Ameliorative Effect of Ethanolic Echinacea purpurea against Hyperthyroidism-Induced Oxidative Stress via AMRK and PPAR Signal Pathway Using Transcriptomics and Network Pharmacology Analysis
Abstract
:1. Introduction
2. Results
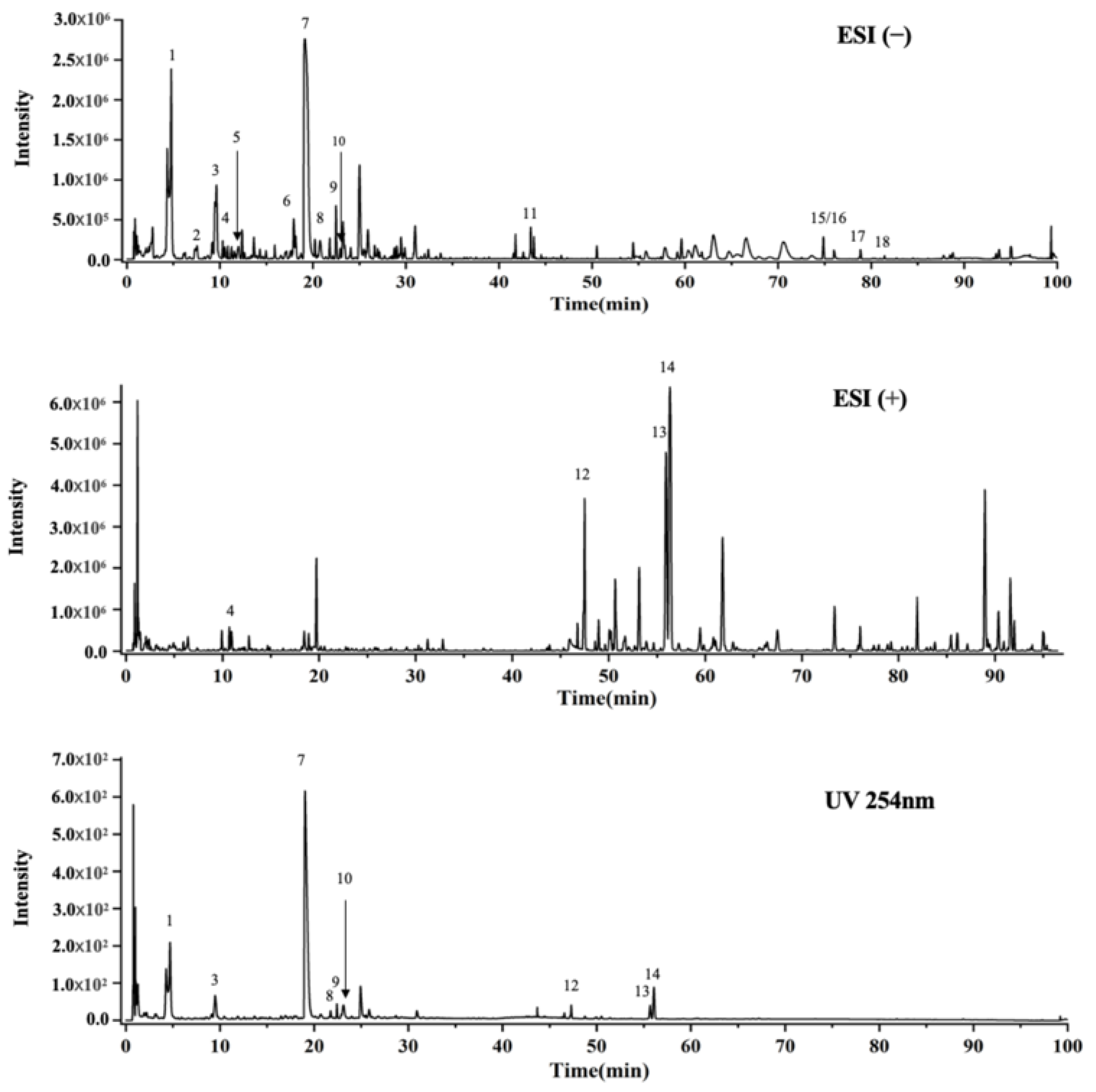
2.1. UPLC-Q-TOF-MS Analysis
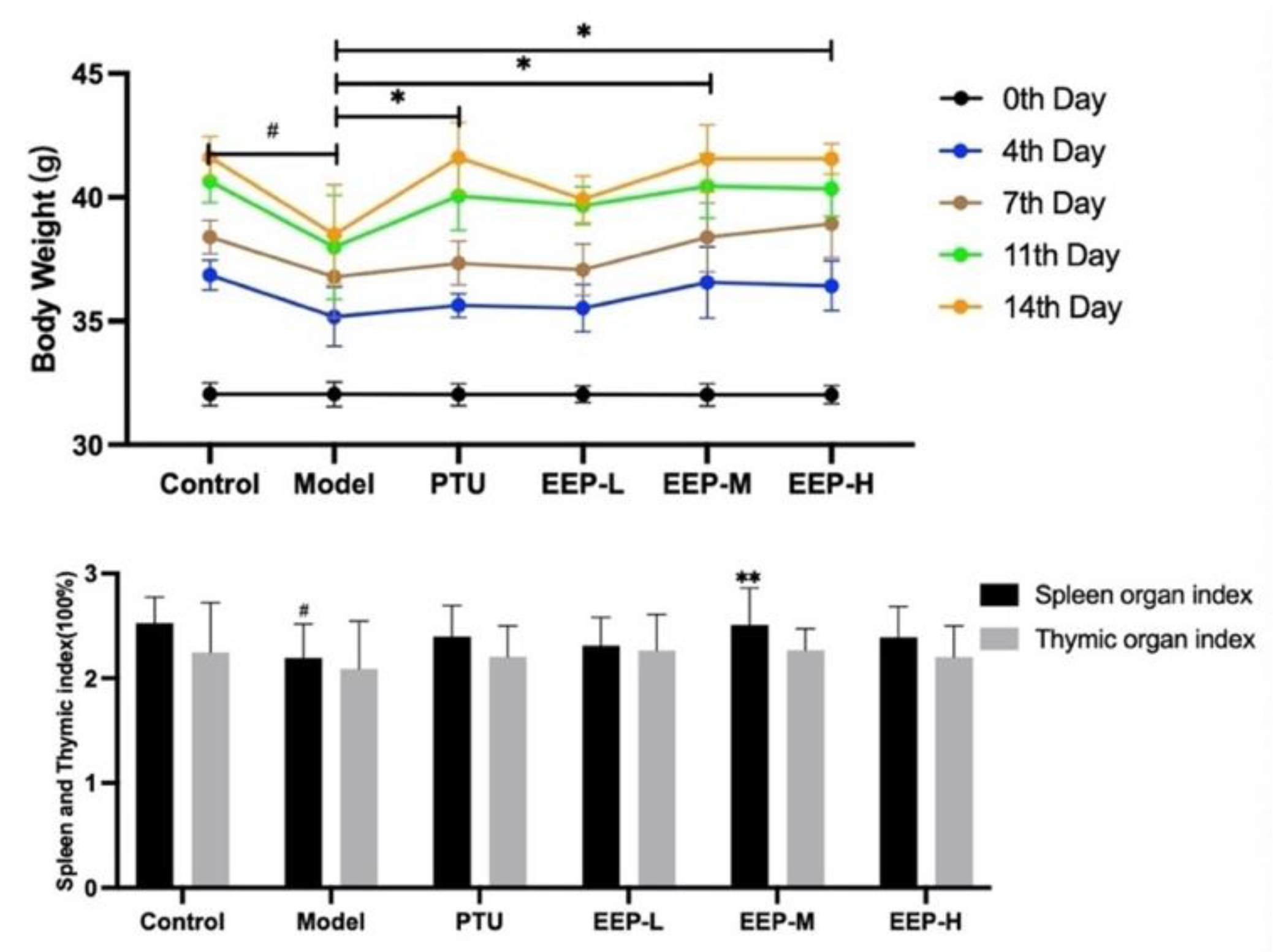
2.2. Effects of EEP on Body Weight Changes and Different Organ Weights in Hyperthyroidism Mice
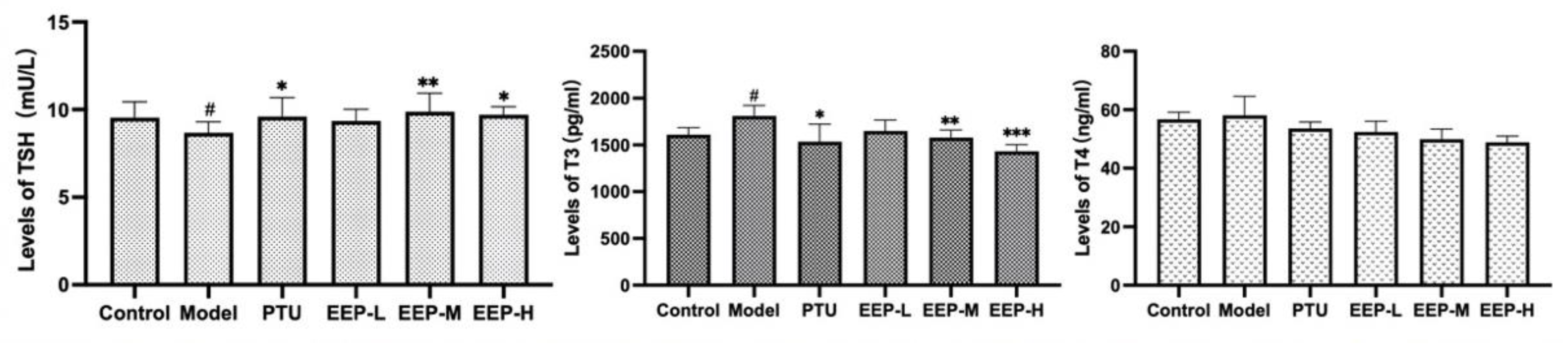
2.3. Effects of EEP on Thyroid Hormone Levels in Hyperthyroidism Mice
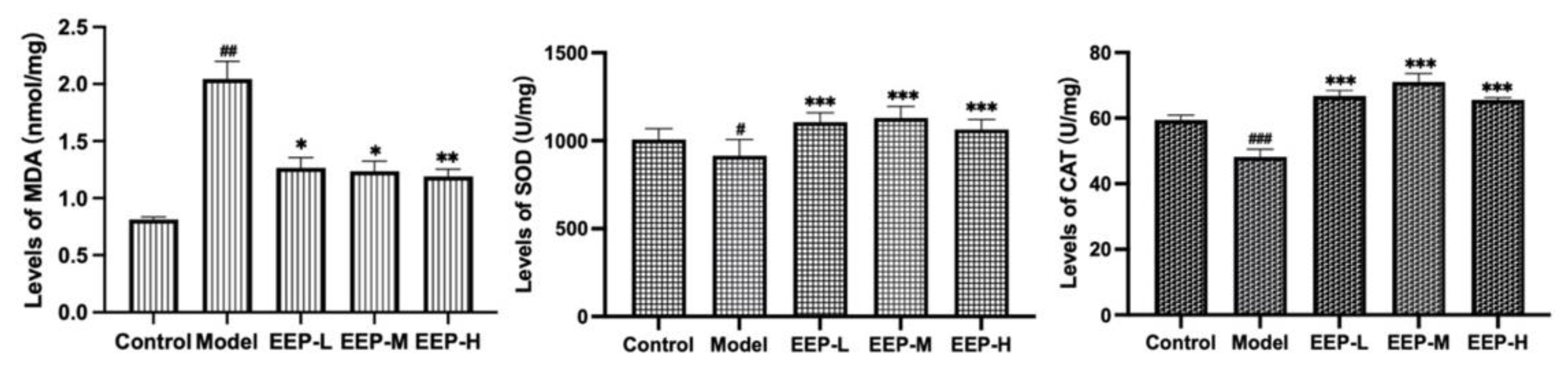
2.4. Effects of EEP on Antioxidative Markers in Hyperthyroidism Mice
2.5. Transcriptomics Analysis the Antioxidative Mechanism of EEP in Hyperthyroidism Mice
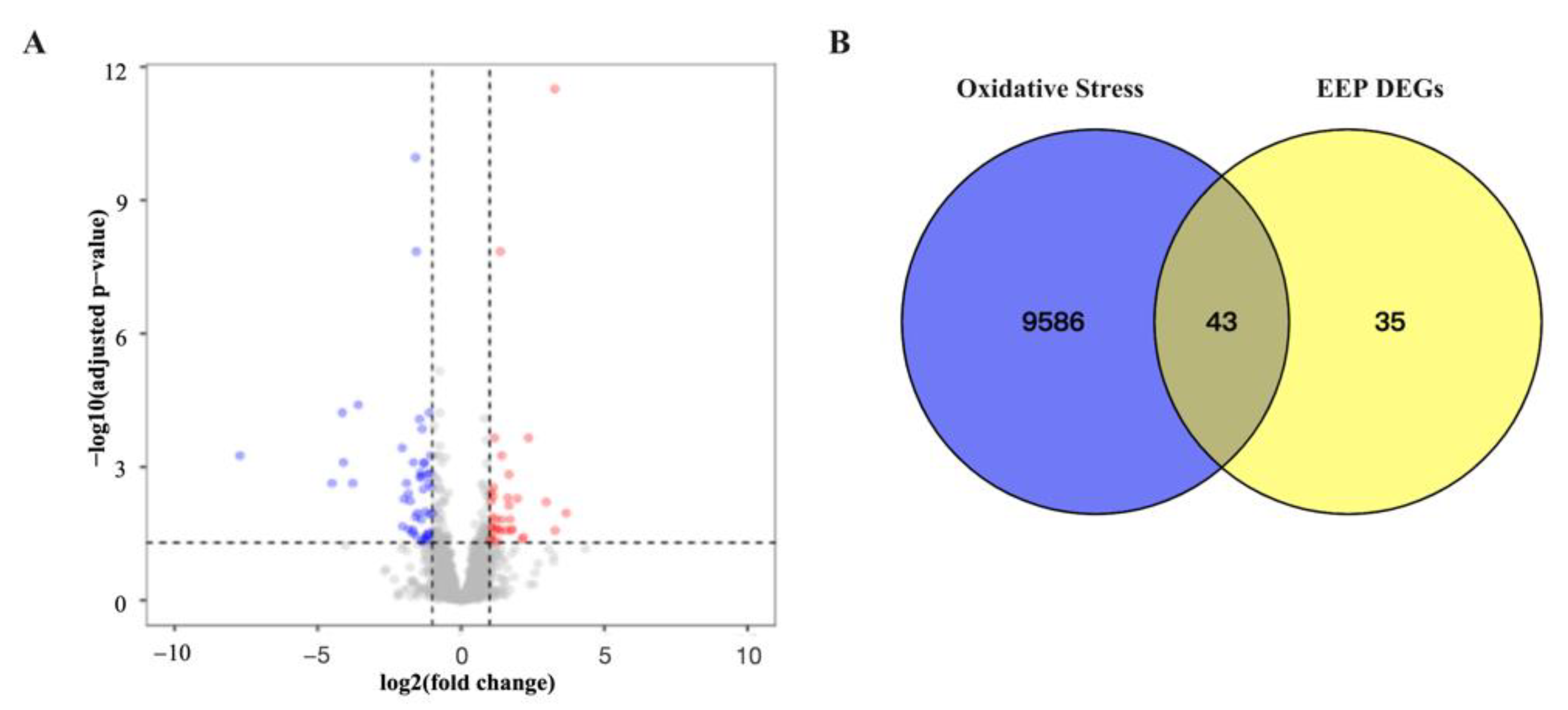
2.5.1. Identification Related Antioxidative Genes of Differentially Expressed Genes of EEP in Hyperthyroidism Mice
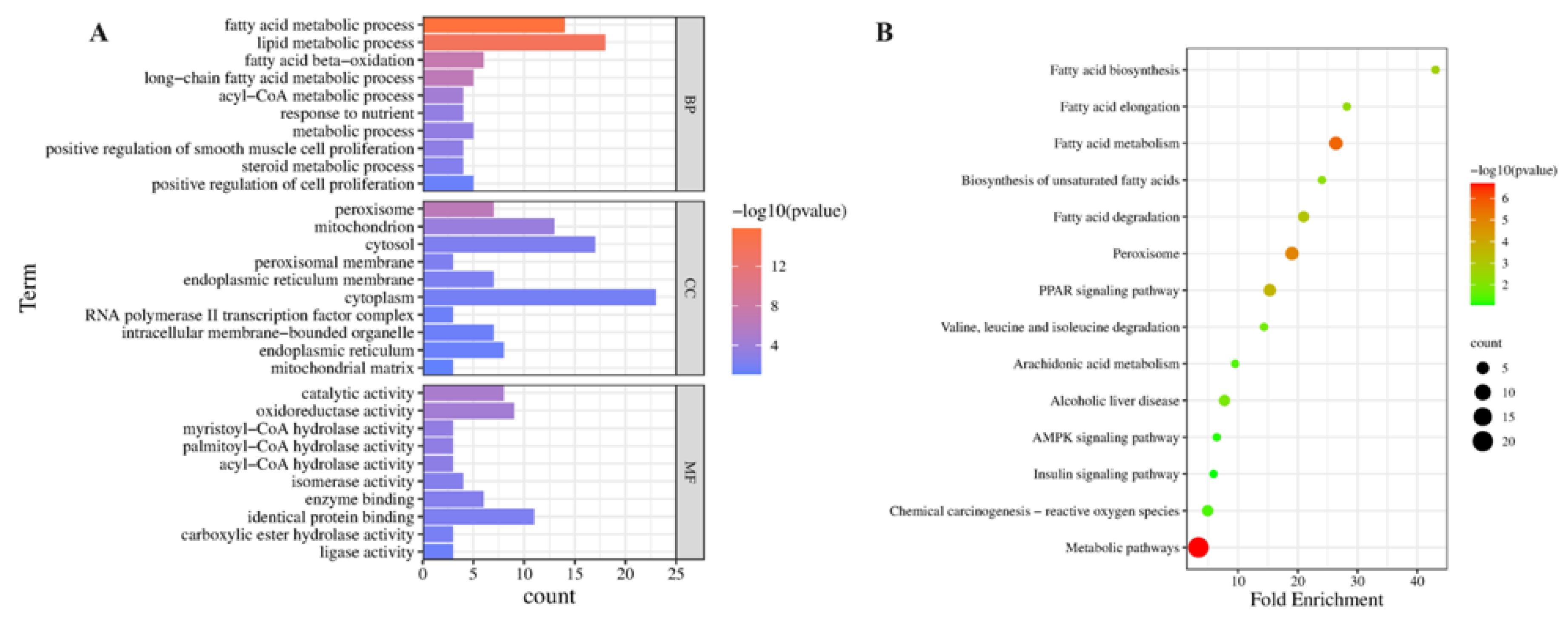
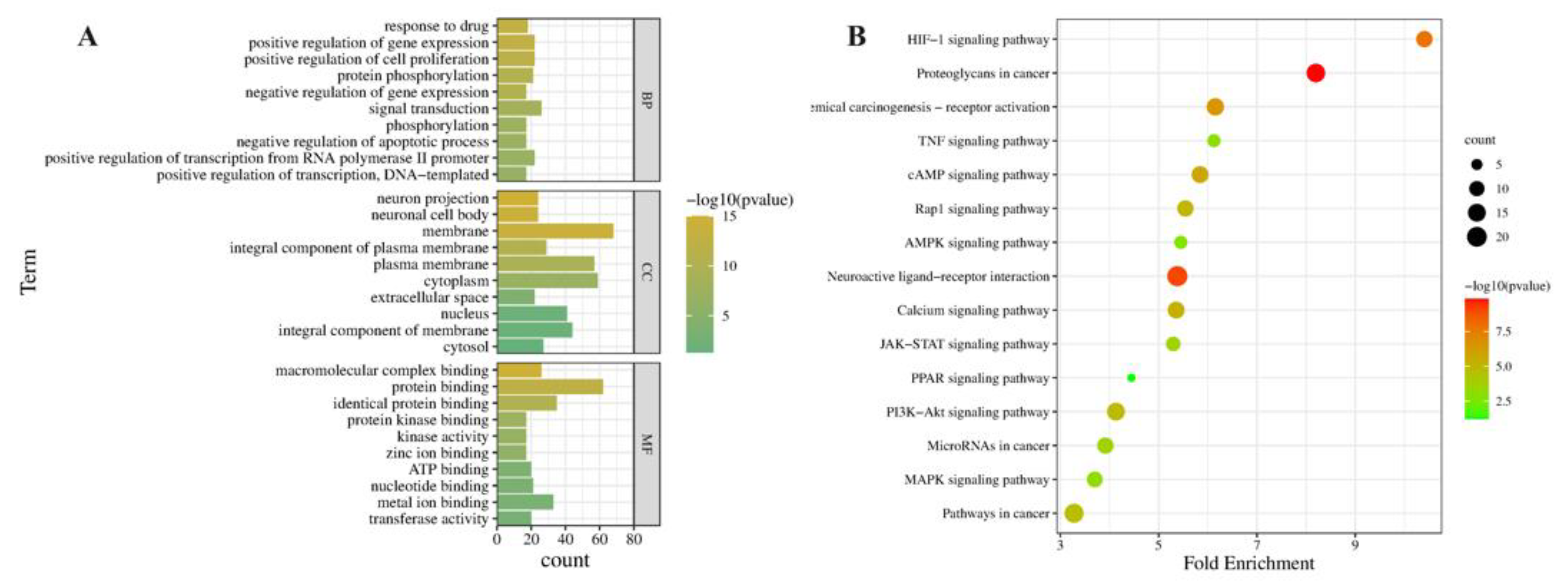
2.5.2. GO and KEGG Pathway Enrichment Analysis of Related Antioxidative Perturbation Genes of EEP
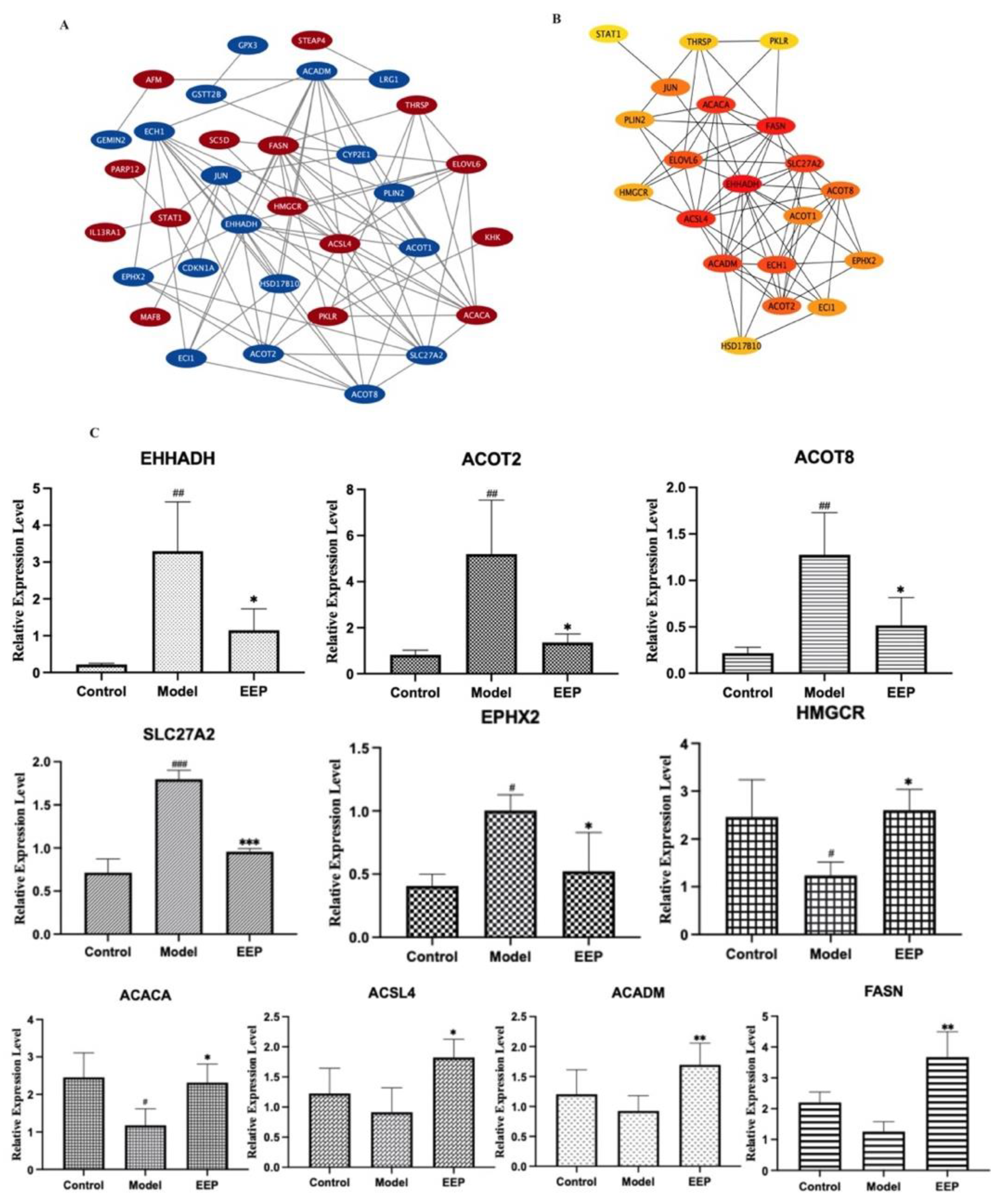
2.5.3. Identification and Validation of PPI Network and Related Key Antioxidative Genes of EEP
2.6. Network Pharmacology Analysis of the Antioxidative Mechanism of EEP
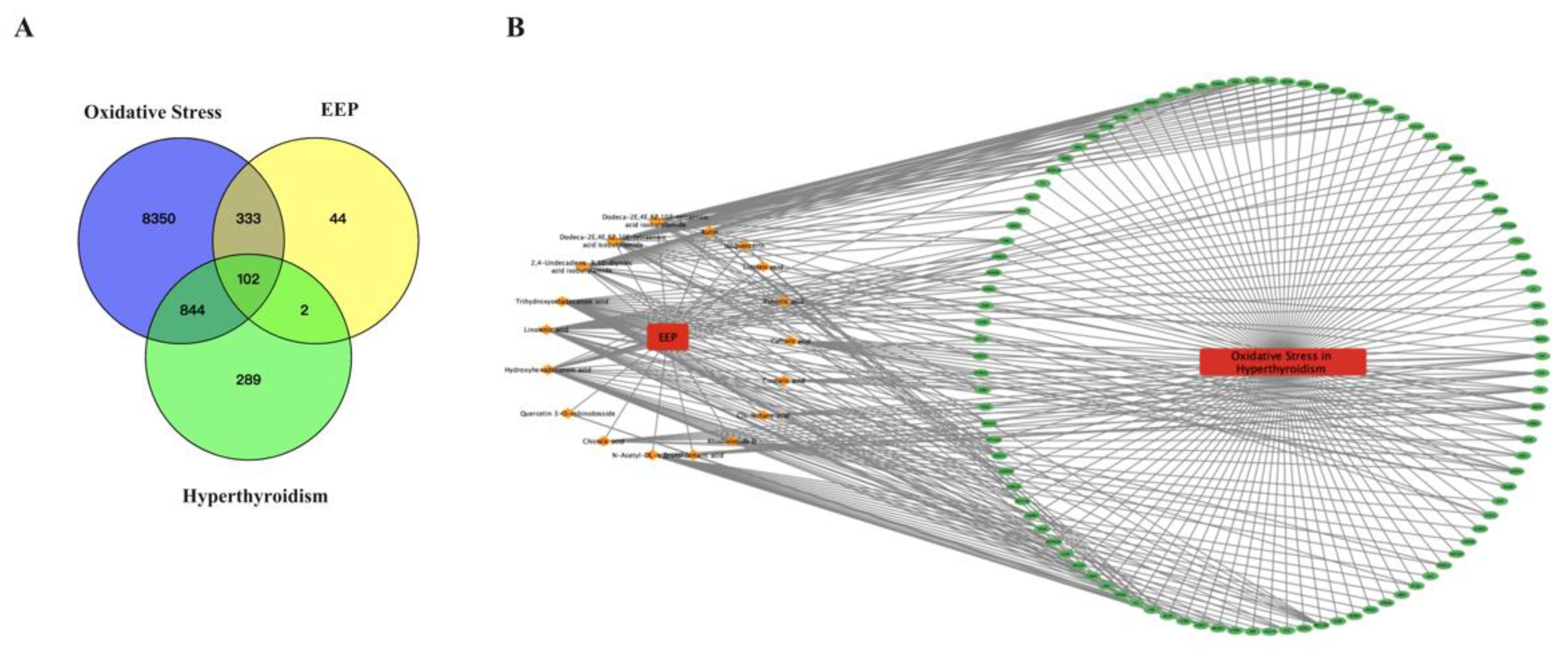
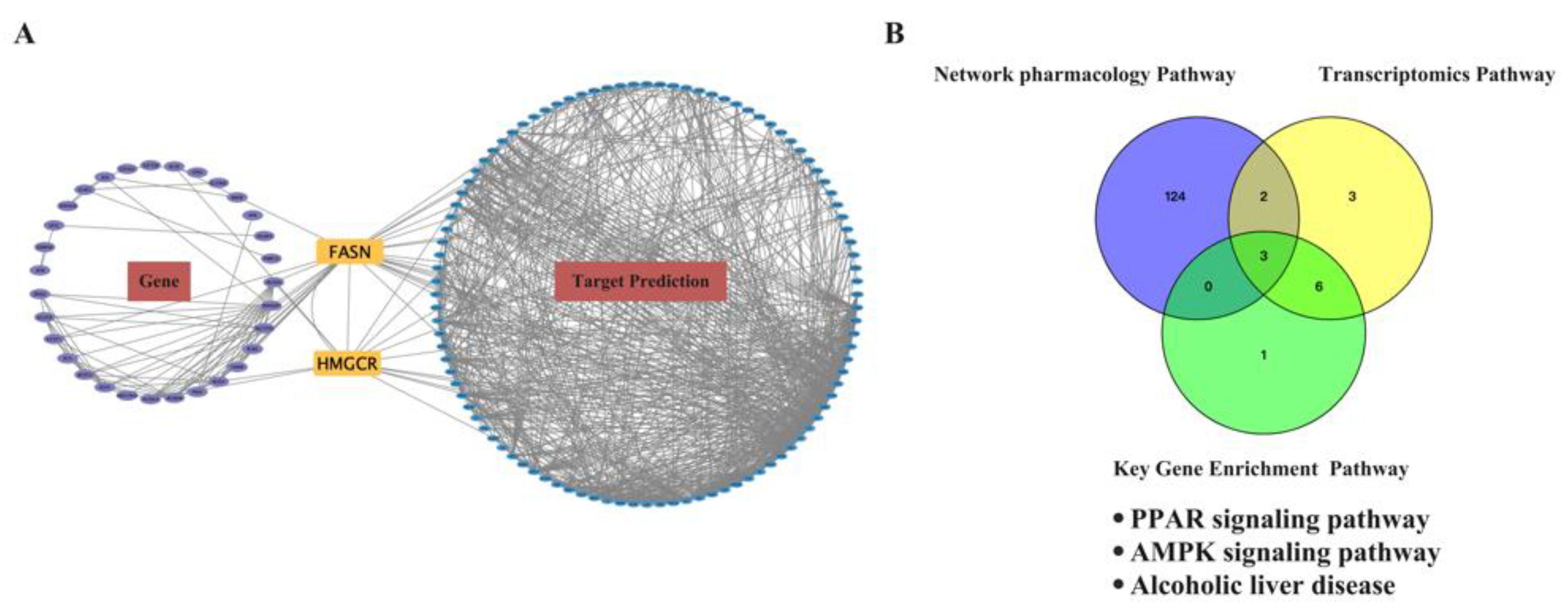
2.6.1. Prediction of the Target Proteins of EEP and Construction of the EEP-Compound–Hyperthyroidism-Oxidative Stress-Target Network
2.6.2. Functional Enrichment and Pathway Analysis of the Predicted Targets for EEP Antioxidative against Hyperthyroidism
2.7. Conjoint Analysis of the Antioxidant Effect of EEP on Hyperthyroidism-Induced Oxidative Stress by Transcriptomics and Network Pharmacology Analysis
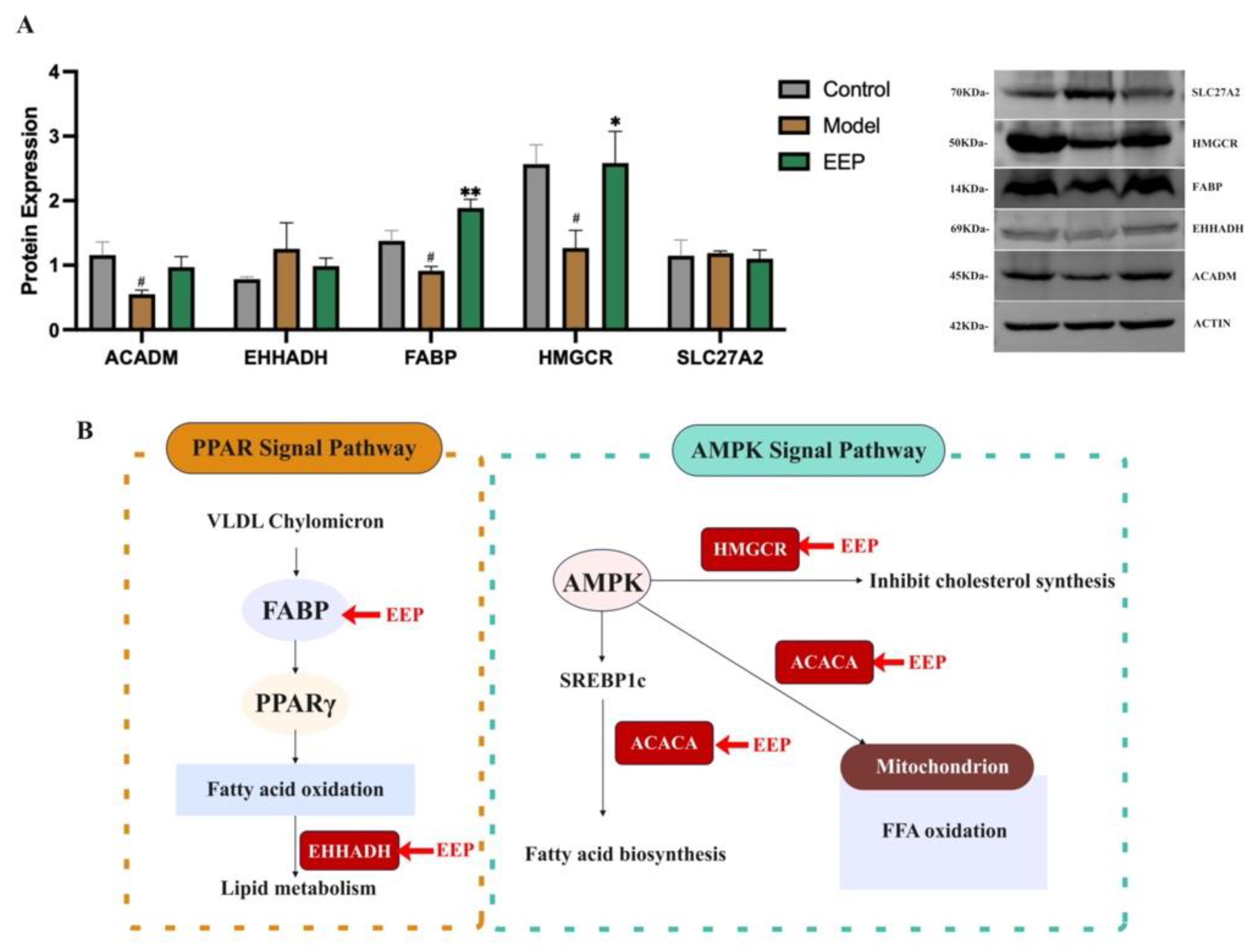
2.8. EEP Activate Protective Antioxidant Mechanisms on Oxidative Stress Induced by Hyperthyroidism via Ampk and Ppar Signalling Pathways
3. Discussion
4. Materials and Methods
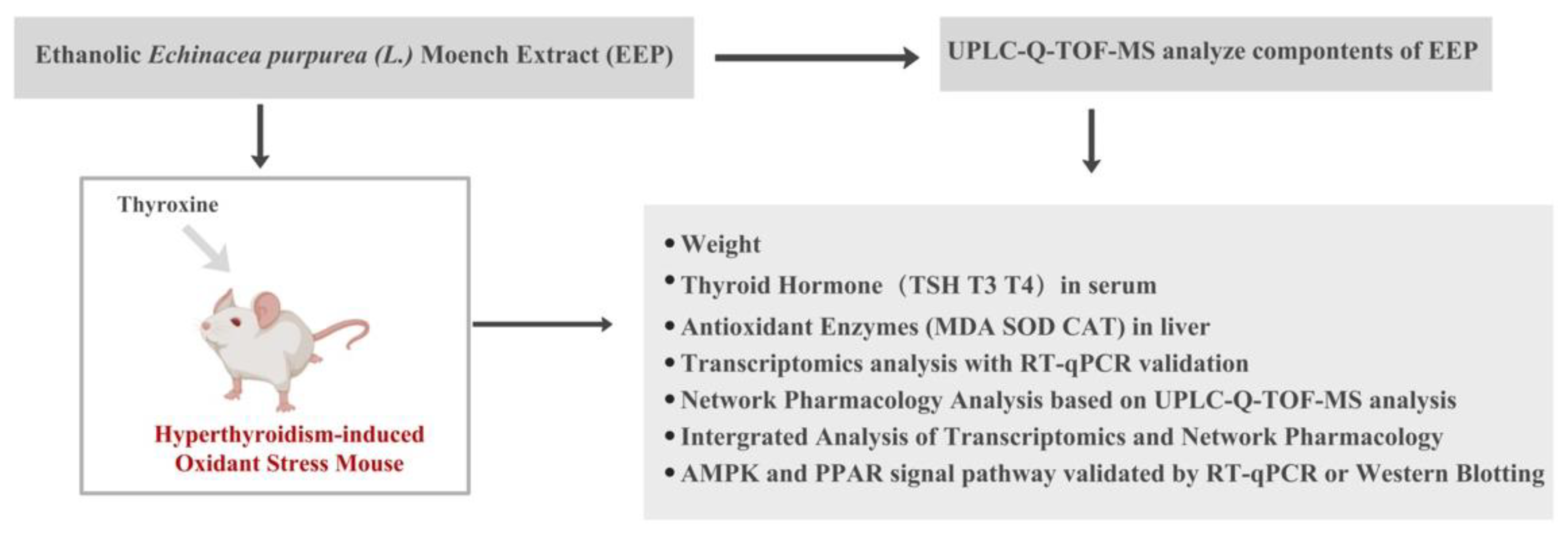
4.1. Experimental Design
4.2. Preparation of Ethanolic Echinacea purpurea Extracts
4.3. UPLC-Q-TOF-MS Analysis
4.4. Animal Experiments
4.4.1. Induction of Hyperthyroidism
4.4.2. Body Weight and Organ Index
4.4.3. Sample Collection
4.4.4. Assessment of Thyroid Hormone in Hyperthyroidism Mice
4.4.5. Determination of Hepatic Oxidative Stress Markers in Hyperthyroidism Mice
4.5. Transcriptome Experiment and Analysis
4.5.1. Identifying the Antioxidant Effect of EEP on Hyperthyroidism-Induced Oxidative Stress Genes
4.5.2. GO and KEGG Enrichment Analysis
4.5.3. Construction of a PPI Network and Analysis of Modules
4.5.4. Validation of Key Genes mRNA Expressions in the Mouse Liver
4.6. Network Pharmacology Analysis
4.6.1. Target Prediction of the Active Compounds
4.6.2. Collection of Oxidative Stress Genes Caused by Hyperthyroidism
4.6.3. GO and KEGG Enrichment Analysis
4.7. Combined Analysis of Transcriptomics and Network Pharmacology
4.8. Determination of Protein Target in AMRK Signal Pathway and PPAR Signal Pathway Using Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; Del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, D.M.; Gordon, N.P.; Jensen, C.D.; Avins, A.L. Nonvitamin, nonmineral supplement use over a 12-month period by adult members of a large health maintenance organization. J. Am. Diet. Assoc. 2003, 103, 1500–1505. [Google Scholar] [CrossRef]
- Jeurissen, S.M.F.; Buurma-Rethans, E.J.M.; Beukers, M.H.; Jansen-van der Vliet, M.; van Rossum, C.T.M.; Sprong, R.C. Consumption of plant food supplements in the Netherlands. Food Funct. 2018, 9, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Tsui, B.; Dennehy, C.E.; Tsourounis, C. A survey of dietary supplement use during pregnancy at an academic medical center. Am. J. Obstet. Gynecol. 2001, 185, 433–437. [Google Scholar] [CrossRef]
- Wilson, K.M.; Klein, J.D.; Sesselberg, T.S.; Yussman, S.M.; Markow, D.B.; Green, A.E.; West, J.C.; Gray, N.J. Use of complementary medicine and dietary supplements among U.S. adolescents. J. Adolesc. Health 2006, 38, 385–394. [Google Scholar] [CrossRef]
- Ryan, E.A.; Pick, M.E.; Marceau, C. Use of alternative medicines in diabetes mellitus. Diabet. Med. 2001, 18, 242–245. [Google Scholar] [CrossRef]
- Pakzad, K.; Boucher, B.A.; Kreiger, N.; Cotterchio, M. The use of herbal and other non-vitamin, non-mineral supplements among pre- and post-menopausal women in Ontario. Can. J. Public Health 2007, 98, 383–388. [Google Scholar] [CrossRef]
- Ashwell, E. The endocrine system and associated disorders. Br. J. Nurs. 2022, 31, 316–320. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Teng, D.; Shi, X.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; Li, Y.; et al. Hyperthyroidism prevalence in China after universal salt iodization. Front. Endocrinol. 2021, 12, 651534. [Google Scholar] [CrossRef] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Lee, S.Y.; Braverman, L.E. Hyperthyroidism. Lancet 2016, 388, 906–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asayama, K.; Dobashi, K.; Hayashibe, H.; Megata, Y.; Kato, K. Lipid peroxidation and free radical scavengers in thyroid dysfunction in the rat: A possible mechanism of injury to heart and skeletal muscle in hyperthyroidism. Endocrinology 1997, 121, 21128. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Costa Rosa, L.F.; Safi, D.A.; Bechara, E.J.; Curi, R. Control of superoxide dismutase, catalase and glutathione peroxidase activities in rat lymphoid organs by thyroid hormones. J. Endocrinol. 1994, 140, 73–77. [Google Scholar] [CrossRef]
- Mogulkoc, R.; Baltaci, A.K.; Oztekin, E.; Aydin, L.; Sivrikaya, A. Melatonin prevents oxidant damage in various tissues of rats with hyperthyroidism. Life Sci. 2006, 79, 311–315. [Google Scholar] [CrossRef]
- Venditti, P.; Balestrieri, M.; Di Meo, S.; De Leo, T. Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J. Endocrinol. 1997, 155, 1517. [Google Scholar] [CrossRef] [Green Version]
- Baydas, B.; Meral, I. Effects of melatonin on lipid peroxidation and anti-oxidant enzyme activity in rats with experimentally induced hyperthyroidism. Clin. Exp. Pharmacol. Physiol. 2005, 32, 541–544. [Google Scholar] [CrossRef]
- Seven, A.; Seymen, O.; Hatemi, S.; Hatemi, H.; Yiğit, G.; Candan, G. Antioxidant status in experimental hyperthyroidism: Effect of vitamin E supplementation. Clin. Chim. Acta 1996, 256, 65–74. [Google Scholar] [CrossRef]
- Mohamadin, A.M.; Hammad, L.N.; El-Bab, M.F.; Abdel Gawad, H.S. Attenuation of oxidative stress in plasma and tissues of rats with experimentally induced hyperthyroidism by caffeic acid phenylethyl ester. Basic Clin. Pharmacol. Toxicol. 2007, 100, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.; Türk, A.; Aydın, H.; Erten, M.; Kırıcı, P. Vitamin D improves oxidative stress and histopathological damage in rat ovaries caused by hyperthyroidism. J. Obstet. Gynaecol. Res. 2021, 47, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S.; Burch, H.S.; Cooper, D.S.; Garber, J.R.; Greenlee, C.M.; Klein, I.L. The role of propylthiouracil in the management of Graves’ Disease in adults: Report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 2009, 19, 673–674. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef]
- Liu, C.Z.; Abbasi, B.H.; Gao, M.; Murch, S.J.; Saxena, P.K. Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J. Agric. Food. Chem. 2006, 54, 8456–8460. [Google Scholar] [CrossRef]
- Chiou, S.Y.; Sung, J.M.; Huang, P.W.; Lin, S.D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea Flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Chen, Q.; Li, Z.; Zhou, Z. An evidence update on the protective mechanism of tangeretin against neuroinflammation based on network pharmacology prediction and transcriptomic analysis. Eur. J. Pharmacol. 2021, 906, 174094. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, Z.; Zhao, W.; Chen, Q.; Bai, H.; Wang, S.; Yang, L.; Bi, C.; Shi, Y.; Liu, Y. Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxue Decoction in the treatment of diabetic nephropathy in type 2 diabetes mellitus. J. Ethnopharmacol. 2022, 283, 114699. [Google Scholar] [CrossRef]
- Gao, F.; Niu, Y.; Sun, L.; Li, W.; Xia, H.; Zhang, Y.; Geng, S.; Guo, Z.; Lin, H.; Du, G. Integrating network pharmacology and transcriptomic validation to investigate the efficacy and mechanism of Mufangji decoction preventing lung cancer. J. Ethnopharmacol. 2022, 298, 115573. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell Mol. Med. 2019, 23, 4063–4075. [Google Scholar] [CrossRef]
- Liu, G.; Kuang, S.; Cao, R.; Wang, J.; Peng, Q.; Sun, C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019, 33, 10089–10103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation. Can. J. Physiol. Pharmacol. 2014, 92, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hu, Z.; Ma, W.; Zang, L.; Tian, Z.; Hou, Q. Quercetin alleviates hyperthyroidism-induced liver damage via Nrf2 Signaling pathway. Biofactors 2020, 46, 608–619. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging. Prostaglandins Leukot Essent Fat. Acids 2016, 114, 28–34. [Google Scholar] [CrossRef]
- Wågbø, A.M.; Cangialosi, M.V.; Cicero, N.; Letcher, R.J.; Arukwe, A. Perfluorooctane sulfonamide-mediated modulation of hepatocellular lipid homeostasis and oxidative stress responses in Atlantic salmon hepatocytes. Chem. Res. Toxicol. 2012, 25, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Evron, J.M.; Papaleontiou, M. Decision making in subclinical thyroid disease. Med. Clin. N. Am. 2021, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Lewinski, A. The role of oxidative stress in physiological and pathological processes in the thyroid gland; possible involvement in pineal-thyroid interactions. Neuro. Endocrinol. Lett. 2003, 24, 293–303. [Google Scholar]
- Papachristos, D.A.; Huynh, J.; Grossman, M.; MacIsaac, R.J. Liver dysfunction and anti-thyroid therapy. SAGE Open Med. Case Rep. 2015, 3, 2050313X14568335. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, K.; Weiner, J.; Hönes, S.; Klöting, N.; Rijntjes, E.; Heiker, J.T.; Gebhardt, C.; Köhrle, J.; Führer, D.; Steinhoff, K.; et al. The effects of thyroid hormones on gene expression of Acyl-Coenzyme A thioesterases in adipose tissue and liver of mice. Eur. Thyroid. J. 2015, 4 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, M.C.; Alexson, S.E. The role Acyl-CoA thioesterases play in mediating intracellular lipid metabolism. Prog. Lipid Res. 2002, 41, 99–130. [Google Scholar] [CrossRef] [PubMed]
- Li, L.O.; Klett, E.L.; Coleman, R.A. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momose, A.; Fujita, M.; Ohtomo, T.; Umemoto, N.; Tanonaka, K.; Toyoda, H.; Morikawa, M.; Yamada, J. Regulated expression of acyl-CoA thioesterases in the differentiation of cultured rat brown adipocytes. Biochem. Biophys. Res. Commun. 2011, 404, 74–78. [Google Scholar] [CrossRef]
- Wanders, R.J.; Waterham, H.R. Peroxisomal disorders: The single peroxisomal enzyme deficiencies. Biochim. Biophys. Acta 2006, 1763, 1707–1720. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Ma, R.; Li, H.; Yin, K.; Du, G.; Chen, X.; Liu, Z.; Yin, D. Upregulated SLC27A2/FATP2 in differentiated thyroid carcinoma promotes tumor proliferation and migration. J. Clin. Lab. Anal. 2022, 36, e24148. [Google Scholar] [CrossRef]
- Koeners, M.P.; Wesseling, S.; Ulu, A.; Sepúlveda, R.L.; Morisseau, C.; Braam, B.; Hammock, B.D.; Joles, J.A. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats. Am. J. Physiol. Endocrinol. Met. 2011, 300, E691–E698. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, X.; Guan, A.; Zhou, H.; Zhu, Y.; Wang, R.; Li, R. EPHX2 Inhibits colon cancer progression by promoting fatty acid degradation. Front. Oncol. 2022, 12, 870721. [Google Scholar] [CrossRef]
- Panigrahy, D.; Edin, M.L.; Lee, C.R.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Inv. 2012, 122, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Ranea-Robles, P.; Violante, S.; Argmann, C.; Dodatko, T.; Bhattacharya, D.; Chen, H.; Yu, C.; Friedman, S.L.; Puchowicz, M.; Houten, S.M. Murine deficiency of peroxisomal L-bifunctional protein (EHHADH) causes medium-chain 3-hydroxydicarboxylic aciduria and perturbs hepatic cholesterol homeostasis. Cell. Mol. Life Sci. 2021, 78, 5631–5646. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Ruddy, M.; Chakravarthy, M.; et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab. 2017, 26, 394–406.e6. [Google Scholar] [CrossRef]
- Azizidoost, S.; Babaahmadi-Rezaei, H.; Nazeri, Z.; Cheraghzadeh, M.; Kheirollah, A. Amyloid beta increases ABCA1 and HMGCR protein expression, and cholesterol synthesis and accumulation in mice neurons and astrocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2022, 1867, 159069. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Liu, W.; Wu, H.; Li, Y.; Yang, E.; Wang, Z.; Xiao, H.; Quan, R.; Hu, X. The active ingredients and mechanisms of Longchai Jiangxue Formula in treating PV, based on UPLC/Q-TOF-MS/MS, systematic pharmacology, and molecular biology validation. Biomed Pharmacother. 2021, 140, 111767. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kang, S.Y.; Kang, A.N.; Jung, H.W.; Jung, C.; Jeong, J.H.; Park, Y.K. MOK, a pharmacopuncture medicine, regulates thyroid dysfunction in L-thyroxin-induced hyperthyroidism in rats through the regulation of oxidation and the TRPV1 ion channel. BMC Complement Altern Med. 2017, 17, 535. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Wang, Z.; Wang, Y.; Hou, B.; Sun, J.; Tian, H.; Li, B.; Shui, G.; Yang, X.; Yang, X.; et al. Integration of Metabolomics and Transcriptomics Reveals Ketone Body and Lipid Metabolism Disturbance Related to ER Stress in the Liver. J. Proteome Res. 2021, 20, 3875–3888. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Yang, Z.; Wang, J.; Li, W.; Zhou, J.; Zhang, J. Hematopoietic Effects of Paeoniflorin and Albiflorin on Radiotherapy-Induced Myelosuppression Mice. Evid. Based Complement. Altern. Med. 2016, 2016, 5789381. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Liu, Y.; Lai, Z.; Huang, J.; Li, C.; Zhang, Y.; Gong, X.; Deng, J.; Ye, X.; Li, X. An integrated network pharmacology and transcriptomic method to explore the mechanism of the total Rhizoma Coptidis alkaloids in improving diabetic nephropathy. J. Ethnopharmacol. 2021, 270, 113806. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Wang, L.Y.; Zhao, D.P.; Wang, C.L.; Zhang, R.; Fei, W.T.; Wang, J.X.; Zhang, J.J. Antidepressant-like effects of albiflorin involved the NO signaling pathway in rats model of chronic restraint stress. Chin. J. Nat. Med. 2020, 18, 872–880. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhang, J.; Wang, C.; Zheng, T.; Di, S.; Wang, Y.; Fei, W.; Liang, W.; Wang, L. Ameliorative Effect of Ethanolic Echinacea purpurea against Hyperthyroidism-Induced Oxidative Stress via AMRK and PPAR Signal Pathway Using Transcriptomics and Network Pharmacology Analysis. Int. J. Mol. Sci. 2023, 24, 187. https://doi.org/10.3390/ijms24010187
Zhu Y, Zhang J, Wang C, Zheng T, Di S, Wang Y, Fei W, Liang W, Wang L. Ameliorative Effect of Ethanolic Echinacea purpurea against Hyperthyroidism-Induced Oxidative Stress via AMRK and PPAR Signal Pathway Using Transcriptomics and Network Pharmacology Analysis. International Journal of Molecular Sciences. 2023; 24(1):187. https://doi.org/10.3390/ijms24010187
Chicago/Turabian StyleZhu, Yingli, Jianjun Zhang, Chun Wang, Ting Zheng, Songrui Di, Yinyin Wang, Wenting Fei, Weican Liang, and Linyuan Wang. 2023. "Ameliorative Effect of Ethanolic Echinacea purpurea against Hyperthyroidism-Induced Oxidative Stress via AMRK and PPAR Signal Pathway Using Transcriptomics and Network Pharmacology Analysis" International Journal of Molecular Sciences 24, no. 1: 187. https://doi.org/10.3390/ijms24010187




