Hypothermia Does Not Boost the Neuroprotection Promoted by Umbilical Cord Blood Cells in a Neonatal Hypoxia-Ischemia Rat Model
Abstract
:1. Introduction
2. Results
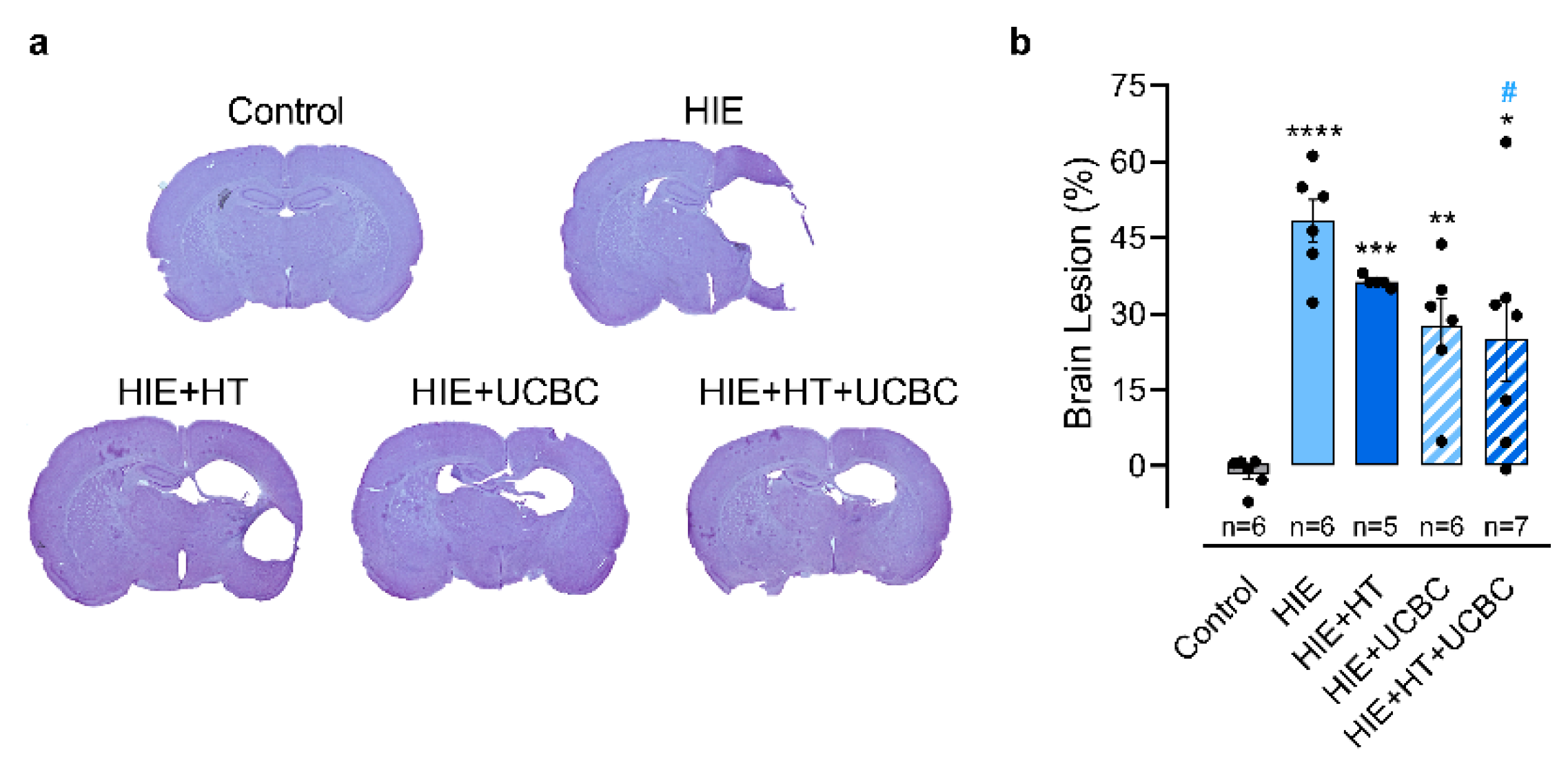
2.1. Effect of Hypothermia and Umbilical Cord Blood Cells on Neonatal HI Brain Injury
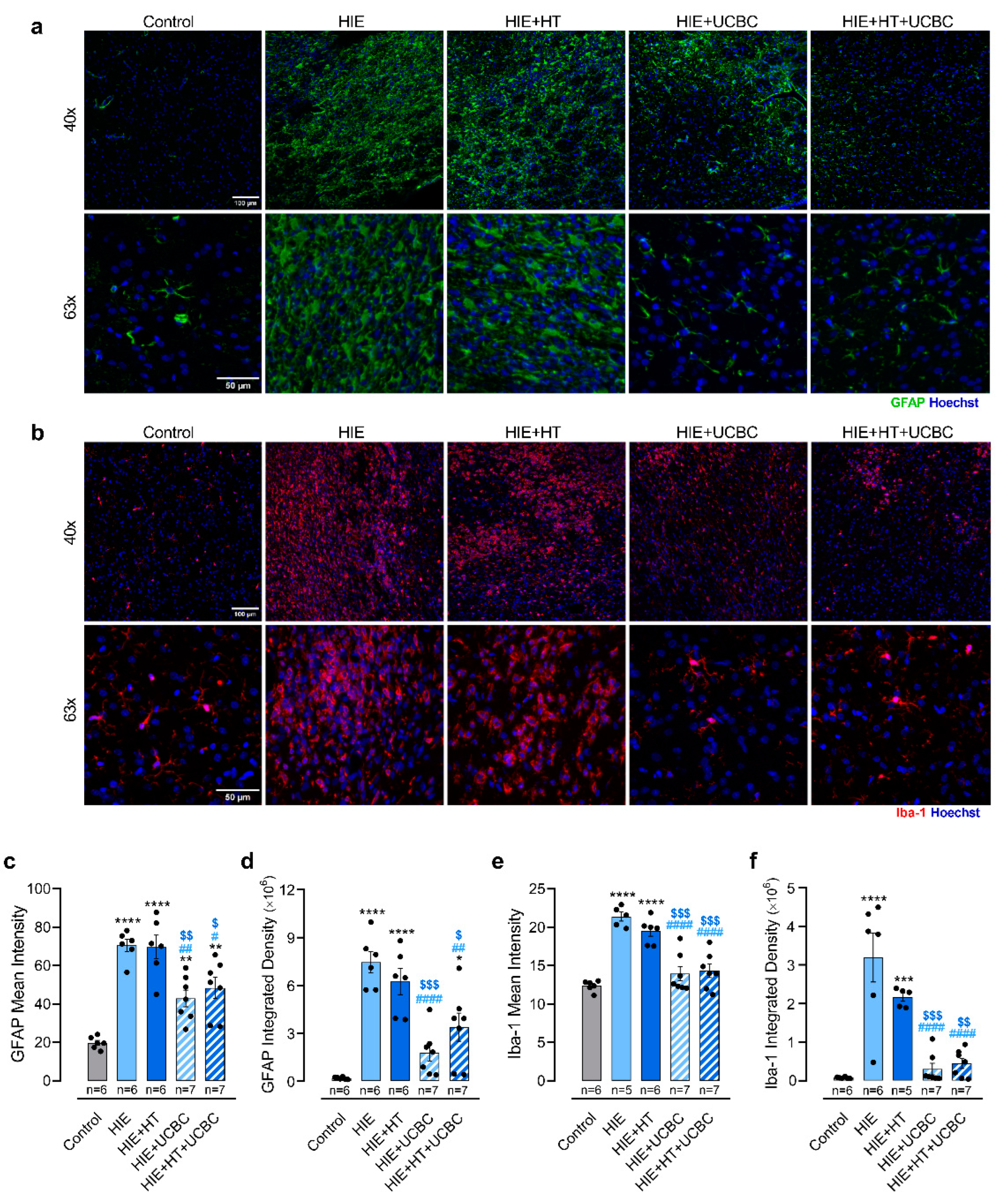
2.2. Impact of Hypothermia and Umbilical Cord Blood Cells on Glial Reactivity Triggered by Neonatal HI Brain Injury
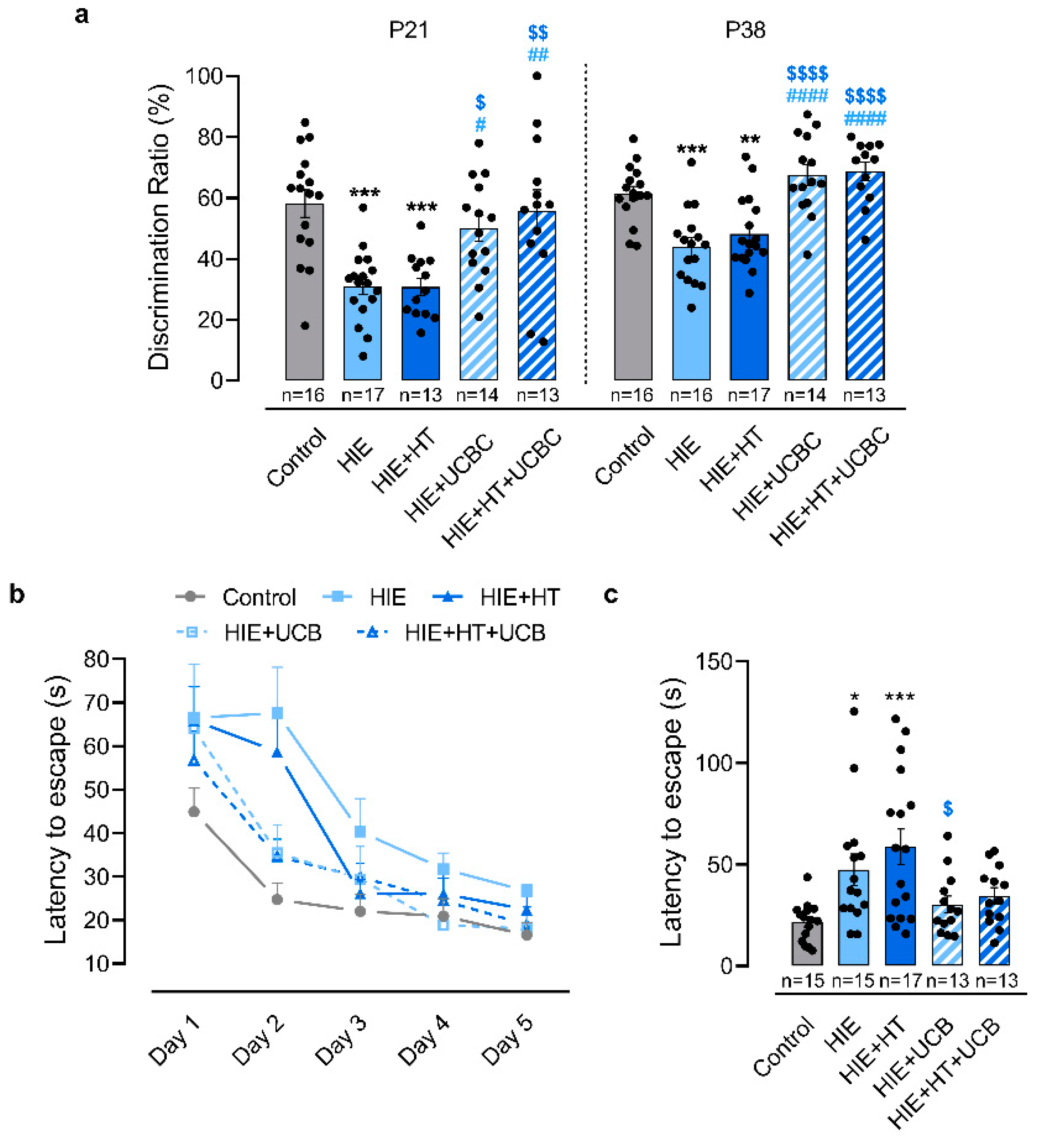
2.3. Impact of Hypothermia and Umbilical Cord Blood Cells in the Sensorimotor and Motor Deficits Caused by Neonatal HI Brain Injury
2.4. Impact of Hypothermia and Umbilical Cord Blood Cells in the Cognitive Deficits Caused by Neonatal HI Brain Injury
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animals
4.3. Induction of Neonatal Hypoxic-Ischemic Brain Injury
4.4. Hypothermia
4.5. Preparation and Administration of UCBC
4.6. Behavioral Analysis
4.6.1. Negative Geotaxis Reflex
4.6.2. Novel Object Recognition Test
4.6.3. Footprint Test
4.6.4. Ladder Rung Walking Test
4.6.5. Barnes Maze Test
4.7. Histology and Fluorescence Microscopy
4.7.1. Cresyl Violet Staining
4.7.2. Immunohistochemistry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 1, CD003311. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74 (Suppl. 1), 50–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, A.J.; Thoresen, M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb. Clin. Neurol. 2019, 162, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Phuc, P.V.; Ngoc, V.B.; Lam, D.H.; Tam, N.T.; Viet, P.Q.; Ngoc, P.K. Isolation of three important types of stem cells from the same samples of banked umbilical cord blood. Cell Tissue Bank. 2012, 13, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Cotten, C.M.; Murtha, A.P.; Goldberg, R.N.; Grotegut, C.A.; Smith, P.B.; Goldstein, R.F.; Fisher, K.A.; Gustafson, K.E.; Waters-Pick, B.; Swamy, G.K.; et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014, 164, 973–979.e971. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, M.; Sawada, M.; Watabe, S.; Sano, H.; Kanai, M.; Tanaka, E.; Ohnishi, S.; Sato, Y.; Sobajima, H.; Hamazaki, T.; et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: A pilot study for feasibility and safety. Sci. Rep. 2020, 10, 4603. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Coelho, P.M.; Magalhaes, E.S.; Lopes, L.M.; deAzevedo, L.C.; Santiago, M.F.; Mendez-Otero, R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: Functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010, 19, 351–358. [Google Scholar] [CrossRef] [PubMed]
- de Paula, S.; Greggio, S.; Marinowic, D.R.; Machado, D.C.; DaCosta, J.C. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience 2012, 210, 431–441. [Google Scholar] [CrossRef]
- Penny, T.R.; Pham, Y.; Sutherland, A.E.; Mihelakis, J.G.; Lee, J.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; McDonald, C.A. Multiple doses of umbilical cord blood cells improve long-term brain injury in the neonatal rat. Brain Res. 2020, 1746, 147001. [Google Scholar] [CrossRef]
- Wasielewski, B.; Jensen, A.; Roth-Harer, A.; Dermietzel, R.; Meier, C. Neuroglial activation and Cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Res. 2012, 1487, 39–53. [Google Scholar] [CrossRef]
- Greggio, S.; de Paula, S.; Azevedo, P.N.; Venturin, G.T.; Dacosta, J.C. Intra-arterial transplantation of human umbilical cord blood mononuclear cells in neonatal hypoxic-ischemic rats. Life Sci. 2014, 96, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Grandvuillemin, I.; Garrigue, P.; Ramdani, A.; Boubred, F.; Simeoni, U.; Dignat-George, F.; Sabatier, F.; Guillet, B. Long-Term Recovery After Endothelial Colony-Forming Cells or Human Umbilical Cord Blood Cells Administration in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2017, 6, 1987–1996. [Google Scholar] [CrossRef]
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflamm. 2018, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yan, Y.; Luo, Z.; Luo, P.; Xiao, N.; Sun, X.; Cheng, L. Effects of human umbilical cord blood CD34(+) cell transplantation in neonatal hypoxic-ischemia rat model. Brain Dev. 2019, 41, 173–181. [Google Scholar] [CrossRef]
- Serrenho, I.; Rosado, M.; Dinis, A.; Cardoso, M.C.; Graos, M.; Manadas, B.; Baltazar, G. Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 3142. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Park, W.S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 2018, 8, 7665. [Google Scholar] [CrossRef] [Green Version]
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Yoo, H.S.; Sung, D.K.; Im, G.H.; Choi, S.J.; Chang, Y.S. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 2015, 10, e0120893. [Google Scholar] [CrossRef] [Green Version]
- Herz, J.; Koster, C.; Reinboth, B.S.; Dzietko, M.; Hansen, W.; Sabir, H.; van Velthoven, C.; Bendix, I.; Felderhoff-Muser, U. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2018, 70, 118–130. [Google Scholar] [CrossRef]
- Bhalala, U.S.; Koehler, R.C.; Kannan, S. Neuroinflammation and neuroimmune dysregulation after acute hypoxic-ischemic injury of developing brain. Front. Pediatr. 2014, 2, 144. [Google Scholar] [CrossRef]
- Halpin, S.; McCusker, C.; Fogarty, L.; White, J.; Cavaliere, E.; Boylan, G.; Murray, D. Long-term neuropsychological and behavioral outcome of mild and moderate hypoxic ischemic encephalopathy. Early Hum. Dev. 2022, 165, 105541. [Google Scholar] [CrossRef]
- de Vries, L.S.; Jongmans, M.J. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F220–F224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Handel, M.; Swaab, H.; de Vries, L.S.; Jongmans, M.J. Behavioral outcome in children with a history of neonatal encephalopathy following perinatal asphyxia. J. Pediatr. Psychol. 2010, 35, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Schreglmann, M.; Ground, A.; Vollmer, B.; Johnson, M.J. Systematic review: Long-term cognitive and behavioural outcomes of neonatal hypoxic-ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr. 2020, 109, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.L.; Laughlin, M.J. UC blood hematopoietic stem cells and therapeutic angiogenesis. Cytotherapy 2007, 9, 4–13. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, F.; Qu, Y.; Zhang, L.; Wang, Y.; Mu, D. Animal models of hypoxic-ischemic encephalopathy: Optimal choices for the best outcomes. Rev. Neurosci. 2017, 28, 31–43. [Google Scholar] [CrossRef]
- Sabir, H.; Scull-Brown, E.; Liu, X.; Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012, 43, 3364–3370. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Concepcion, K.; Meng, X.; Zhang, L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog. Neurobiol. 2017, 159, 50–68. [Google Scholar] [CrossRef]
- Bregere, C.; Schwendele, B.; Radanovic, B.; Guzman, R. Microglia and Stem-Cell Mediated Neuroprotection after Neonatal Hypoxia-Ischemia. Stem Cell Rev. Rep. 2022, 18, 474–522. [Google Scholar] [CrossRef]
- Hamdy, N.; Eide, S.; Sun, H.S.; Feng, Z.P. Animal models for neonatal brain injury induced by hypoxic ischemic conditions in rodents. Exp. Neurol. 2020, 334, 113457. [Google Scholar] [CrossRef]
- Ohshima, M.; Taguchi, A.; Sato, Y.; Ogawa, Y.; Saito, S.; Yamahara, K.; Ihara, M.; Harada-Shiba, M.; Ikeda, T.; Matsuyama, T.; et al. Evaluations of Intravenous Administration of CD34+ Human Umbilical Cord Blood Cells in a Mouse Model of Neonatal Hypoxic-Ischemic Encephalopathy. Dev. Neurosci. 2016, 38, 331–341. [Google Scholar] [CrossRef]
- Kidani, Y.; Miki, Y.; Nomimura, N.; Minakawa, S.; Tanaka, N.; Miyoshi, H.; Wakabayashi, K.; Kudo, Y. The therapeutic effect of CD133(+) cells derived from human umbilical cord blood on neonatal mouse hypoxic-ischemic encephalopathy model. Life Sci. 2016, 157, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Newcomb, J.; Garbuzova-Davis, S.; Davis Sanberg, C.; Sanberg, P.R.; Willing, A.E. Human Umbilical Cord Blood Cells Have Trophic Effects on Young and Aging Hippocampal Neurons in Vitro. Aging Dis. 2010, 1, 173–190. [Google Scholar]
- Fan, C.G.; Zhang, Q.J.; Tang, F.W.; Han, Z.B.; Wang, G.S.; Han, Z.C. Human umbilical cord blood cells express neurotrophic factors. Neurosci. Lett. 2005, 380, 322–325. [Google Scholar] [CrossRef]
- Burnsed, J.C.; Chavez-Valdez, R.; Hossain, M.S.; Kesavan, K.; Martin, L.J.; Zhang, J.; Northington, F.J. Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain--a longitudinal study. PLoS ONE 2015, 10, e0118889. [Google Scholar] [CrossRef]
- Patel, S.D.; Pierce, L.; Ciardiello, A.; Hutton, A.; Paskewitz, S.; Aronowitz, E.; Voss, H.U.; Moore, H.; Vannucci, S.J. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: Characterization of a translational preclinical model. Pediatr. Res. 2015, 78, 264–271. [Google Scholar] [CrossRef]
- Wood, T.R.; Gundersen, J.K.; Falck, M.; Maes, E.; Osredkar, D.; Loberg, E.M.; Sabir, H.; Walloe, L.; Thoresen, M. Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic-ischaemic brain injury: A single laboratory meta-analysis. Sci. Rep. 2020, 10, 10833. [Google Scholar] [CrossRef]
- Pignataro, G.; Baba, N.; Wang, F.; Iizuka, M.; Shen, Y.; Yamashita, T.; Takaishi, K.; Tsuru, E.; Matsushima, S.; Miyamura, M.; et al. Induction of regional chemokine expression in response to human umbilical cord blood cell infusion in the neonatal mouse ischemia-reperfusion brain injury model. PLoS ONE 2019, 14, e0221111. [Google Scholar] [CrossRef]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wertman, V.; Gromova, A.; La Spada, A.R.; Cortes, C.J. Low-Cost Gait Analysis for Behavioral Phenotyping of Mouse Models of Neuromuscular Disease. J. Vis. Exp. 2019, 149, e59878. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrenho, I.; Cardoso, C.M.; Grãos, M.; Dinis, A.; Manadas, B.; Baltazar, G. Hypothermia Does Not Boost the Neuroprotection Promoted by Umbilical Cord Blood Cells in a Neonatal Hypoxia-Ischemia Rat Model. Int. J. Mol. Sci. 2023, 24, 257. https://doi.org/10.3390/ijms24010257
Serrenho I, Cardoso CM, Grãos M, Dinis A, Manadas B, Baltazar G. Hypothermia Does Not Boost the Neuroprotection Promoted by Umbilical Cord Blood Cells in a Neonatal Hypoxia-Ischemia Rat Model. International Journal of Molecular Sciences. 2023; 24(1):257. https://doi.org/10.3390/ijms24010257
Chicago/Turabian StyleSerrenho, Inês, Carla M. Cardoso, Mário Grãos, Alexandra Dinis, Bruno Manadas, and Graça Baltazar. 2023. "Hypothermia Does Not Boost the Neuroprotection Promoted by Umbilical Cord Blood Cells in a Neonatal Hypoxia-Ischemia Rat Model" International Journal of Molecular Sciences 24, no. 1: 257. https://doi.org/10.3390/ijms24010257




