Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications
Abstract
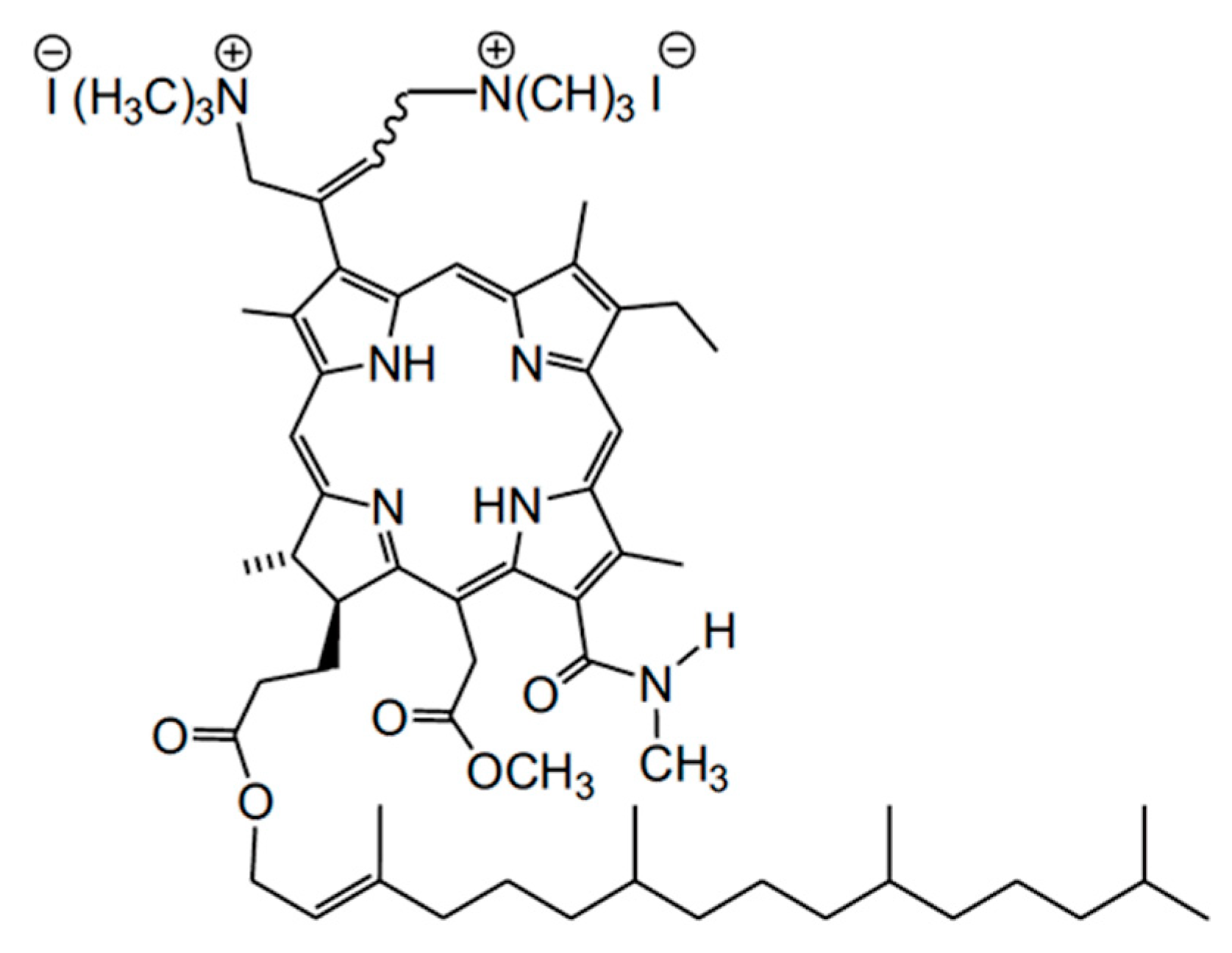
:1. Introduction
2. Results and Discussion
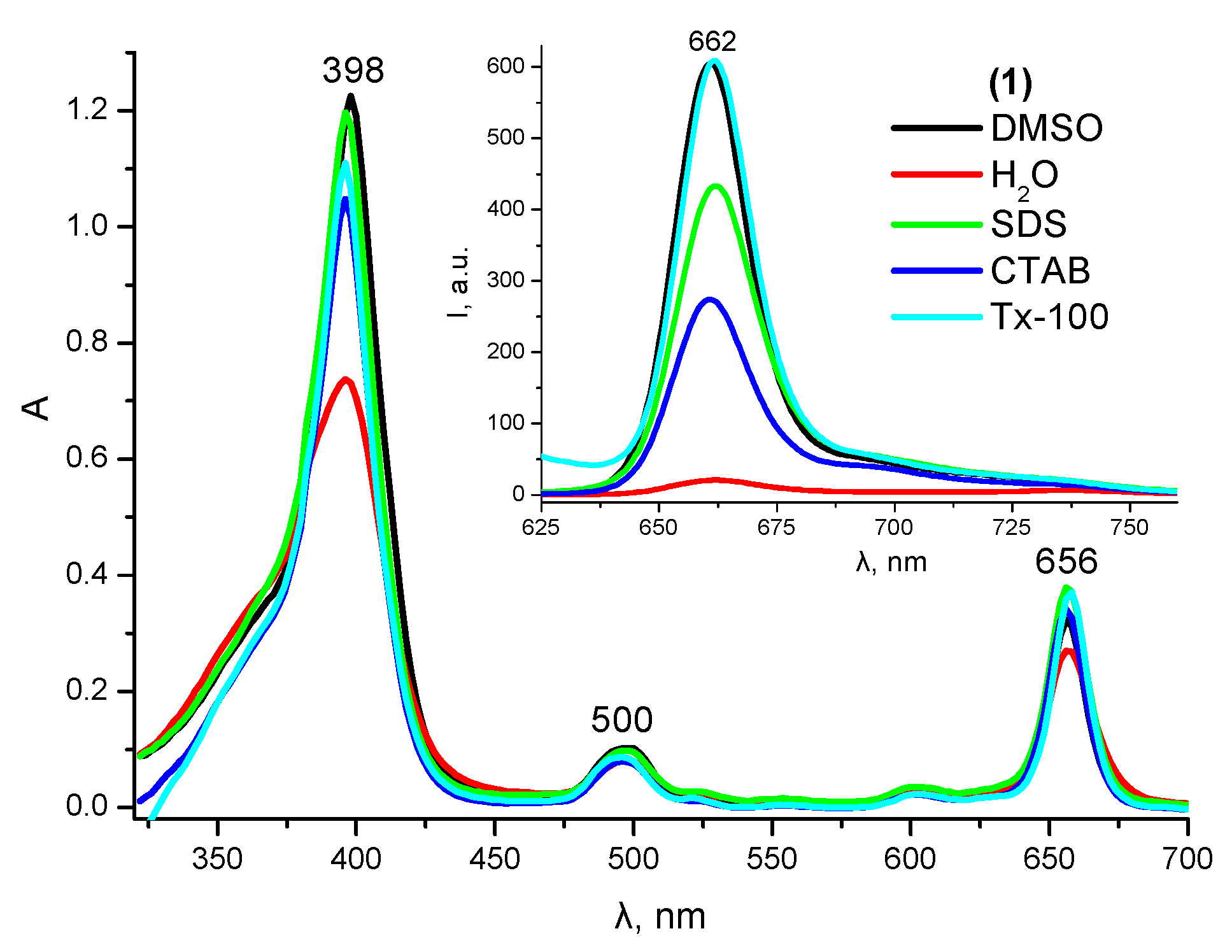
2.1. Solubilization Studies
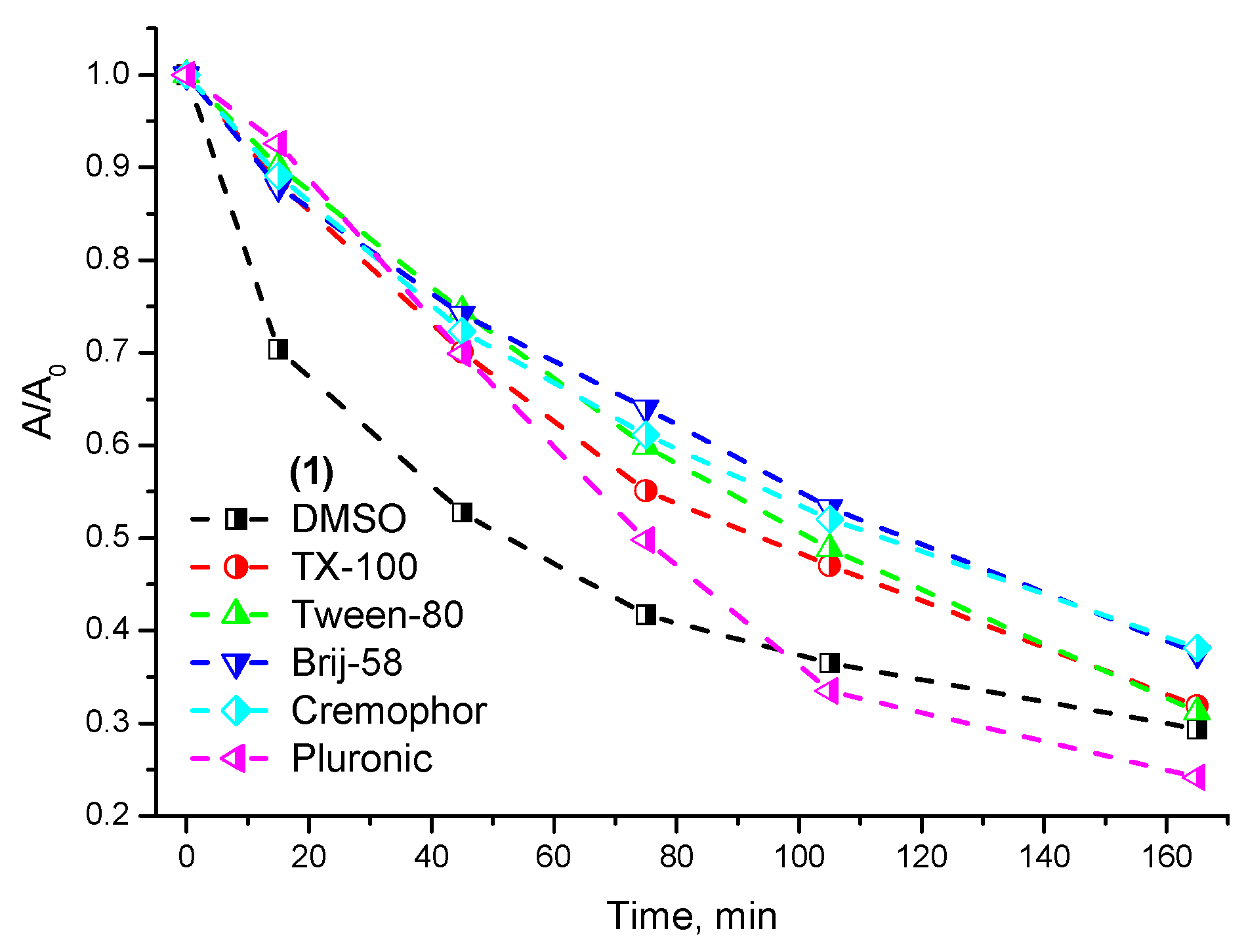
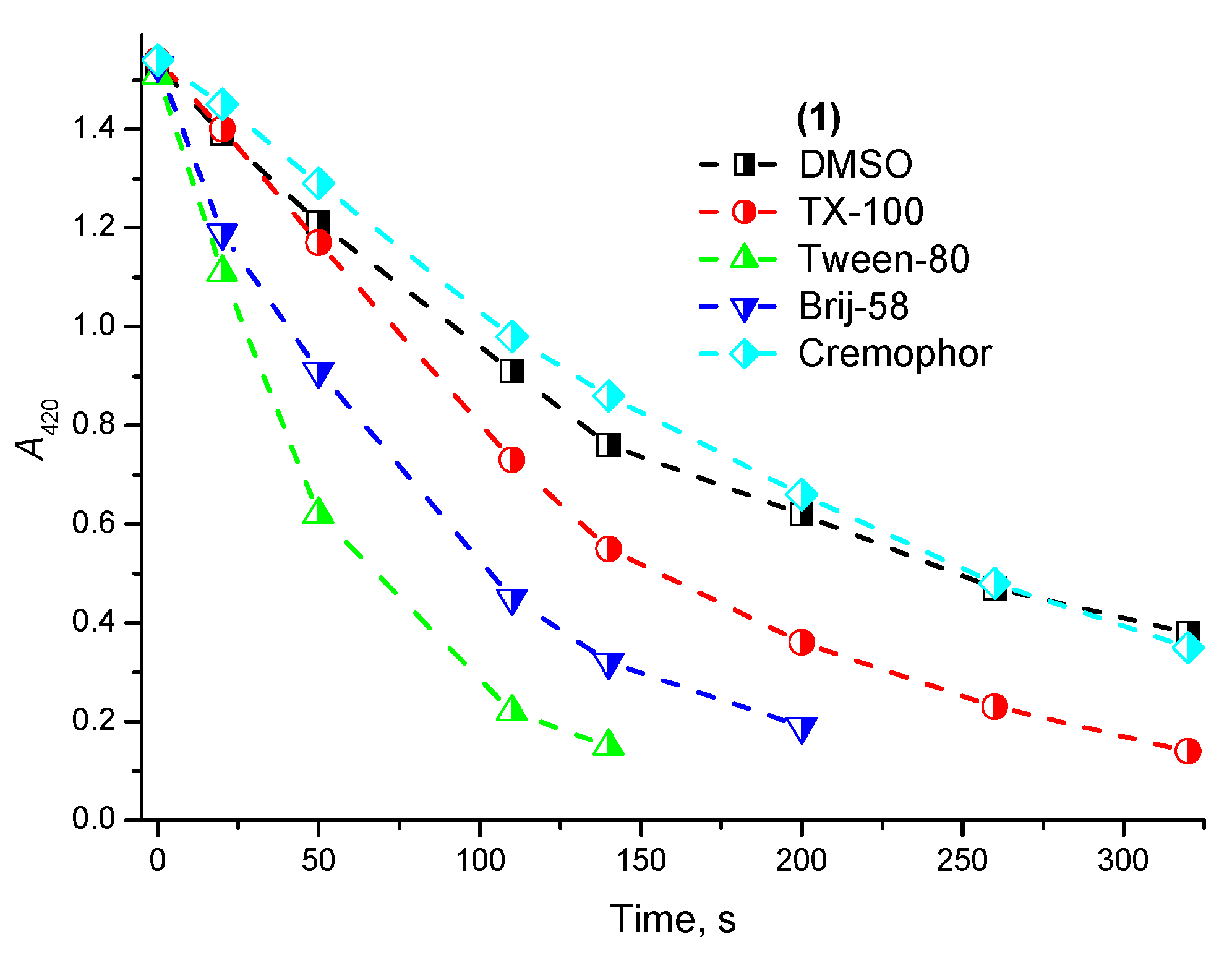
2.2. Photostability and Photochemical Activity
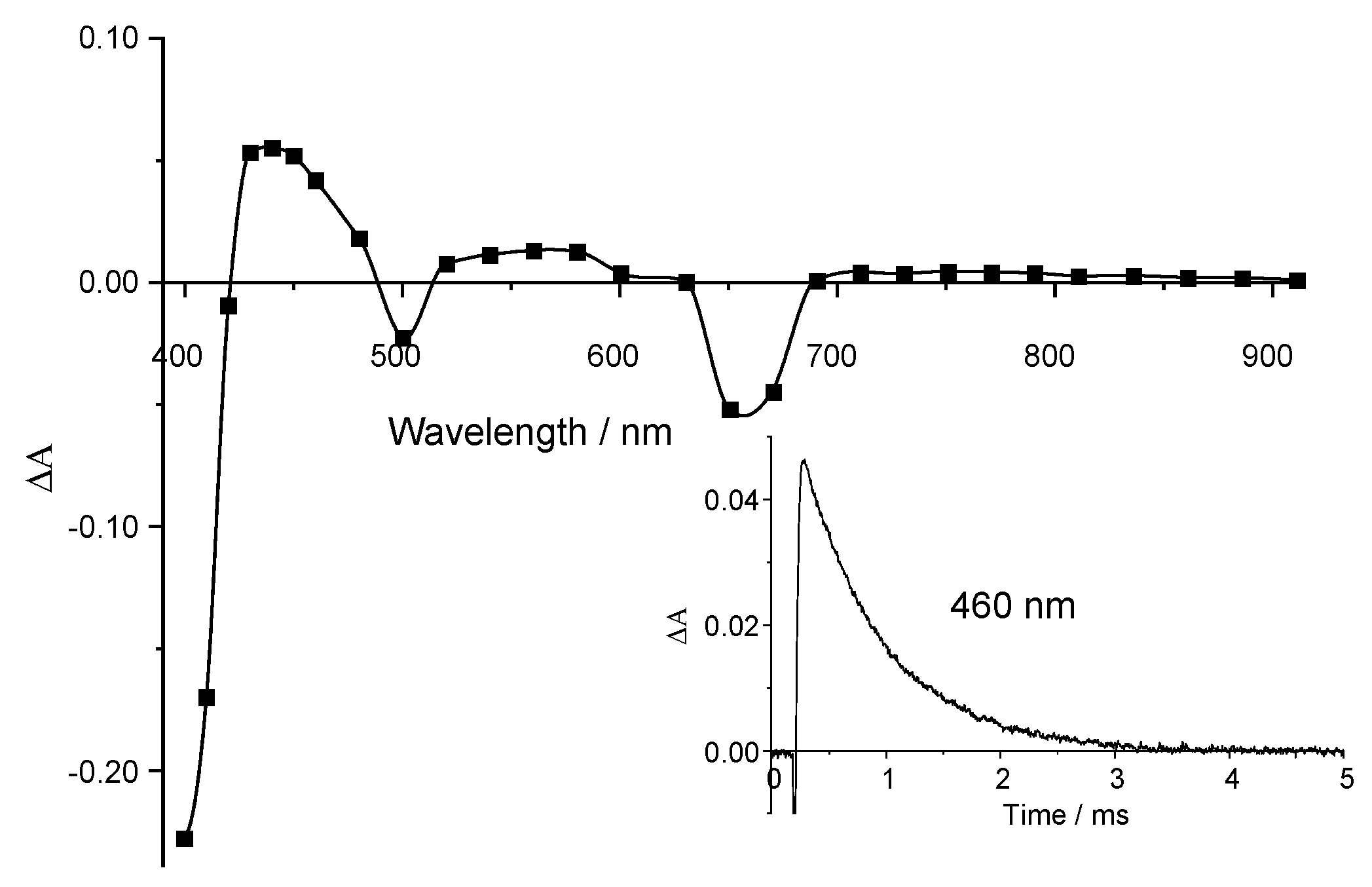
2.3. Triplet State Investigation
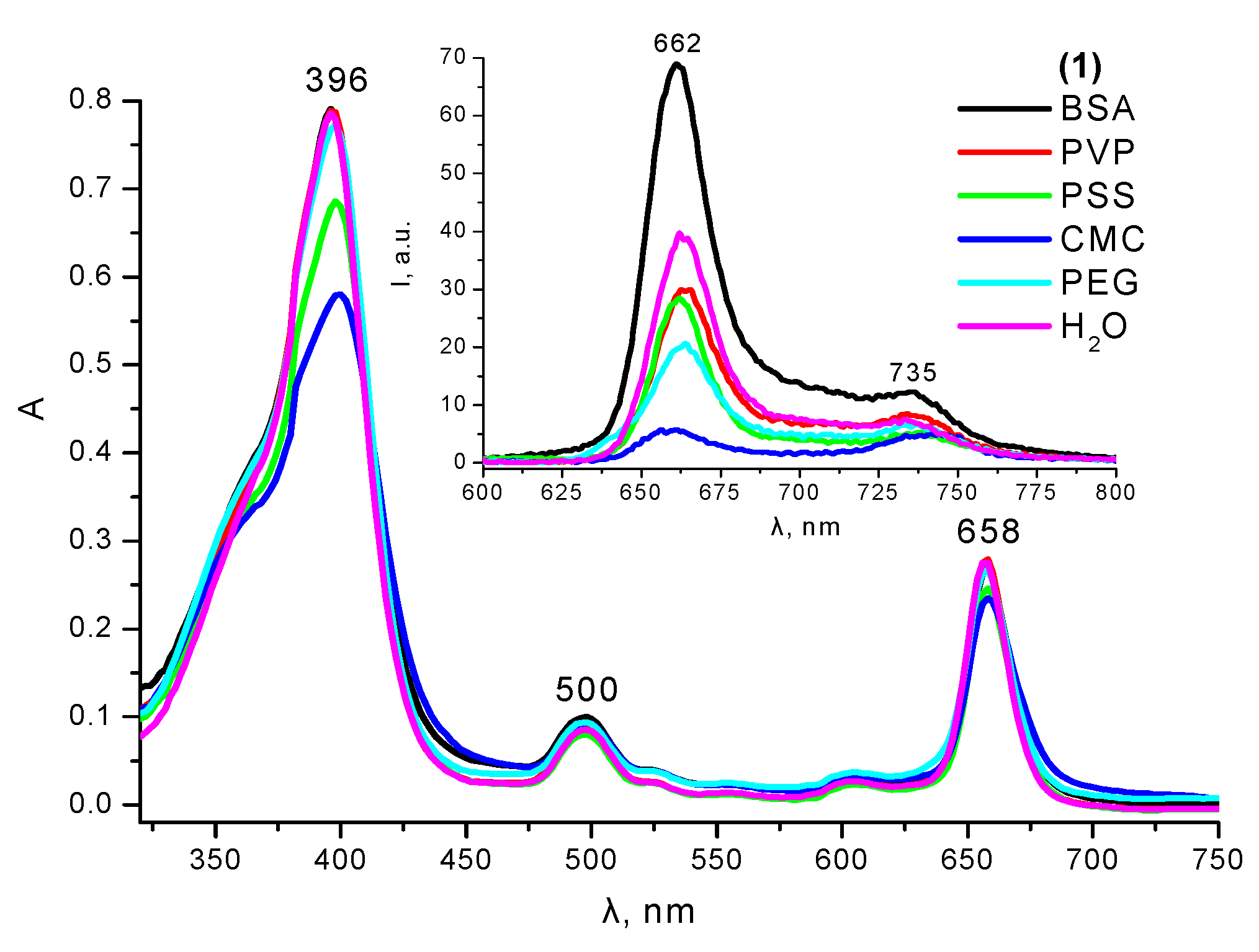
2.4. Interaction with Polymers
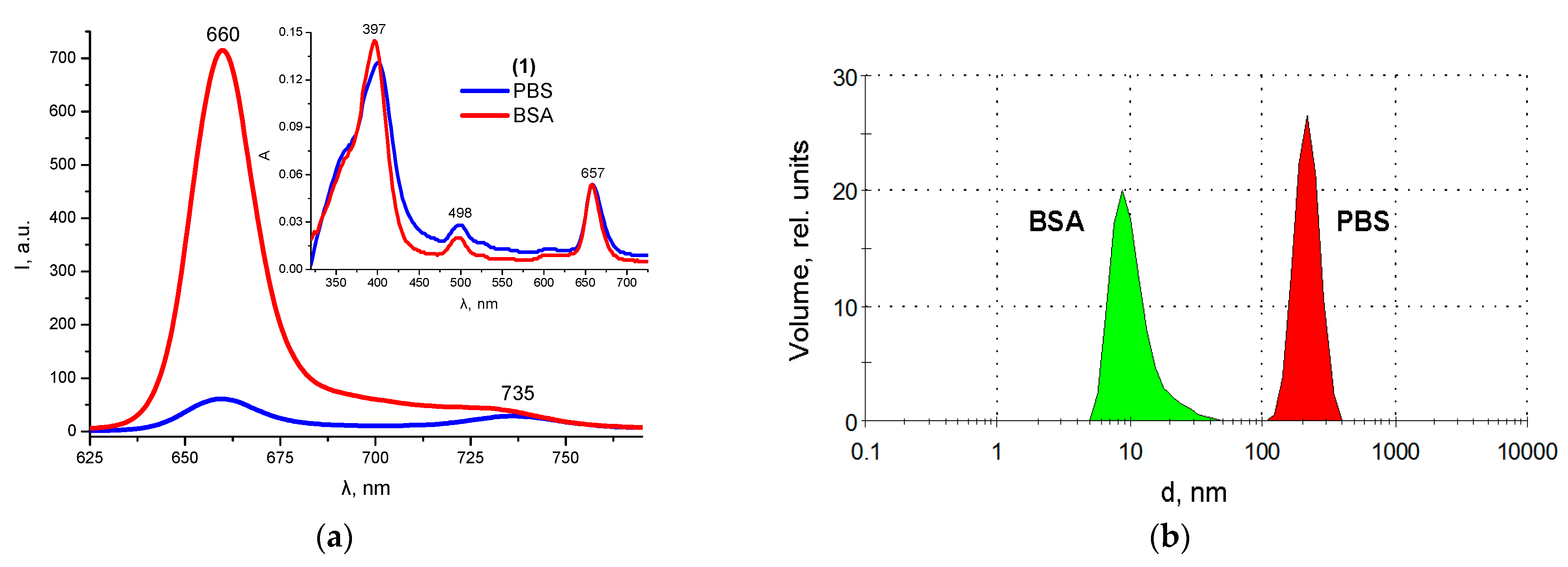
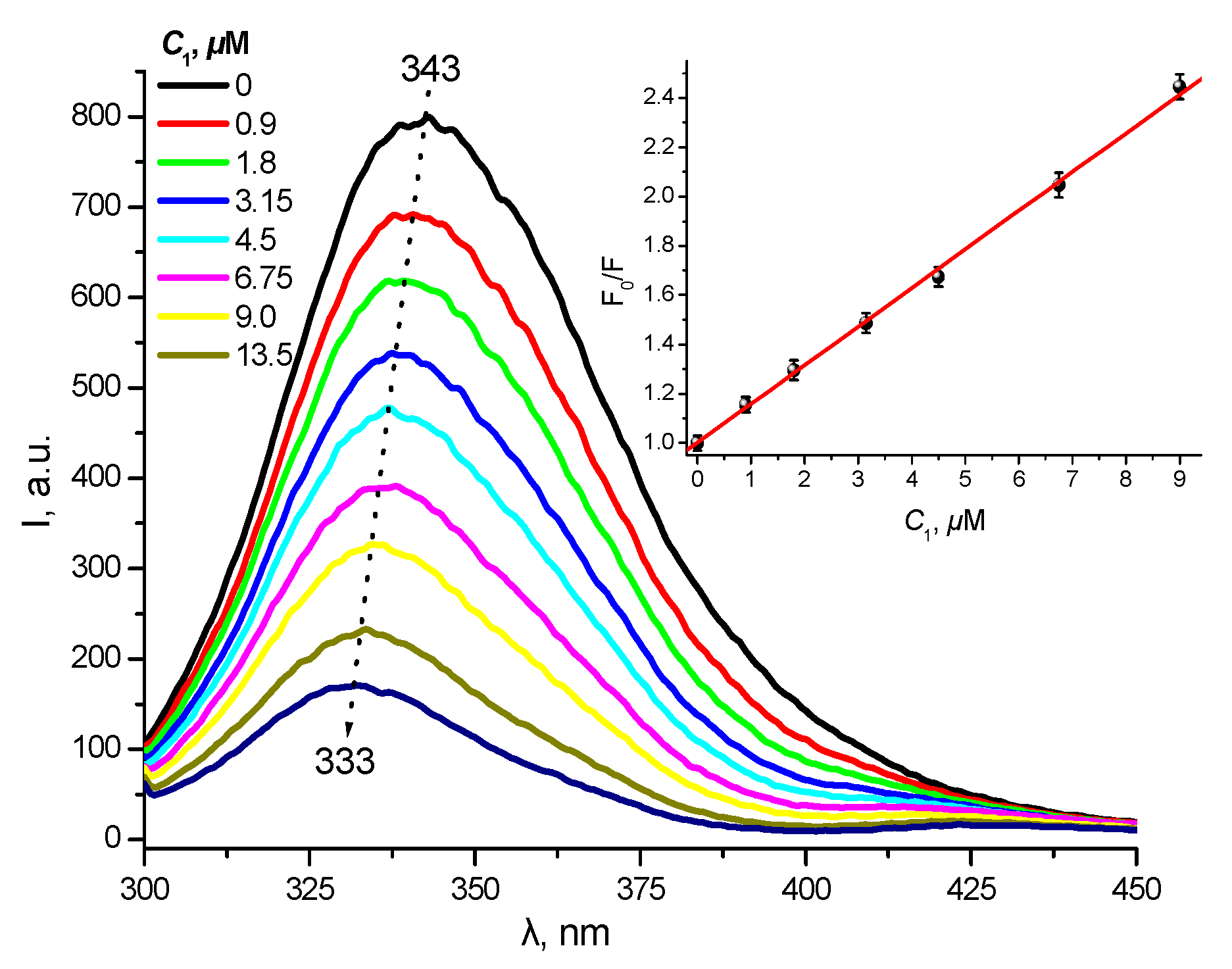
2.5. Protein Binding
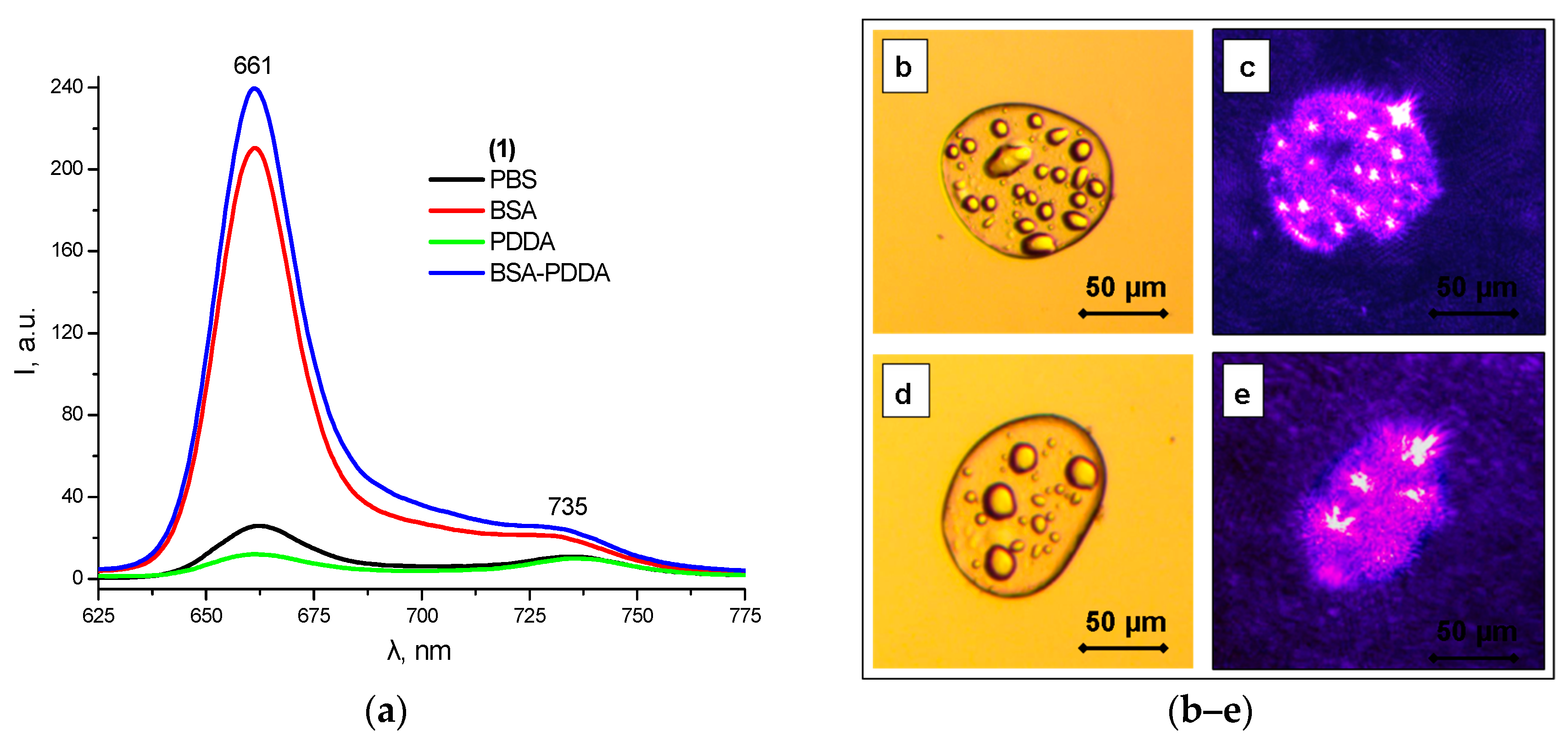
2.6. Polyelectrolyte Complexes
2.7. In Vitro Biological Tests
3. Materials and Methods
3.1. Synthesis of Compound 1
3.2. Solubilization Studies
3.3. Instrumental Characterization
3.3.1. UV–Vis Spectroscopy
3.3.2. Flash Photolysis
3.3.3. Laser Flash Photolysis
3.3.4. DLS Measurements
3.3.5. Optical Microscopy
3.4. Photochemical Measurements
3.4.1. Photostability
3.4.2. Singlet Oxygen Generation
3.5. Biological Tests
3.5.1. Reagents
3.5.2. Cell Culture
3.5.3. Intracellular Distribution Analysis
3.5.4. Cytotoxicity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Ortel, B.; Solban, N.; Pogue, B. Photodynamic therapy of cancer. Cancer Med. 2003, 7, 537–548. [Google Scholar]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment-an update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Zellweger, M.; Wagnières, G.; Van Den Bergh, H.; Cook, S.; Giraud, M.N. Photodynamic therapy for the treatment of atherosclerotic plaque: Lost in translation? Cardiovasc. Ther. 2017, 35, e12238. [Google Scholar] [CrossRef] [Green Version]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I. Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis Photodyn. Ther. 2020, 29, 101568. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Gilaberte, Y.; Rezusta, A.; Juarranz, A.; Hamblin, M.R. Antimicrobial photodynamic therapy: A new paradigm in the fight against infections. Front. Med. 2021, 8, 788888. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy–what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy—current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Garland, M.J.; Cassidy, C.M.; Woolfson, D.; Donnelly, R.F. Designing photosensitizers for photodynamic therapy: Strategies, challenges and promising developments. Future Med. Chem. 2009, 1, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Gushchina, O.I.; Larkina, E.A.; Mironov, A.F. Synthesis of Cationic Derivatives of Chlorin e6. Macroheterocycles 2014, 7, 414–416. [Google Scholar] [CrossRef] [Green Version]
- Pylina, Y.I.; Khudyaeva, I.S.; Startseva, O.M.; Shadrin, D.M.; Shevchenko, O.G.; Velegzhaninov, I.O.; Kukushkina, N.V.; Berezin, D.B.; Belykh, D.V. Dark and photoinduced cytotoxicity of cationic chlorin e6 derivatives with different numbers of charged groups. Macroheterocycles 2021, 14, 317–322. [Google Scholar] [CrossRef]
- Batov, D.V.; Kustov, A.V.; Kruchin, S.O.; Makarov, V.V.; Berezin, D.B. Aggregation of cationic chlorin e6 derivatives in water and aqueous solutions of polyvinilpyrrolidone. J. Struct. Chem. 2019, 60, 443–448. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Huang, Y.Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive cationic functionalization of chlorin derivatives for antimicrobial photodynamic inactivation and related vancomycin conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef]
- Gradova, M.A.; Movchan, T.G.; Khudyaeva, I.S.; Chernyad’ev, A.Y.; Plotnikova, E.V.; Lobanov, A.V.; Belykh, D.V. Synthesis of the Novel Cationic Chlorin Derivatives with a Phytol Fragment on the Periphery of the Macrocycle and Their Aggregation State in Aqueous Surfactant Solutions. Macroheterocycles 2020, 13, 23–32. [Google Scholar] [CrossRef]
- Mironov, A.F.; Zhdanova, K.A.; Bragina, N.A. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859–881. [Google Scholar] [CrossRef]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, A.; Nabi, B.; Kotta, S.; Narang, J.K.; Baboota, S.; Ali, J. Role of nanocarriers in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 30, 101782. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, L. Functional polymer nanocarriers for photodynamic therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.S.D.; Abrahamse, H. Recent advances in the development of biocompatible nanocarriers and their cancer cell targeting efficiency in photodynamic therapy. Front. Chem. 2022, 10, 969809. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Nanocarriers in photodynamic therapy—in vitro and in vivo studies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1509. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Abrahamse, H. Biocompatible Nanocarriers for Enhanced Cancer Photodynamic Therapy Applications. Pharmaceutics 2021, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Berezin, D.B.; Kustov, A.V.; Krest’yaninov, M.A.; Shukhto, O.V.; Batov, D.V.; Kukushkina, N.V. The behavior of monocationic chlorin in water and aqueous solutions of non-ionic surfactant Tween-80 and potassium iodide. J. Mol. Liq. 2019, 283, 532–536. [Google Scholar] [CrossRef]
- Kustov, A.V.; Krestyaninov, M.A.; Kruchin, S.O.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Khudyaeva, I.S.; Koifman, M.O.; Razgovorov, P.B.; Berezin, D.B. Interaction of cationic chlorin photosensitizers with non-ionic surfactant Tween 80. Mendeleev Commun. 2021, 31, 65–67. [Google Scholar] [CrossRef]
- Krasnovsky, A.A.; Benditkis, A.S.; Kozlov, A.S. Kinetic measurements of singlet oxygen phosphorescence in hydrogen-free solvents by time-resolved photon counting. Biochemistry 2019, 84, 153–163. [Google Scholar] [CrossRef]
- Wu, H.; Song, Q.; Ran, G.; Lu, X.; Xu, B. Recent developments in the detection of singlet oxygen with molecular spectroscopic methods. Trends Anal. Chem. 2011, 30, 133–141. [Google Scholar] [CrossRef]
- You, Y. Chemical tools for the generation and detection of singlet oxygen. Org. Biomol. Chem. 2018, 16, 4044–4060. [Google Scholar] [CrossRef]
- Isakau, H.A.; Parkhats, M.V.; Knyukshto, V.N.; Dzhagarov, B.M.; Petrov, E.P.; Petrov, P.T. Toward understanding the high PDT efficacy of chlorin e6–polyvinylpyrrolidone formulations: Photophysical and molecular aspects of photosensitizer–polymer interaction in vitro. J. Photochem. Photobiol. B Biol. 2008, 92, 165–174. [Google Scholar] [CrossRef]
- Kustov, A.V.; Morshnev, P.K.; Kukushkina, N.Y.V.; Smirnova, N.L.; Berezin, D.B.; Karimov, D.R.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Malshakova, M.V.; et al. Solvation, cancer cell photoinactivation and the interaction of chlorin photosensitizers with a potential passive carrier non-ionic surfactant Tween 80. Int. J. Mol. Sci. 2022, 23, 5294. [Google Scholar] [CrossRef] [PubMed]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical intermediates in photoinduced reactions on TiO2 (an EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, E.W.; Demas, J.N.; DeGraff, B.A. Viscosity and Temperature Effects on the Rate of Oxygen Quenching of Tris-(2, 2′-bipyridine) ruthenium (II). J. Fluoresc. 2013, 23, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Militello, M.P.; Hernández-Ramírez, R.E.; Lijanova, I.V.; Previtali, C.M.; Bertolotti, S.G.; Arbeloa, E.M. Novel PAMAM dendrimers with porphyrin core as potential photosensitizers for PDT applications. J. Photochem. Photobiol. A: Chem. 2018, 353, 71–76. [Google Scholar] [CrossRef]
- Kustov, A.V.; Berezin, D.B.; Kruchin, S.O.; Batov, D.V. Interaction of macrocyclic dicationic photosensitizers with Tween-80. Russ. J. Phys. Chem. A 2022, 96, 793–799. [Google Scholar] [CrossRef]
- Berezin, D.B.; Solodukhin, T.N.; Shukhto, O.V.; Belykh, D.V.; Startseva, O.M.; Khudyaeva, I.S.; Kustov, A.V. Association of hydrophilic derivatives of chlorophyll a in ethanol–water and ethanol–water–solubilizer systems. Russ. Chem. Bull. 2018, 67, 1273–1279. [Google Scholar] [CrossRef]
- Kumar, B.P.; Fothergill, J.; Bretherton, J.; Tian, L.; Patil, A.J.; Davis, S.A.; Mann, S. Chloroplast-containing coacervate micro-droplets as a step towards photosynthetically active membrane-free protocells. Chem. Commun. 2018, 54, 3594–3597. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Qiao, Y. Engineering coacervate droplets towards the building of multiplex biomimetic protocells. Supramol. Mater. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Gurevich, L. Drug delivery with polymeric nanocarriers—cellular uptake mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Wang, X.; Su, X.; Liu, Q. Involvement of mitochondrial and reactive oxygen species in the sonodynamic toxicity of chlorin e6 in human leukemia K562 cells. Ultrasound Med. Biol. 2014, 40, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Álvarez, C.; Aragon-Martinez, O.H. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar] [CrossRef]
- Xiao, H.; Zhu, B.; Wang, D.; Pang, Y.; He, L.; Ma, X.; Wang, R.; Jin, C.; Chen, Y.; Zhu, X. Photodynamic effects of chlorin e6 attached to single wall carbon nanotubes through noncovalent interactions. Carbon 2012, 50, 1681–1689. [Google Scholar] [CrossRef]
- Tarabukina, I.S.; Startseva, O.M.; Patov, S.A.; Belykh, D.V. Novel dicationic chlorin e6 derivatives. Macroheterocycles 2015, 8, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Venediktov, E.A.; Tulikova, E.Y.; Rozhkova, E.P.; Khudyaeva, I.S.; Belykh, D.V.; Berezin, D.B. Synthesis, spectral, luminescence and photochemical properties of the chlorin e6 tricationic derivative with trimethylammonio groups. Macroheterocycles 2017, 10, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, N.A.; Gretsova, N.S.; Kalmykova, E.A.; Makarova, E.A.; Dashkevich, S.N.; Negrimovsky, V.M.; Lukyanets, E.A. Structure-photochemical properties relationship for porphyrins and related compounds. Russ. J. Gen. Chem. 2000, 70, 140–148. [Google Scholar]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent effects on the photochemical and fluorescence properties of zinc phthalocyanine derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 6, 469–471. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradova, M.A.; Gradov, O.V.; Lobanov, A.V.; Bychkova, A.V.; Nikolskaya, E.D.; Yabbarov, N.G.; Mollaeva, M.R.; Egorov, A.E.; Kostyukov, A.A.; Kuzmin, V.A.; et al. Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications. Int. J. Mol. Sci. 2023, 24, 345. https://doi.org/10.3390/ijms24010345
Gradova MA, Gradov OV, Lobanov AV, Bychkova AV, Nikolskaya ED, Yabbarov NG, Mollaeva MR, Egorov AE, Kostyukov AA, Kuzmin VA, et al. Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications. International Journal of Molecular Sciences. 2023; 24(1):345. https://doi.org/10.3390/ijms24010345
Chicago/Turabian StyleGradova, Margarita A., Oleg V. Gradov, Anton V. Lobanov, Anna V. Bychkova, Elena D. Nikolskaya, Nikita G. Yabbarov, Mariia R. Mollaeva, Anton E. Egorov, Alexey A. Kostyukov, Vladimir A. Kuzmin, and et al. 2023. "Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications" International Journal of Molecular Sciences 24, no. 1: 345. https://doi.org/10.3390/ijms24010345





