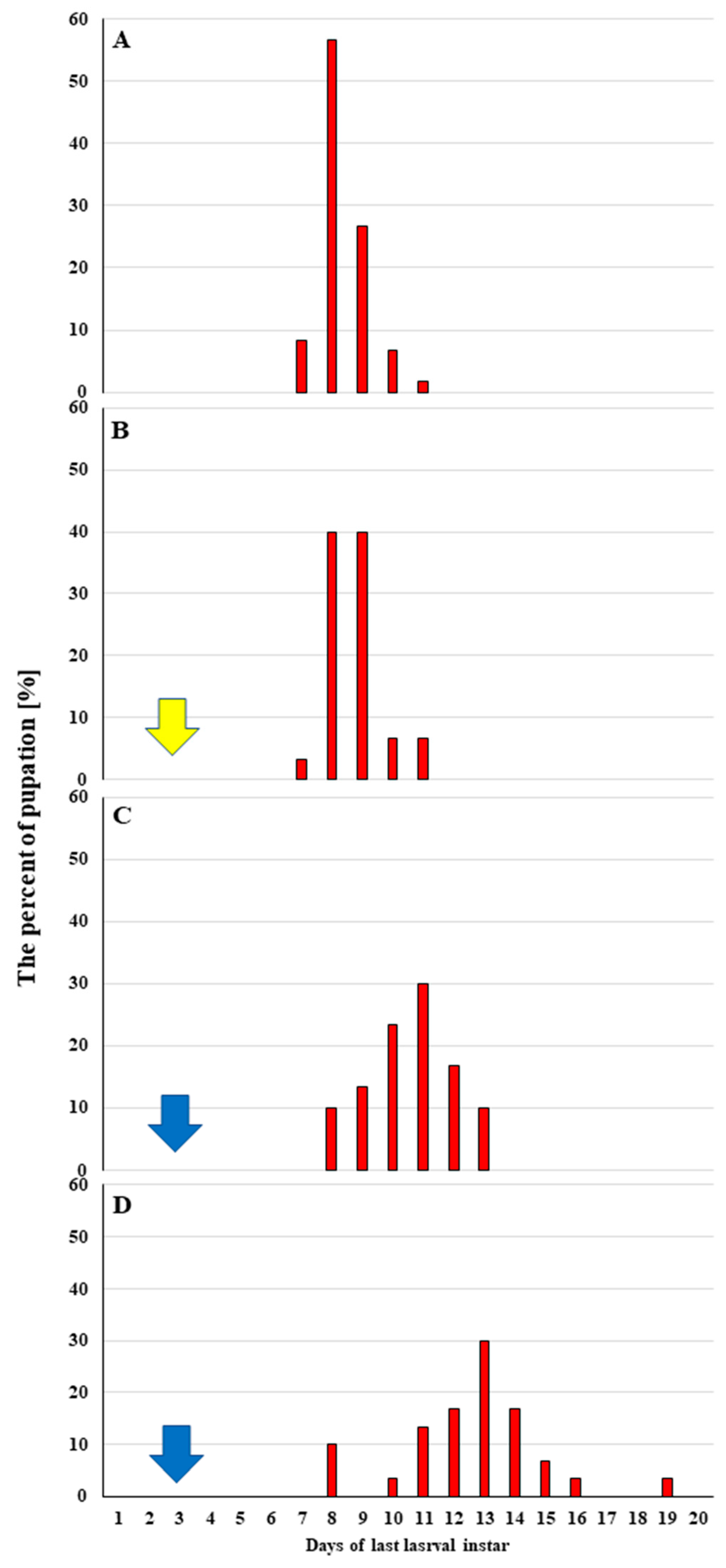
2.3.1. Effect of Octanoic Acid on the Survival and Further Development of G. mellonella Larvae
2.3.2. The LD50 and LD100 Values of Octanoic Acid for G. mellonella Larvae and Adults
2.4. The Changes in Cuticular FFA Profiles Observed in G. mellonella after Topical Application of Octanoic Acid (LD50 or LD100 Dose)
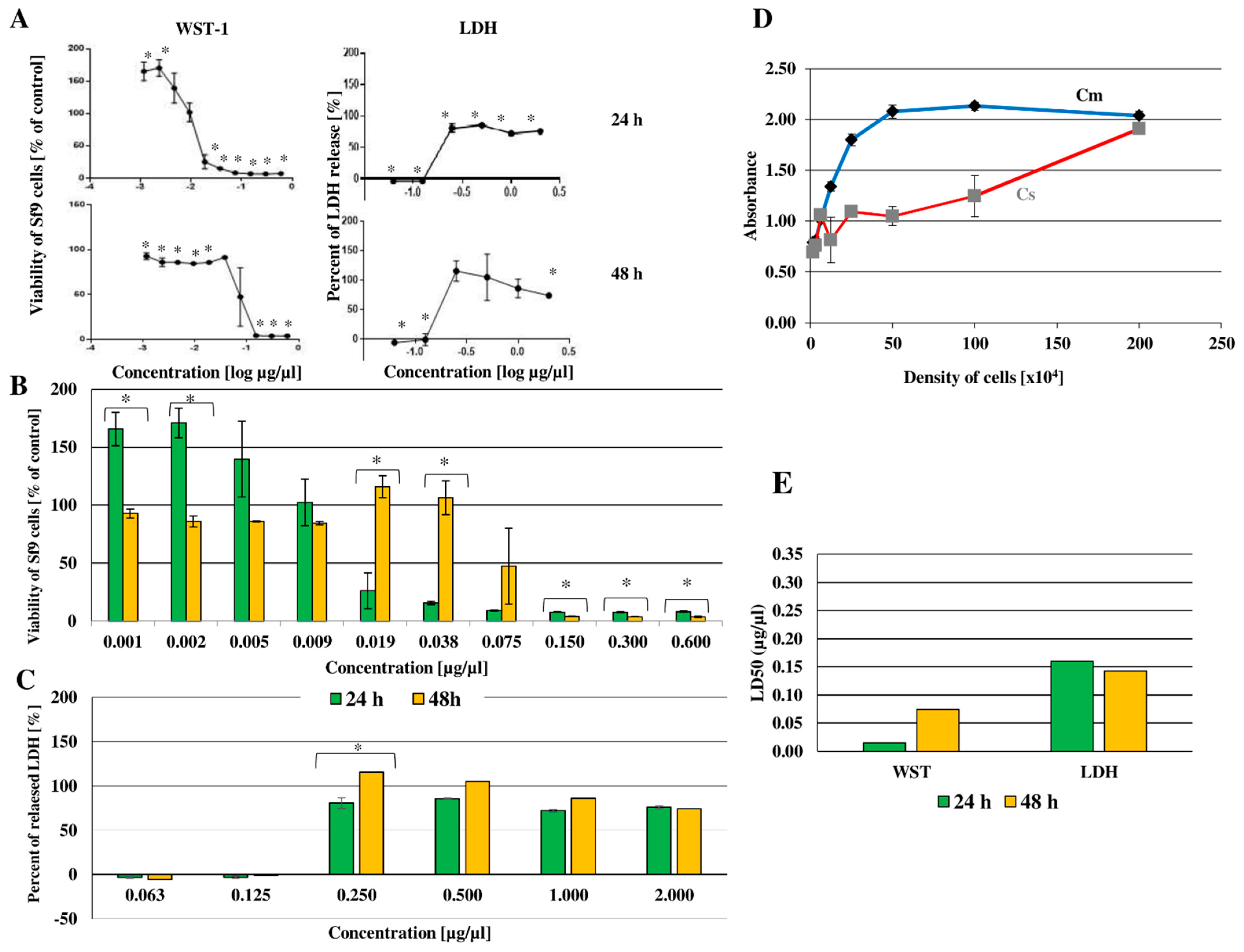
2.5. Cytotoxic Effect of Octanoic Acid on Sf-9 Cell Line
Lethal Concentration (LC50)
2.6. The Changes in G. mellonella Hemocytes Observed after Octanoic Acid Application
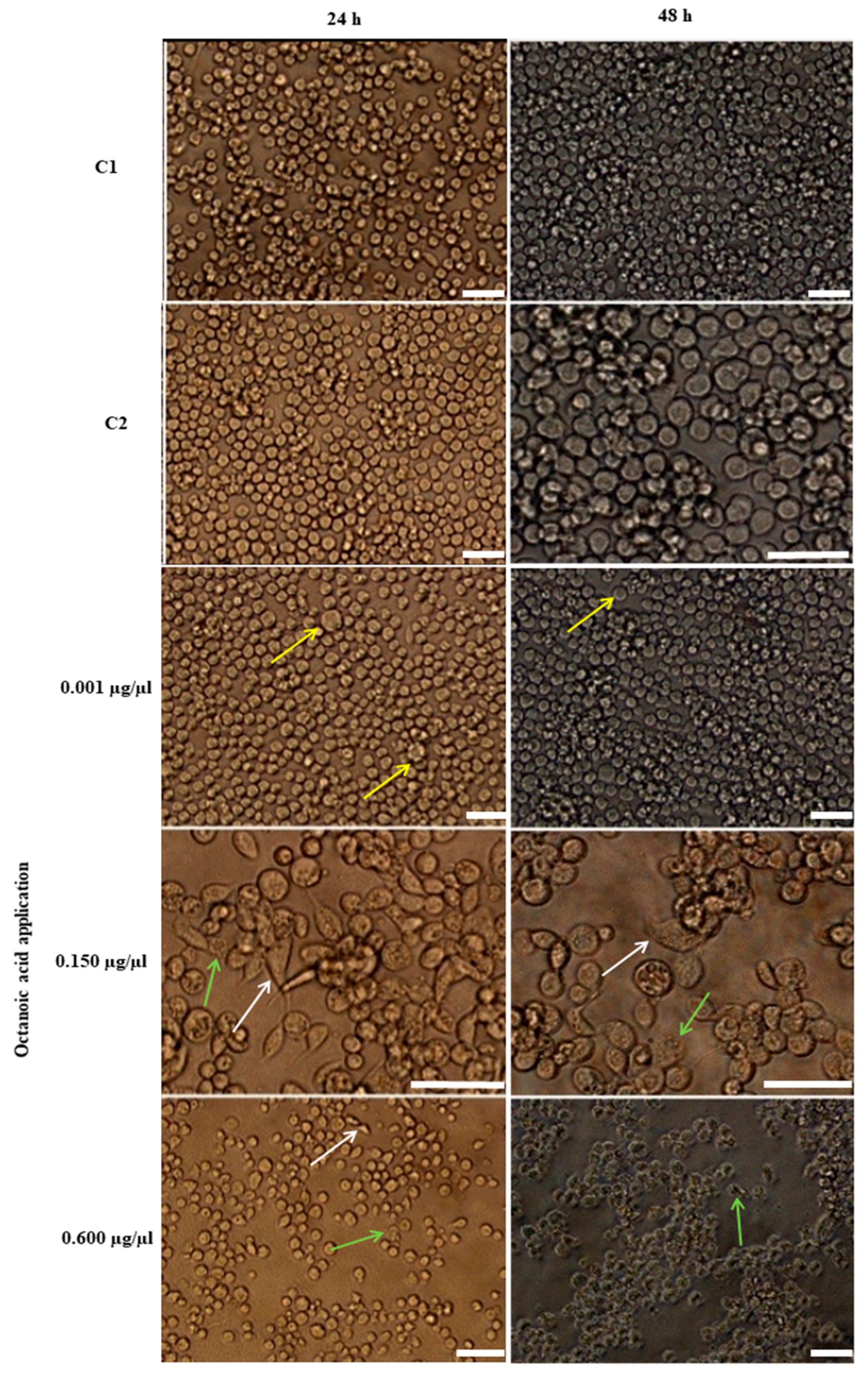
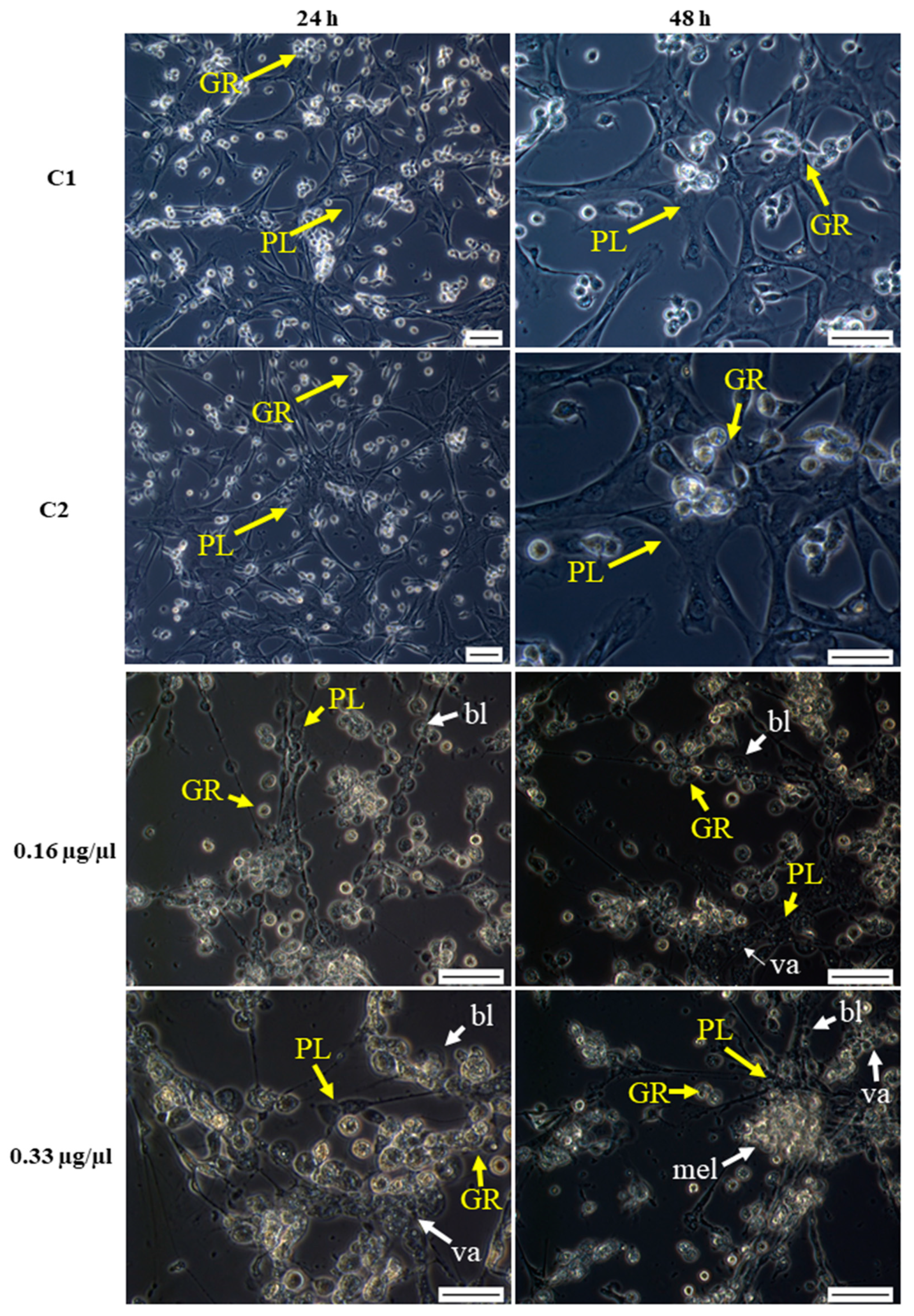
2.6.1. Morphological Changes in Hemocytes after Octanoic Acid Application
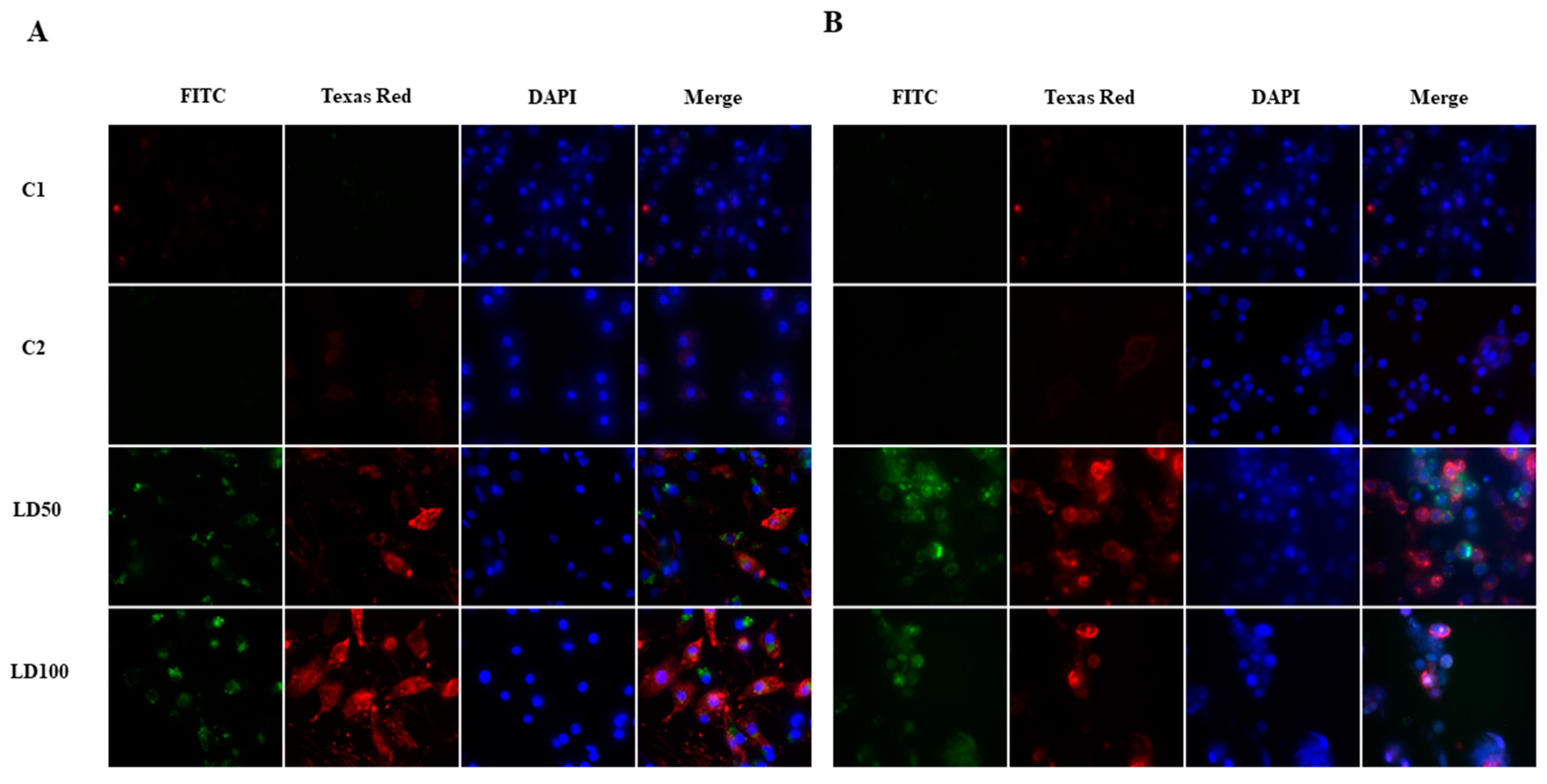
2.6.2. Detection of Apoptosis in G. mellonella Hemocytes after Octanoic Acid Application
2.6.3. Identification and Measurement of Caspase Activity in G. mellonella Hemocyte Cultures after Octanoic Acid Application
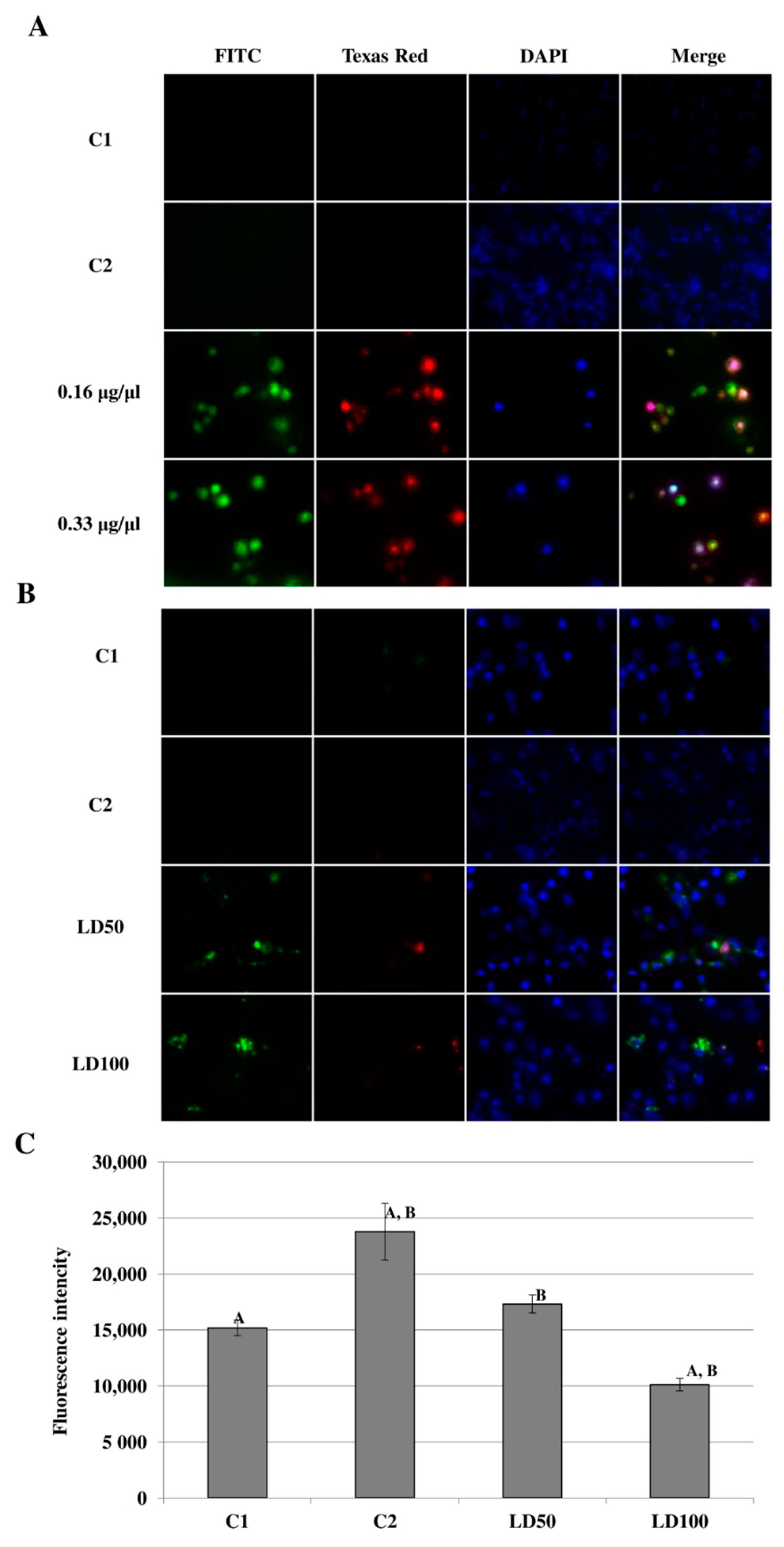
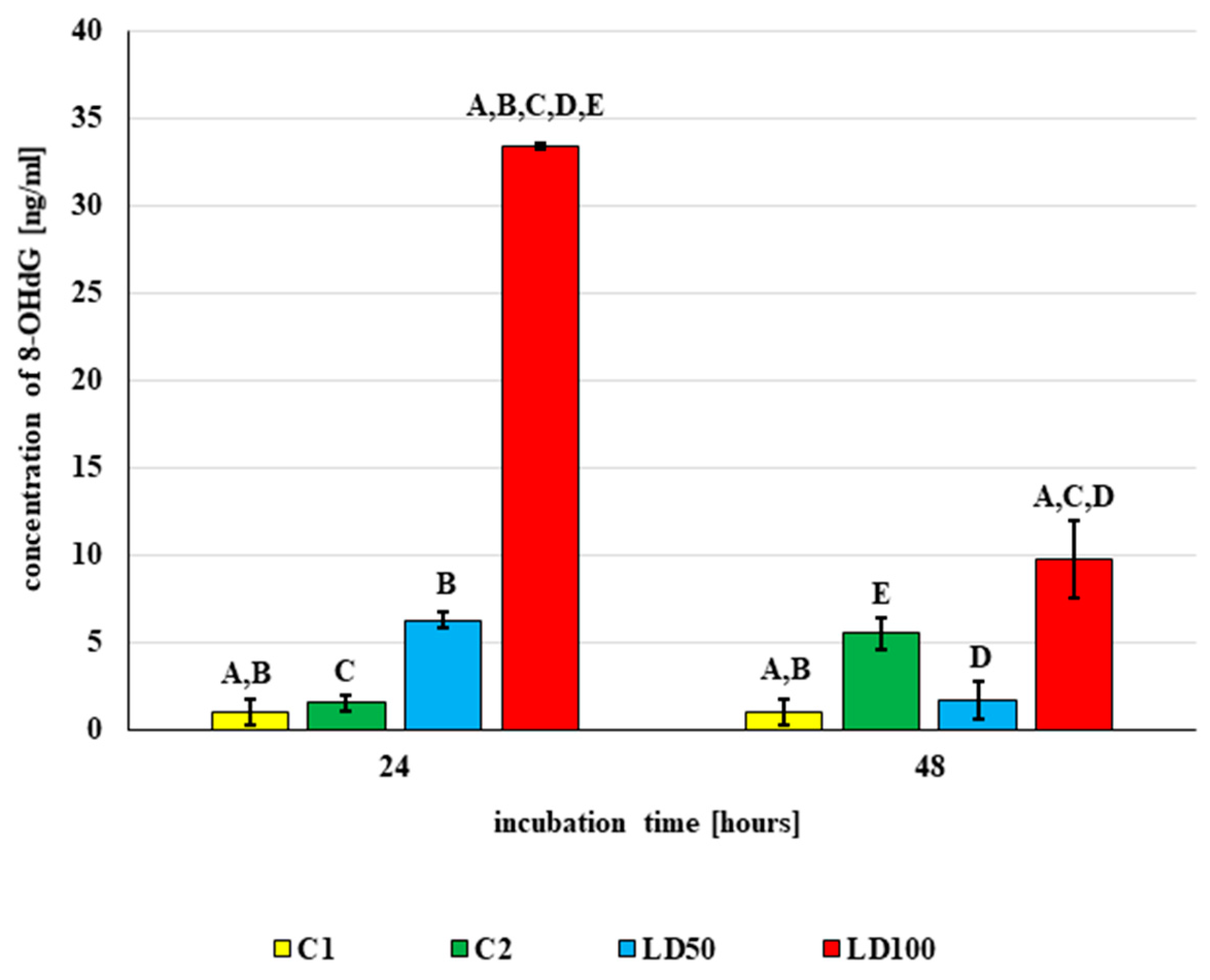
2.6.4. The DNA Damage Observed in G. mellonella Hemocytes after In Vivo Application of Octanoic Acid to Larvae
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Insects
Hemolymph Collection
4.3. Fungus
4.4. Extractions Method
4.4.1. Extraction of C. coronatus Filtrate
4.4.2. Extraction of Free Fatty Acids (FFAs)
4.5. GC-MS Analysis
4.5.1. Derivatization Method
4.5.2. GC-MS Analysis
4.6. The Evaluation of Insecticidal Activity of the Fractions Extracted from Fungal Filtrate and of Octanoic Acid against G. mellonella
4.7. The Determination of the LD50 and LD100 Doses
4.8. The Determination of Morphological and Viability Changes of Sf-9 Cell Cultures after In Vitro Octanoic Acid Treatment
4.8.1. The Determination of Sf-9 Cell Viability
- S—test sample;
- Cs—control of spontaneous release of cellular LDH;
- Cm—control of the maximum release of LDH from the cell;
- A490—absorbance value at 490 nm;
- A660—absorbance value at 660 nm.
4.8.2. The Calculation of Lethal Concentration (LC50)
4.9. Detection of Apoptosis after In Vivo and In Vitro Administration of Octanoic Acid
4.10. The Detection and Measurement of Caspase Activity
4.11. The Changes in 8-Hydroxy-2’-Deoxyguanosine (8-OHdG) Concentration in G. mellonella Hemocytes after In Vivo Application of Octanoic Acid to Larvae
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Valen, L. A New Evolutionary Law (1973). In Foundations of Macroecology: Classic Papers with Commentaries; Smith, F.A., Gittleman, J.L., Brown, J.H., Eds.; University of Chicago Press: Chicago, IL, USA, 2014; pp. 284–314. [Google Scholar] [CrossRef]
- Boguś, M.I.; Wieloch, W.; Ligȩza-Żuber, M. Coronatin-2 from the entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella larvae and incapacitates hemocytes. Bull. Entomol. Res. 2017, 107, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, A.K.; Boguś, M.I.; Włóka, E.; Kazek, M.; Kaczmarek, A.; Zalewska, K. Cuticular fatty acids of Galleria mellonella (Lepidoptera) inhibit fungal enzymatic activities of pathogenic Conidiobolus coronatus. PLoS ONE 2018, 13, e0192715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bania, J.; Samborski, J.; Bogus, M.; Polanowski, A. Specificity of an extracellular proteinase from Conidiobolus coronatus and its inhibition by an inhibitor from insect hemolymph. Arch. Insect Biochem. Physiol. 2006, 62, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Classification and infection mechanism of entomopathogenic fungi. Arq. Inst. Biol. 2018, 84, e0552015. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, A.; Boguś, M. The metabolism and role of free fatty acids in key physiological processes in insects of medical, veterinary and forensic importance. PeerJ 2021, 9, e12563. [Google Scholar] [CrossRef]
- Wieloch, W.; Boguś, M.I.; Ligeza, M.; Koszela-Piotrowska, I.; Szewczyk, A. Coronatin-1 isolated from entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella hemocytes in vitro and forms potassium channels in planar lipid membrane. Toxicon 2011, 58, 369–379. [Google Scholar] [CrossRef]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Dodecanol, metabolite of entomopathogenic fungus Conidiobolus coronatus, affects fatty acid composition and cellular immunity of Galleria mellonella and Calliphora vicina. Sci. Rep. 2021, 11, 15963. [Google Scholar] [CrossRef]
- Wrońska, A.K.; Boguś, M.I. Harman and norharman, metabolites of the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales), affect the serotonin levels and phagocytic activity of hemocytes, insect immunocompetent cells, in Galleria mellonella (Lepidoptera). Cell Biosci. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Wrońska, A.K.; Boguś, M.I.; Kaczmarek, A.; Kazek, M. Harman and norharman, metabolites of entomopathogenic fungus Conidiobolus coronatus (Entomopthorales), disorganize development of Galleria mellonella (Lepidoptera) and affect serotonin-regulating enzymes. PLoS ONE 2018, 13, e0204828. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, M.; Tyma, M.; Ligęza-Żuber, M.; Włóka, E.; Boguś, M. Trichothecenes production by entomopathogenic fungus Conidiobolus coronatus. Adv. Toxicol. Toxic Eff. 2016, 1, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Boguś, M.I.; Wrońska, A.K.; Kaczmarek, A.; Boguś-Sobocińska, M. In vitro screening of 65 mycotoxins for insecticidal potential. PLoS ONE 2021, 16, e0248772. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, N.; Kaltner, F.; Maul, R.; Gareis, M.; Schwaiger, K.; Gottschalk, C. Distribution of T-2 toxin and HT-2 toxin during experimental feeding of yellow mealworm (Tenebrio molitor). Mycotoxin Res. 2021, 37, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.; et al. Re-evaluation of fatty acids (E 570) as a food additive. EFSA J. 2017, 15, e04785. [Google Scholar] [CrossRef] [Green Version]
- Skinner, W.A.; Tong, H.C.; Maibach, H.I.; Skidmore, D. Human skin-surface lipid fatty acids—Mosquito repellents. Experientia 1970, 26, 728–730. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Schultz, G.W.; Axelrod, H.; Kramer, W.L.; Mulla, M.S. Ovipositional Repellency of Fatty Acids and Their Derivatives Against Culex and Aedes Mosquitoes. Environ. Entomol. 1982, 11, 223–226. [Google Scholar] [CrossRef]
- Hou, X.-Q.; Zhang, D.-D.; Powell, D.; Wang, H.-L.; Andersson, M.N.; Löfstedt, C. Ionotropic receptors in the turnip moth Agrotis segetum respond to repellent medium-chain fatty acids. bioRxiv 2021, 27, 1–22. [Google Scholar] [CrossRef]
- Sá-Correia, I. Synergistic effects of ethanol, octanoic, and decanoic acids on the kinetics and the activation parameters of thermal death in Saccharomyces bayanus. Biotechnol. Bioeng. 1986, 28, 761–763. [Google Scholar] [CrossRef]
- Viegas, C.A.; Rosa, M.F.; Correia, I.S.; Novais, J.M. Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl. Environ. Microbiol. 1989, 55, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Kwadha, C.A.; Ong’Amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The biology and control of the greater wax moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzi, F.; Bortolomeazzi, R.; Della Vedova, G.; Del Piccolo, F.; Annoscia, D.; Milani, N. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften 2009, 96, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Lim, J.; Harman, K.; Rowley, A.F.; Griffiths, D.J.; Emery, H.; Layton, W. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol. Toxicol. 2019, 35, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.Q.; Casadevall, A. Fungal immunity and pathogenesis in mammals versus the invertebrate model organism Galleria mellonella. Pathog. Dis. 2021, 79, ftab013. [Google Scholar] [CrossRef] [PubMed]
- Sugeçti, S. Pathophysiological effects of Klebsiella pneumoniae infection on Galleria mellonella as an invertebrate model organism. Arch. Microbiol. 2021, 203, 3509–3517. [Google Scholar] [CrossRef]
- Kaya, S.; Akkuş, G.; Türkdoğan, S.; Gündüz, B. Influence of Helichrysum arenarium on hemocyte-mediated immune responses and phenoloxidase enzyme activity of model organism Galleria mellonella (L.). Int. J. Trop. Insect Sci. 2021, 41, 2521–2528. [Google Scholar] [CrossRef]
- Wojda, I.; Staniec, B.; Sułek, M.; Kordaczuk, J. The greater wax moth Galleria mellonella: Biology and use in immune studies. Pathog. Dis. 2020, 78, ftaa057. [Google Scholar] [CrossRef]
- Vertyporokh, L.; Wojda, I. Immune response of Galleria mellonella after injection with non-lethal and lethal dosages of Candida albicans. J. Invertebr. Pathol. 2020, 170, 107327. [Google Scholar] [CrossRef]
- Vertyporokh, L.; Hułas-Stasiak, M.; Wojda, I. Host–pathogen interaction after infection of Galleria mellonella with the filamentous fungus Beauveria bassiana. Insect Sci. 2019, 27, 1079–1089. [Google Scholar] [CrossRef]
- Friedrich, A.; Pechstein, J.; Berens, C.; Lührmann, A. Modulation of host cell apoptotic pathways by intracellular pathogens. Curr. Opin. Microbiol. 2017, 35, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Dudka, M.; Andrejko, M. Galleria mellonella hemocytes destruction after infection with Pseudomonas aeruginosa. J. Basic Microbiol. 2014, 54, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.; Rossi, C.C.; Vieira de Queiroz, M.; Martins, G.F.; Isaac, C.; Bossé, J.T.; Li, Y.; Wren, B.W.; Terra, V.S.; Cuccui, J.; et al. Galleria mellonella is an effective model to study Actinobacillus pleuropneumoniae infection. Microbiology 2015, 161, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryukov, V.Y.; Kosman, E.; Tomilova, O.; Polenogova, O.; Rotskaya, U.; Tyurin, M.; Alikina, T.; Yaroslavtseva, O.; Kabilov, M.; Glupov, V. Interplay between fungal infection and bacterial associates in the wax moth Galleria mellonella under different temperature conditions. J. Fungi 2020, 6, 170. [Google Scholar] [CrossRef]
- Wrońska, A.K.; Kaczmarek, A.; Kazek, M.; Boguś, M.I. Infection of Galleria mellonella (Lepidoptera) Larvae With the Entomopathogenic Fungus Conidiobolus coronatus (Entomophthorales) Induces Apoptosis of Hemocytes and Affects the Concentration of Eicosanoids in the Hemolymph. Front. Physiol. 2022, 12, 2132. [Google Scholar] [CrossRef]
- Friedrich, A.; Beare, P.A.; Schulze-Luehrmann, J.; Cordsmeier, A.; Pazen, T.; Sonnewald, S.; Lührmann, A. The Coxiella burnetii effector protein CaeB modulates endoplasmatic reticulum (ER) stress signalling and is required for efficient replication in Galleria mellonella. Cell. Microbiol. 2021, 23, e13305. [Google Scholar] [CrossRef]
- Chapman, R.F.; Chapman, R.F. The Insects: Structure and Function; Cambridge Low Price Editions; Cambridge University Press: Cambridge, UK, 1998; ISBN 9780521578905. [Google Scholar]
- Arteaga Blanco, L.A.; Crispim, J.S.; Fernandes, K.M.; de Oliveira, L.L.; Pereira, M.F.; Bazzolli, D.M.S.; Martins, G.F. Differential cellular immune response of Galleria mellonella to Actinobacillus pleuropneumoniae. Cell Tissue Res. 2017, 370, 153–168. [Google Scholar] [CrossRef]
- Pech, L.L.; Strand, M.R. Plasmatocytes from the moth Pseudoplusia includens induce apoptosis of granular cells. J. Insect Physiol. 2000, 46, 1565–1573. [Google Scholar] [CrossRef]
- Sung, H.-H.; Kao, W.-Y.; Su, Y.-J. Effects and toxicity of phthalate esters to hemocytes of giant freshwater prawn, Macrobrachium rosenbergii. Aquat. Toxicol. 2003, 64, 25–37. [Google Scholar] [CrossRef]
- Sung, H.-H.; Lin, S.-C.; Chen, W.-L.; Ting, Y.-Y.; Chao, W.-L. Influence of TimsenTM on Vibrio populations of culture pond water and hepatopancreas and on the hemocytic activity of tiger shrimp (Penaeus monodon). Aquaculture 2003, 219, 123–133. [Google Scholar] [CrossRef]
- Feig, C.; Peter, M.E. How apoptosis got the immune system in shape. Eur. J. Immunol. 2007, 37 (Suppl. 1), S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.L.; Deepe, G.S.J. Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum. J. Clin. Investig. 2005, 115, 2875–2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, A.J.; Vass, E. Hydrogen peroxide production in immune-reactive Drosophila melanogaster. J. Parasitol. 1998, 84, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Slepneva, I.A.; Glupov, V.V.; Sergeeva, S.V.; Khramtsov, V.V. EPR detection of reactive oxygen species in hemolymph of Galleria mellonella and Dendrolimus superans sibiricus (Lepidoptera) larvae. Biochem. Biophys. Res. Commun. 1999, 264, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Davis, A.J.; Giebultowicz, J.M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008, 374, 299–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitten, M.M.A.; Mello, C.B.; Gomes, S.A.O.; Nigam, Y.; Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Role of superoxide and reactive nitrogen intermediates in Rhodnius prolixus (Reduviidae)/Trypanosoma rangeli interactions. Exp. Parasitol. 2001, 98, 44–57. [Google Scholar] [CrossRef]
- Lanz-Mendoza, H.; Hernández-Martínez, S.; Ku-López, M.; Del Carmen Rodríguez, M.; Herrera-Ortiz, A.; Rodríguez, M.H. Superoxide anion in Anopheles albimanus hemolymph and midgut is toxic to Plasmodium berghei ookinetes. J. Parasitol. 2002, 88, 702–706. [Google Scholar] [CrossRef]
- Sugeçti, S. Biochemical and immune responses of model organism Galleria mellonella after infection with Escherichia coli. Entomol. Exp. Appl. 2021, 169, 911–917. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Martemyanov, V.V.; Vorontsova, Y.L.; Rantala, M.J.; Gryzanova, E.V.; Glupov, V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Physiol.—C Toxicol. Pharmacol. 2008, 148, 1–5. [Google Scholar] [CrossRef]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M. Conidiobolus coronatus induces oxidative stress and autophagy response in Galleria mellonella larvae. PLoS ONE 2020, 15, e0228407. [Google Scholar] [CrossRef]
- Yucel, M.S.; Kayis, T. Imidacloprid induced alterations in oxidative stress, biochemical, genotoxic, and immunotoxic biomarkers in non-mammalian model organism Galleria mellonella L. (Lepidoptera: Pyralidae). J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.; Reeves, E.P.; Renwick, J.; Wientjes, F.B.; Kavanagh, K. Superoxide production in Galleria mellonella hemocytes: Identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect. Immun. 2005, 73, 4161–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bánfi, B.; Clark, R.A.; Steger, K.; Krause, K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiszt, M.; Lekstrom, K.; Witta, J.; Leto, T.L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 2003, 278, 20006–20012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, N.; Hyršl, P.; Šimek, V. Nitric oxide production by hemocytes of larva and pharate prepupa of Galleria mellonella in response to bacterial lipopolysaccharide: Cytoprotective or cytotoxic? Comp. Biochem. Physiol.—C Toxicol. Pharmacol. 2006, 142, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide—Biol. Chem. 2000, 4, 423–430. [Google Scholar] [CrossRef]
- Komarov, D.A.; Slepneva, I.A.; Glupov, V.V.; Khramtsov, V.V. Superoxide and hydrogen peroxide formation during enzymatic oxidation of DOPA by phenoloxidase. Free Radic. Res. 2005, 39, 853–858. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, A.; Boguś, M.I. Fungi of entomopathogenic potential in Chytridiomycota and Blastocladiomycota, and in fungal allies of the Oomycota and Microsporidia. IMA Fungus 2021, 12, 29. [Google Scholar] [CrossRef]
- Boguś, M.I.; Scheller, K. Extraction of an insecticidal protein fraction from the parasitic fungus Conidiobolus coronatus (Entomophthorales). Acta Parasitol. 2002, 47, 66–72. [Google Scholar]
- Legal, L.; Plawecki, M. Comparative sensitivity of various insects to toxic compounds from Morinda citrifolia. Entomol. Probl. 1995, 26, 155–159. [Google Scholar]
- Boch, R.; Shearer, D.A.; Shuel, R.W. Octanoic and Other Volatile Acids in the Mandibular Glands of the Honeybee and in Royal Jelly. J. Apic. Res. 1979, 18, 250–253. [Google Scholar] [CrossRef]
- Page, R.E.; Blum, M.S.; Fales, H.M. o-Aminoacetophenone, a pheromone that repels honeybees (Apis mellifera L.). Experientia 1988, 44, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Legal, L.; Moulin, B.; Jallon, J.M. The relation between structures and toxicity of oxygenated aliphatic compounds homologous to the insecticide octanoic acid and the chemotaxis of two species of Drosophila. Pestic. Biochem. Physiol. 1999, 65, 90–101. [Google Scholar] [CrossRef]
- Globally Harmonized System of Classification and Labelling of Chemicals. GHS. Available online: https://unece.org/about-ghs (accessed on 28 April 2022).
- Opdyke, D.L.J. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1977, 15, 611–638. [Google Scholar] [CrossRef]
- European Chemicals Agency. Available online: https://echa.europa.eu (accessed on 28 April 2022).
- Pedrini, N.; Crespo, R.; Juárez, M.P. Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp. Biochem. Physiol.—C Toxicol. Pharmacol. 2007, 146, 124–137. [Google Scholar] [CrossRef]
- Crespo, R.; Patricia Juárez, M.; Cafferata, L.F.R. Biochemical interaction between entomopathogenous fungi and their insect-host-like hydrocarbons. Mycologia 2000, 92, 528–536. [Google Scholar] [CrossRef]
- Pedrini, N.; Ortiz-Urquiza, A.; Huarte-Bonnet, C.; Zhang, S.; Keyhani, N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Gołębiowski, M.; Maliński, E.; Boguś, M.I.; Kumirska, J.; Stepnowski, P. The cuticular fatty acids of Calliphora vicina, Dendrolimus pini and Galleria mellonella larvae and their role in resistance to fungal infection. Insect Biochem. Mol. Biol. 2008, 38, 619–627. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Wieloch, W.; Włóka, E.; Stepnowski, P. The composition of the cuticular and internal free fatty acids and alcohols from Lucilia sericata males and females. Lipids 2012, 47, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Stepnowski, P. The composition of the free fatty acids from Dendrolimus pini exuviae. J. Insect Physiol. 2010, 56, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Cerkowniak, M.; Boguś, M.I.; Włóka, E.; Dawgul, M.; Kamysz, W.; Stepnowski, P. Free fatty acids in the cuticular and internal lipids of Calliphora vomitoria and their antimicrobial activity. J. Insect Physiol. 2013, 59, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Cerkowniak, M.; Urbanek, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Stepnowski, P. Identification and antifungal activity of novel organic compounds found in cuticular and internal lipids of medically important flies. Microbiol. Res. 2015, 170, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Cerkowniak, M.; Urbanek, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Sosnowska, D.; Stepnowski, P. Antimicrobial activity of untypical lipid compounds in the cuticular and internal lipids of four fly species. J. Appl. Microbiol. 2014, 116, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Boguś, M.I.; Włóka, E.; Wrońska, A.K.; Krawiel, A.; Kazek, M.; Zalewska, K.; Kłocinska-Biały, K.; Sobocinska, M.; Gliniewicz, A.; et al. The interaction between cuticle free fatty acids (FFAs) of the cockroaches Blattella germanica and Blatta orientalis and hydrolases produced by the entomopathogenic fungus Conidiobolus coronatus. PLoS ONE 2020, 15, e0235785. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Metamorphosis-related changes in the free fatty acid profiles of Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830). Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Sandoval, N.R.; Papoutsakis, E.T. Engineering membrane and cell-wall programs for tolerance to toxic chemicals: Beyond solo genes. Curr. Opin. Microbiol. 2016, 33, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Chernyshov, A.; Najdi, T.; Fu, Y.; Dickerson, J.; Sandmeyer, S.; Jarboe, L. Membrane stress caused by octanoic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 3239–3251. [Google Scholar] [CrossRef] [Green Version]
- Besada-Lombana, P.B.; Fernandez-Moya, R.; Fenster, J.; Da Silva, N.A. Engineering Saccharomyces cerevisiae fatty acid composition for increased tolerance to octanoic acid. Biotechnol. Bioeng. 2017, 114, 1531–1538. [Google Scholar] [CrossRef]
- Jatsch, A.S.; Ruther, J. Acetone application for administration of bioactive substances has no negative effects on longevity, fitness, and sexual communication in a parasitic wasp. PLoS ONE 2021, 16, e0245698. [Google Scholar] [CrossRef]
- Endemann, G.; Goetz, P.G.; Edmond, J.; Brunengraber, H. Lipogenesis from ketone bodies in the isolated perfused rat liver. Evidence for the cytosolic activation of acetoacetate. J. Biol. Chem. 1982, 257, 3434–3440. [Google Scholar] [CrossRef]
- Kissebah, A.H.; Tulloch, B.R.; Fraser, T.R. Interrelationship between glucose and acetoacetate metabolism in human adipose tissue. Diabetologia 1974, 10, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Pan, Y.; Dorfman, R.G.; Yin, Y.; Zhou, Q.; Huang, S.; Liu, J.; Zhao, S. Sirtinol promotes PEPCK1 degradation and inhibits gluconeogenesis by inhibiting deacetylase SIRT2. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, H.M.; Killian, K.A. Response of the insect immune system to three different immune challenges. J. Insect Physiol. 2015, 81, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Krautz, R.; Arefin, B.; Theopold, U. Damage signals in the insect immune response. Front. Plant Sci. 2014, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef]
- Neuwirth, M. The structure of the hemocytes of Galleria mellonella (Lepidoptera). J. Morphol. 1973, 139, 105–123. [Google Scholar] [CrossRef]
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: Isolation, structure and function. J. Physiol. 2011, 589, 4413–4421. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Seth, R.K.; Dwarakanath, B.S.; Chandna, S. Mitochondrial regulation of insect cell apoptosis: Evidence for permeability transition pore-independent cytochrome-c release in the Lepidopteran Sf9 cells. Int. J. Biochem. Cell Biol. 2009, 41, 1430–1440. [Google Scholar] [CrossRef]
- Huang, J.F.; Tian, M.; Lv, C.J.; Li, H.Y.; Zhong, G.H. Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic. Biochem. Physiol. 2011, 100, 256–263. [Google Scholar] [CrossRef]
- Hou, X.; Han, L.; An, B.; Zhang, Y.; Cao, Z.; Zhan, Y.; Cai, X.; Yan, B.; Cai, J. Mitochondria and Lysosomes Participate in Vip3Aa-Induced Spodoptera frugiperda Sf9 Cell Apoptosis. Toxins 2020, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cheng, X.; Meng, Q.; Wang, P.; Shu, B.; Hu, Q.; Hu, M.; Zhong, G. Azadirachtin-induced apoptosis involves lysosomal membrane permeabilization and cathepsin L release in Spodoptera frugiperda Sf9 cells. Int. J. Biochem. Cell Biol. 2015, 64, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Chidiac, P.; Dixon, S.J.; Trick, C.G. Extracellular organic compounds from the ichthyotoxic red tide alga Heterosigma akashiwo elevate cytosolic calcium and induce apoptosis in Sf9 cells. Harmful Algae 2005, 4, 789–800. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, J.; Jiang, Z.; Cui, G.; Veeran, S.; Zhong, G. Harmine induced apoptosis in Spodoptera frugiperda Sf9 cells by activating the endogenous apoptotic pathways and inhibiting DNA topoisomerase I activity. Pestic. Biochem. Physiol. 2019, 155, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Azuma, M.; Adegawa, S.; Kikuta, S.; Sato, R. Water influx via aquaporin directly determines necrotic cell death induced by the Bacillus thuringiensis Cry toxin. FEBS Lett. 2017, 591, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boguś, M.I.; Włóka, E.; Wrońska, A.; Kaczmarek, A.; Kazek, M.; Zalewska, K.; Ligęza-Żuber, M.; Gołębiowski, M. Cuticle hydrolysis in four medically important fly species by enzymes of the entomopathogenic fungus Conidiobolus coronatus. Med. Vet. Entomol. 2017, 31, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Boguś, M.I.; Kedra, E.; Bania, J.; Szczepanik, M.; Czygier, M.; Jabłoński, P.; Pasztaleniec, A.; Samborski, J.; Mazgajska, J.; Polanowski, A. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J. Insect Physiol. 2007, 53, 909–922. [Google Scholar] [CrossRef]
- Casal, M.; Paiva, S.; Queirós, O.; Soares-Silva, I. Transport of carboxylic acids in yeasts. FEMS Microbiol. Rev. 2008, 32, 974–994. [Google Scholar] [CrossRef] [Green Version]
- Mira, N.P.; Teixeira, M.C.; Sá-Correia, I. Adaptive response and tolerance to weak acids in Saccharomyces cerevisiae: A genome-wide view. Omi. A J. Integr. Biol. 2010, 14, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Piper, P.; Calderon, C.O.; Hatzixanthis, K.; Mollapour, M. Weak acid adaptation: The stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 2001, 147, 2635–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtczak, L.; Wiȩckowski, M.R. The mechanisms of fatty acid-induced proton permeability of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 1999, 31, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Nasution, O.; Lee, Y.M.; Kim, E.; Lee, Y.; Kim, W.; Choi, W. Overexpression of OLE1 enhances stress tolerance and constitutively activates the MAPK HOG pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.C.; de Barros, P.P.; de Oliveira Fugisaki, L.R.; Rossoni, R.D.; Ribeiro, F.D.C.; de Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J. Fungi 2018, 4, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Cheng, J.; Wang, S.; Wang, X.; Jiang, H.; Yang, Y.; Wang, Y.; Zhang, C.; Liang, W.; Feng, H. α-Lipoic acid attenuates oxidative stress and neurotoxicity via the ERK/Akt-dependent pathway in the mutant hSOD1 related Drosophila model and the NSC34 cell line of amyotrophic lateral sclerosis. Brain Res. Bull. 2018, 140, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wollin, S.D.; Jones, P.J.H. α-Lipoic Acid and Cardiovascular Disease. J. Nutr. 2003, 133, 3327–3330. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Medina-Leendertz, S.; Villalobos, V.; Molero, L.; Bohórquez, A. Paraquat-induced oxidative stress in Drosophila melanogaster: Effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem. Res. 2006, 31, 1425–1432. [Google Scholar] [CrossRef]
- Lang, W.; Gertner, D.; Radhakrishnan, P. Dietary antioxidants reduce damage and rescue sperm viability and fertility following oxidative stress in Drosophila melanogaster. Entomol. Exp. Appl. 2021, 169, 491–498. [Google Scholar] [CrossRef]
- McCarthy, E.L.; Booker, S.J. The Biosynthesis of Lipoic Acid. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–23. [Google Scholar]
- Sree, K.S.; Padmaja, V. Destruxin from Metarhizium anisopliae induces oxidative stress effecting larval mortality of the polyphagous pest Spodoptera litura. J. Appl. Entomol. 2008, 132, 68–78. [Google Scholar] [CrossRef]
- Staiger, K.; Staiger, H.; Weigert, C.; Haas, C.; Häring, H.U.; Kellerer, M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-κB activation. Diabetes 2006, 55, 3121–3126. [Google Scholar] [CrossRef]
- Mei, S.; Ni, H.M.; Manley, S.; Bockus, A.; Kassel, K.M.; Luyendyk, J.P.; Copple, B.L.; Ding, W.X. Differential roles of unsaturated and saturated fatty acids on autophagy and apoptosis in hepatocytes. J. Pharmacol. Exp. Ther. 2011, 339, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, T.M.; Kanunfre, C.C.; Pompéia, C.; Verlengia, R.; Curi, R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol. Vitr. 2002, 16, 741–747. [Google Scholar] [CrossRef]
- Qin, S.; Yin, J.; Huang, K. Free Fatty Acids Increase Intracellular Lipid Accumulation and Oxidative Stress by Modulating PPARα and SREBP-1c in L-02 Cells. Lipids 2016, 51, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, D.; Casartelli, M.; Cappellozza, S.; De Eguileor, M.; Tettamanti, G. Roles and regulation of autophagy and apoptosis in the remodelling of the lepidopteran midgut epithelium during metamorphosis. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Clarke, T.E.; Clem, R.J. Insect defenses against virus infection: The role of apoptosis. Int. Rev. Immunol. 2003, 22, 401–424. [Google Scholar] [CrossRef]
- Nainu, F.; Tanaka, Y.; Shiratsuchi, A.; Nakanishi, Y. Protection of Insects against Viral Infection by Apoptosis-Dependent Phagocytosis. J. Immunol. 2015, 195, 5696–5706. [Google Scholar] [CrossRef] [Green Version]
- Clem, R.J. The role of apoptosis in defense against baculovirus infection in insects. Role Apoptosis Infect. 2005, 289, 113–129. [Google Scholar]
- Ramphul, U.N.; Garver, L.S.; Molina-Cruz, A.; Canepa, G.E.; Barillas-Mury, C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc. Natl. Acad. Sci. USA 2015, 112, 1273–1280. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.T.; Fortuna, K.; Mendoza Herrera, A.; Tamborindeguy, C. Liberibacter, A Preemptive Bacterium: Apoptotic Response Repression in the Host Gut at the Early Infection to Facilitate Its Acquisition and Transmission. Front. Microbiol. 2020, 11, 3348. [Google Scholar] [CrossRef]
- Butt, T.M.; Greenfield, B.P.J.; Greig, C.; Maffeis, T.G.G.; Taylor, J.W.D.; Piasecka, J.; Dudley, E.; Abdulla, A.; Dubovskiy, I.M.; Garrido-Jurado, I.; et al. Metarhizium anisopliae pathogenesis of mosquito larvae: A verdict of accidental death. PLoS ONE 2013, 8, e81686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrońska, A.K.; Boguś, M.I. Heat shock proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) hemolymph are affected by infection with Conidiobolus coronatus (Entomophthorales). PLoS ONE 2020, 15, e0228556. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, D.M.; Granville, D.J.; Lowenberger, C. The insect caspases. Apoptosis 2009, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Zibaee, A.; Malagoli, D. The multifaceted activity of insect caspases. J. Insect Physiol. 2015, 76, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ju, X.; Chen, L.; Chen, K. Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection. Z. Naturforsch.—Sect. C J. Biosci. 2017, 72, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Courtiade, J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genom. 2011, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hounsell, C.; Fan, Y. The duality of caspases in cancer, as told through the fly. Int. J. Mol. Sci. 2021, 22, 8927. [Google Scholar] [CrossRef]
- Sehnal, F. A critical study of the biome and biometry of the wax moth Galleria mellonella raised in varying conditions. Z. Wiss. Zool. 1966, 174, 53–82. [Google Scholar]
- Callaghan, A.A. Light and spore discharge in Entomophthorales. Trans. Br. Mycol. Soc. 1969, 53, 87–97. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I.; Kazek, M.; Gliniewicz, A.; Mikulak, E.; Matławska, M. The type of blood used to feed Aedes aegypti females affects their cuticular and internal free fatty acid (FFA) profiles. PLoS ONE 2021, 16, e0251100. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Boguś, M.I. The Impact of the Entomopathogenic Fungus Conidiobolus coronatus on the Free Fatty Acid Profile of the Flesh Fly Sarcophaga argyrostoma. Insects 2021, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Cerkowniak, M.; Puckowski, A.; Stepnowski, P.; Gołębiowski, M. The use of chromatographic techniques for the separation and the identification of insect lipids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 937, 67–78. [Google Scholar] [CrossRef]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Diet influences the bacterial and free fatty acid profiles of the cuticle of Galleria mellonella larvae. PLoS ONE 2019, 14, e0211697. [Google Scholar] [CrossRef]




| Treatments | Dose | N | Percent of Surviving Larvae (% ± SD) | Mean Time to Pupation (Days after Octanoic Acid Application ± SD) | Percent of Pupation (% ± SD) | Mean Time to Emergence (Days after Octanoic Acid Application ± SD) | Percent of Adults (% ± SD) |
|---|---|---|---|---|---|---|---|
| C1 | 0 µg (0 µL) | 65 | 95.33 ± 0.47 A | 5.09 ± 1.22 | 88.33 ± 6.24 A (92.69 ± 6.96 #) | 15.21 ± 0.97 | 49.67 ± 10.96 A (55.96 ± 9.81 ##) |
| C2 | 0 µg (5 µL) | 65 | 95.67 ± 3.30 B | 4.83 ± 1.25 | 91.33 ± 5.19 B (95.43 ± 3.56 #) | 13.98 ± 1.52 | 44.67 ± 8.18 B (49.04 ± 8.91 ##) |
| octanoic acid application | 50 µg (5 µL) | 60 | 93.33 ± 6.24 C | 5.17 ± 0.85 | 81.67 ± 12.47 C (88.33 ± 16.50 #) | 16,00 ± 0.00 | 26.67 ± 6.24 (32.34 ± 3.23 ##) |
| 100 µg (5 µL) | 60 | 28.33 ± 22.48 A,B,C | 3.07 ± 2.17 | 20.00 ± 18.71 A,B,C (65.91 ± 15,91 #) | 10.67 ± 7.54 | 11.67 ± 10.27 A,B (61,11 ± 5,56 ##) |
| FFA | Larvae | Adults | ||||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | Octanoic Acid | C1 | C2 | Octanoic Acid | |||
| LD50 11.45 ± 1.21 (µg/mg of Body Mass) | LD100 33.56 ± 2.57 (µg/mg of Body Mass) | LD50 11.40 ± 1.18 (µg/mg of Body mass) | LD100 18.33 ± 2.49 (µg/mg of Body Mass) | |||||
| C6:0 | 0.18 ± 0.00 | 0.43 ± 0.05 | 0.62 ± 0.09 | 1.15 ± 0.62 | 0.32 ± 0.00 A,B | 0.10 ± 0.04 B,C | 0.08 ± 0.05 A,D | 0.36 ± 0.10 C,D |
| C8:0 | 0.20 ± 0.00 a | 0.74 ± 0.07 b | 179.55 ± 16.79 a,b | 28.57 ± 12.32 | 0.55 ± 0.00 A,B | 0.68 ± 0.20 | 6.88 ± 0.47 A | 7.79 ± 0.83 B |
| C9:0 | 0.29 ± 0.00 | 1.07 ± 0.16 | 2.18 ± 1.41 | 2.33 ± 0.75 | 6.02 ± 0.32 A,B | 4.19 ± 0.26 A,C | 0.51 ± 0.18 A | 0.64 ± 0.15 B,C |
| C10:1 | 1.42 ± 0.00 a,b,c | Nd a | Nd b | Nd c | 0.11 ± 0.00 A,B,C | ND A | ND B | ND C |
| C10:0 | 0.53 ± 0.00 a | 0.91 ± 0.02 b | 5.54 ± 1.75 a,b,c | 1.69 ± 0.48 c | 1.33 ± 0.08 A | 9.29 ± 1.05 A,B,C | 0.36 ± 0.04 B | 1.59 ± 0.32 C |
| C11:0 | nd | nd | 1.99 ± 1.39 | nd | 29.68 ± 1.19 A | 60.69 ± 5.16 A,B | 24.22 ± 1.76 B | 30.03 ± 2.67 A,B |
| C12:0 | 1.77 ± 0.06 a | 16.37 ± 0.40 b | 96.86 ± 9.08 a,b | 47.03 ± 20.49 a | 2.21 ± 0.06 A,B | 4.63 ± 0.46 A,C | 1.47 ± 0.10 C,D | 5.61 ± 0.61 B,D |
| C13:0 | nd a | Nd b | 2.80 ± 1.57 a,b,c | Nd c | 0.51 ± 0.07 | 1.23 ± 0.41 | 1.10 ± 0.22 | 0.65 ± 0.14 |
| C14:1 | 1.08 ± 0.09 a | 2.64 ± 0.83 a,b,c | Nd b | Nd c | 0.59 ± 0.10 A,B,C | ND A | ND B | ND C |
| C14:0 | 7.82 ± 0.13 a | 54.80 ± 3.63 a | 129.69 ± 7.67 a,b | 31.01 ± 12.0 b | 5.70 ± 0.48 A | 25.70 ± 3.79 A,B | 7.80 ± 0.58 B | 13.93 ± 1.12 A |
| C15:0 | 1.62 ± 0.07 a,b | 21.27 ± 3.47 a,c | 30.47 ± 4.22 b,d | 6.2 ± 2.711 c,d | 0.72 ± 0.11 A | 1.68 ± 0.32 B | 2.31 ± 0.19 A | 3.32 ± 0.49 A,B |
| C16:1 | 3.23 ± 0.25 a | 14.89 ± 0.92 | 33.07 ± 10.52 a,b | 16.11 ± 2.29 b | 3.21 ± 0.53 A | 4.32 ± 0.92 B | 41.64 ± 6.60 A,B | 25.82 ± 6.09 A,B |
| C16:0 | 99.04 ± 3.67 a,b,c | 1108.29 ± 183.53 a | 1139.12 ± 78.65 b | 541.71 ± 238.95 c | 68.19 ± 2.59 A | 42.03 ± 4.64 B | 53.03 ± 6.75 C | 110.21 ± 16.72 A,B,C |
| C17:1 | 0.55 ± 0.03 a,b,c | Nd a | Nd b | Nd c | ND | ND | ND | ND |
| C17:0 | 1.55 ± 0.01 | 6.68 ± 2.87 | 9.68 ± 6.94 | nd | 0.60 ± 0.06 A,B,C | ND A | ND B | ND C |
| C18:3 | 3.45 ± 1.34 | nd | nd | nd | 3.82 ± 0.91 A,B,C | ND A | ND B | ND C |
| C18:2 | 84.90 ± 4.08 a,b,c | nda | 130.56 ± 14.93 b | Nd c | 77.55 ± 9.88 A,C | ND A | 35.41 ± 16.56 A | 7.82 ± 5.57 C |
| C18:1 | 72.61 ± 10.14 | 99.18 ± 31.09 | 57.42 ± 31.79 | 73.62 ± 37.08 | 46.15 ± 15.53 A,B | 10.50 ± 6.01 A | 12.08 ± 3.31 B | 27.96 ± 6.46 |
| C18:0 | 15.70 ± 0.74 a,b,c | Nd a | Nd b | Nd c | 22.09 ± 2.09 A,B | 4.62 ± 0.87 A | 1.34 ± 0.22 B | 7.72 ± 2.01 B |
| C20:1 | nd | nd | nd | nd | 2.30 ± 0.10 A,B,C | ND A | ND B | ND C |
| C20:0 | nd | nd | nd | nd | 1.27 ± 0.04 A,B,C | ND A | ND B | ND C |
| C24:0 | nd | nd | nd | nd | 2.31 ± 0.54 A,B,C | ND A | ND B | ND C |
| C26:0 | nd | nd | nd | nd | 1.83 ± 0.40 A,B,C | ND A | ND B | ND C |
| Sum of FFAs | 299.10 ± 18.03 a | 110.61 ± 13.37 b | 1820.25 ± 83.96 a,b,c | 745.11 ± 331.50 c | 277.04 ± 3.508 A | 169.65 ± 16.23 A | 181.26 ± 32.13 | 251.80 ± 40.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Octanoic Acid—An Insecticidal Metabolite of Conidiobolus coronatus (Entomopthorales) That Affects Two Majors Antifungal Protection Systems in Galleria mellonella (Lepidoptera): Cuticular Lipids and Hemocytes. Int. J. Mol. Sci. 2022, 23, 5204. https://doi.org/10.3390/ijms23095204
Kaczmarek A, Wrońska AK, Kazek M, Boguś MI. Octanoic Acid—An Insecticidal Metabolite of Conidiobolus coronatus (Entomopthorales) That Affects Two Majors Antifungal Protection Systems in Galleria mellonella (Lepidoptera): Cuticular Lipids and Hemocytes. International Journal of Molecular Sciences. 2022; 23(9):5204. https://doi.org/10.3390/ijms23095204
Chicago/Turabian StyleKaczmarek, Agata, Anna Katarzyna Wrońska, Michalina Kazek, and Mieczysława Irena Boguś. 2022. "Octanoic Acid—An Insecticidal Metabolite of Conidiobolus coronatus (Entomopthorales) That Affects Two Majors Antifungal Protection Systems in Galleria mellonella (Lepidoptera): Cuticular Lipids and Hemocytes" International Journal of Molecular Sciences 23, no. 9: 5204. https://doi.org/10.3390/ijms23095204






