Ethyl Gallate Inhibits Bovine Viral Diarrhea Virus by Promoting IFITM3 Expression, Lysosomal Acidification and Protease Activity
Abstract
:1. Introduction
2. Results
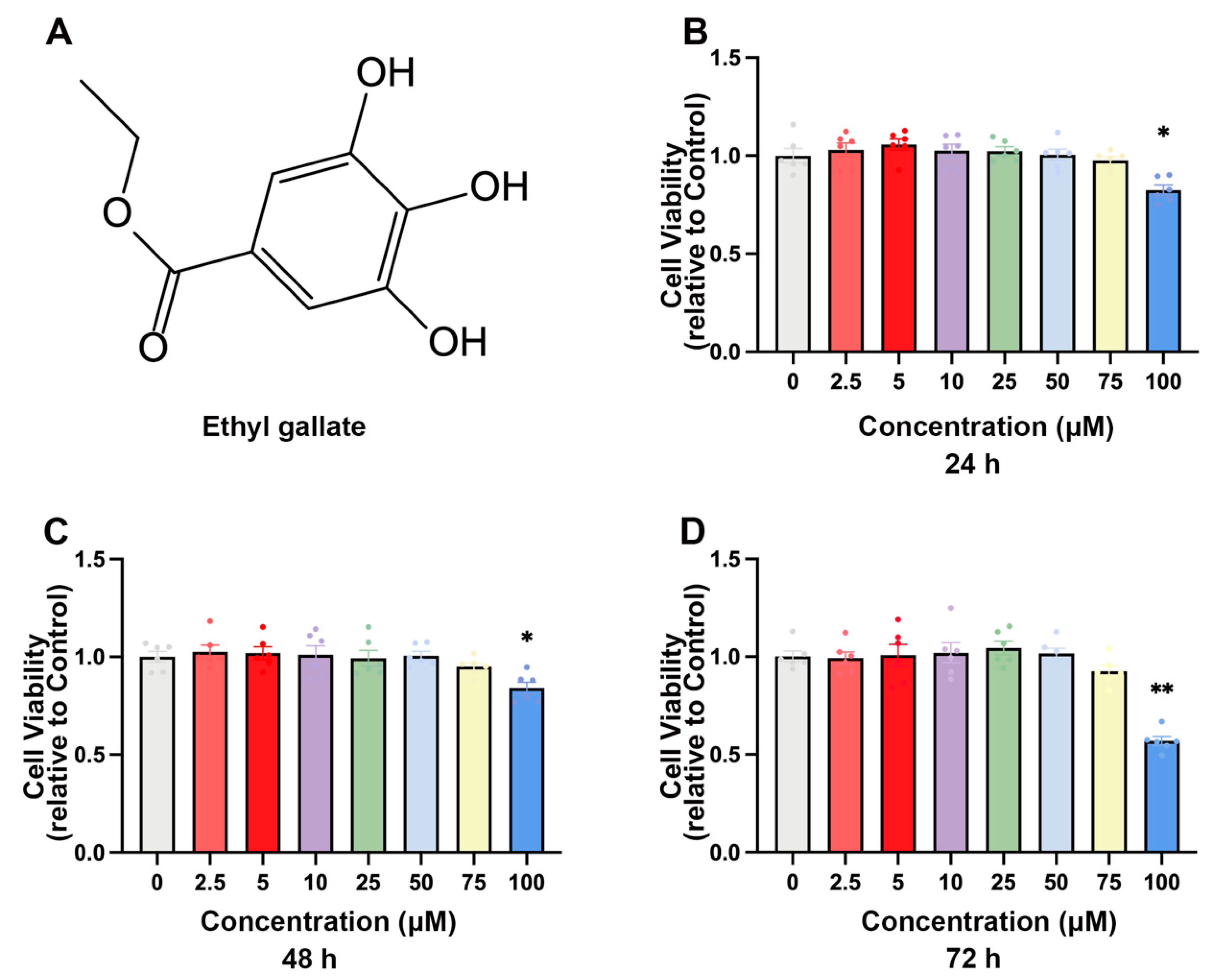
2.1. EG Inhibits BVDV Infection in MDBK Cells
2.2. EG Inhibits BVDV Entry and Replication of Viral Life Cycle
2.3. EG Promotes the Production of IFITM3 in MDBK Cells Infected with BVDV
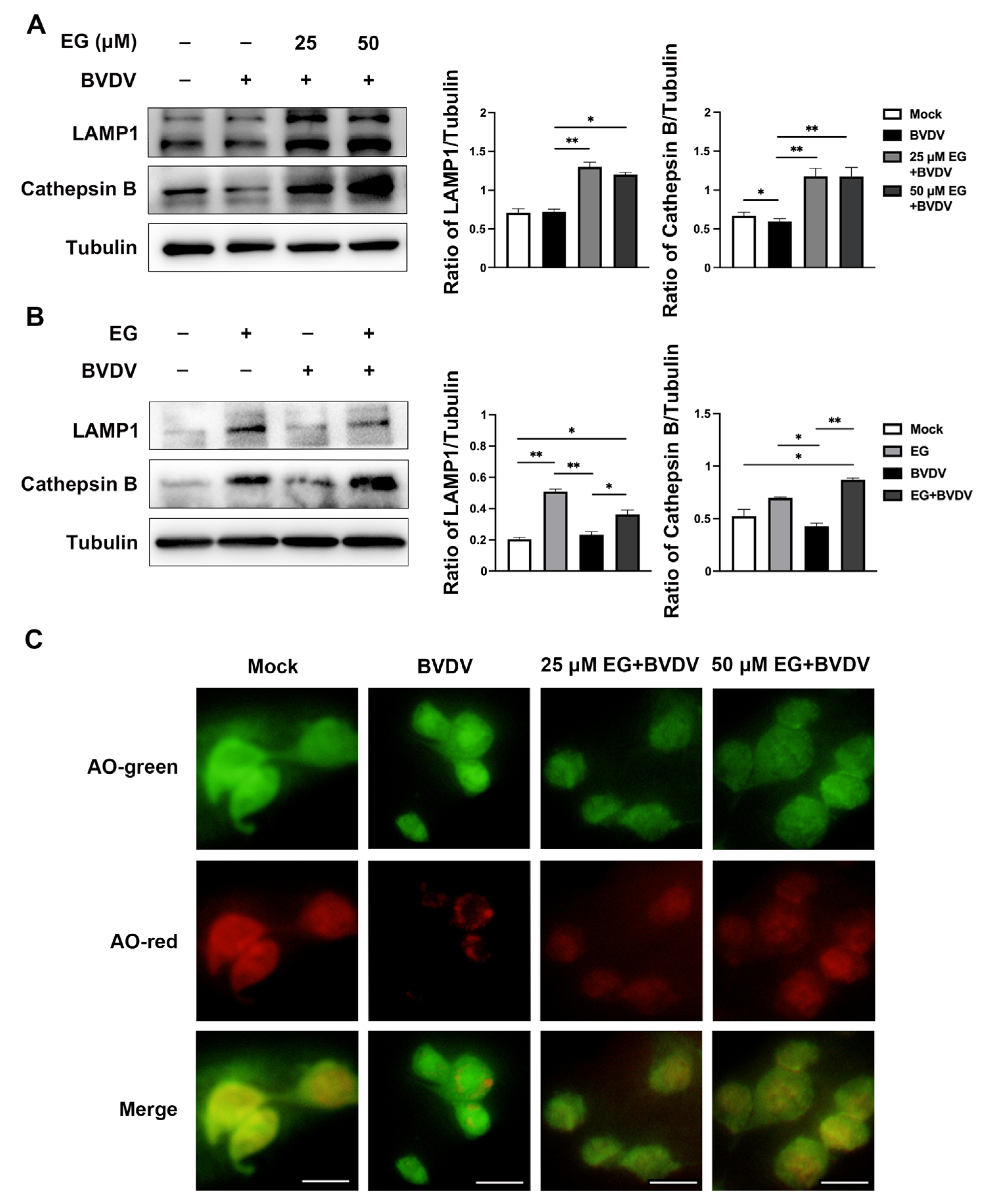
2.4. EG Promotes Lysosomal Acidification and Protease Activity to Prevent BVDV Infection
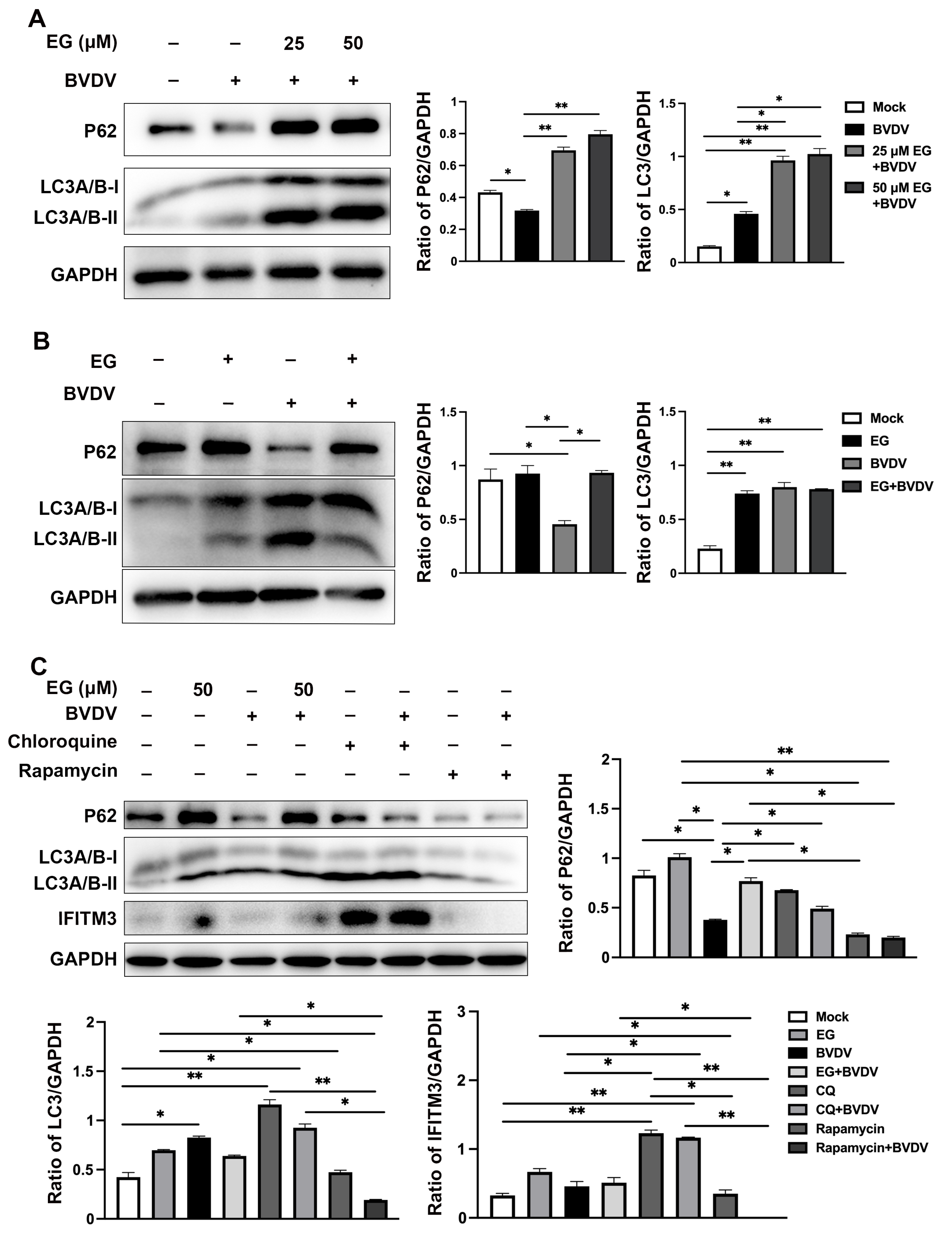
2.5. EG Limited Replication of BVDV by Interfering with Autophagy In Vitro
3. Discussion
4. Materials and Methods
4.1. Reagent and Antibodies
4.2. Cell Culture and Viruses
4.3. The Effect of EG Treatment at Different Times
4.4. Virus Replication Cycle Assay
4.5. Cytotoxicity Assay
4.6. Quantitative Reverse Transcription-PCR (qRT-PCR)
4.7. Western Blotting
4.8. Immunofluorescence Assay (IFA)
4.9. Acridine Orange Staining
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Huang, J.; Zhao, Z.; Yuan, X.; Li, C.; Liu, S.; Cui, Y.; Liu, Y.; Zhou, Y.; Zhu, Z. In Vivo and In Vitro Antiviral Activity of Phlorizin against Bovine Viral Diarrhea Virus. J. Agric. Food Chem. 2022, 70, 14841–14850. [Google Scholar] [CrossRef]
- Kučer, N.; Marković, E.; Prpić, J.; Rudan, D.; Jemeršić, L.; Hajek, Z.; Škaro, K.; Pavlak, M.; Rudan, N. The epidemiology of bovine viral diarrhea virus infection on a dairy farm—Clinical signs, seroprevalence, virus detection and genotyping. Vet. Arh. 2022, 92, 119–126. [Google Scholar] [CrossRef]
- Ridpath, J.F. Bovine viral diarrhea virus: Global status. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 105–121. [Google Scholar] [CrossRef]
- Casey-Bryars, M.; Tratalos, J.A.; Graham, D.A.; Guelbenzu-Gonzalo, M.P.; Barrett, D.; O’Grady, L.; Madden, J.M.; McGrath, G.; More, S.J. Risk factors for detection of bovine viral diarrhoea virus in low-risk herds during the latter stages of Ireland’s eradication programme. Prev. Vet. Med. 2022, 201, 105607. [Google Scholar] [CrossRef]
- Akagami, M.; Seki, S.; Kashima, Y.; Yamashita, K.; Oya, S.; Fujii, Y.; Takayasu, M.; Yaguchi, Y.; Suzuki, A.; Ono, Y.; et al. Risk factors associated with the within-farm transmission of bovine viral diarrhea virus and the incidence of persistently infected cattle on dairy farms from Ibaraki prefecture of Japan. Res. Vet. Sci. 2020, 129, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.L.; Manly, S.P.; McMahon, M.; Kerr, L.M.; Stark, G.R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell 1984, 38, 745–755. [Google Scholar] [CrossRef]
- Siegrist, F.; Ebeling, M.; Certa, U. The small interferon-induced transmembrane genes and proteins. J. Interferon Cytokine Res. 2011, 31, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Feeley, E.M.; Sims, J.S.; John, S.P.; Chin, C.R.; Pertel, T.; Chen, L.M.; Gaiha, G.D.; Ryan, B.J.; Donis, R.O.; Elledge, S.J.; et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011, 7, e1002337. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, H.; Wang, Y.; Dong, W.; Liu, Y.; Zhang, L.; Zhang, Y. Antiviral Role of IFITM Proteins in Classical Swine Fever Virus Infection. Viruses 2019, 11, 126. [Google Scholar] [CrossRef]
- Tartour, K.; Nguyen, X.N.; Appourchaux, R.; Assil, S.; Barateau, V.; Bloyet, L.M.; Burlaud Gaillard, J.; Confort, M.P.; Escudero-Perez, B.; Gruffat, H.; et al. Interference with the production of infectious viral particles and bimodal inhibition of replication are broadly conserved antiviral properties of IFITMs. PLoS Pathog. 2017, 13, e1006610. [Google Scholar] [CrossRef]
- Savidis, G.; Perreira, J.M.; Portmann, J.M.; Meraner, P.; Guo, Z.; Green, S.; Brass, A.L. The IFITMs Inhibit Zika Virus Replication. Cell Rep. 2016, 15, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Schwertz, H.; Hottz, E.D.; Rowley, J.W.; Manne, B.K.; Washington, A.V.; Hunter-Mellado, R.; Tolley, N.D.; Christensen, M.; Eustes, A.S.; et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood 2019, 133, 2013–2026. [Google Scholar] [CrossRef]
- Spence, J.S.; He, R.; Hoffmann, H.H.; Das, T.; Thinon, E.; Rice, C.M.; Peng, T.; Chandran, K.; Hang, H.C. IFITM3 directly engages and shuttles incoming virus particles to lysosomes. Nat. Chem. Biol. 2019, 15, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection—A double-edged sword. Nat. Rev. Microbiol. 2018, 16, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Y.; Ou, J.H. HCV induces the expression of Rubicon and UVRAG to temporally regulate the maturation of autophagosomes and viral replication. PLoS Pathog. 2015, 11, e1004764. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhao, M.; Ye, Z.; Gou, H.; Wang, J.; Yi, L.; Dong, X.; Liu, W.; Luo, Y.; Liao, M.; et al. Autophagy enhances the replication of classical swine fever virus in vitro. Autophagy 2014, 10, 93–110. [Google Scholar] [CrossRef]
- Mehla, K.; Balwani, S.; Kulshreshtha, A.; Nandi, D.; Jaisankar, P.; Ghosh, B. Ethyl gallate isolated from Pistacia integerrima Linn. inhibits cell adhesion molecules by blocking AP-1 transcription factor. J. Ethnopharmacol. 2011, 137, 1345–1352. [Google Scholar] [CrossRef]
- Kim, W.H.; Song, H.O.; Choi, H.J.; Bang, H.I.; Choi, D.Y.; Park, H. Ethyl gallate induces apoptosis of HL-60 cells by promoting the expression of caspases-8, -9, -3, apoptosis-inducing factor and endonuclease G. Int. J. Mol. Sci. 2012, 13, 11912–11922. [Google Scholar] [CrossRef]
- da Silva, J.H.S.; Simas, N.K.; Alviano, C.S.; Alviano, D.S.; Ventura, J.A.; de Lima, E.J.; Seabra, S.H.; Kuster, R.M. Anti-Escherichia coli activity of extracts from Schinus terebinthifolius fruits and leaves. Nat. Prod. Res. 2018, 32, 1365–1368. [Google Scholar] [CrossRef]
- Huan, C.; Xu, W.; Ni, B.; Guo, T.; Pan, H.; Jiang, L.; Li, L.; Yao, J.; Gao, S. Epigallocatechin-3-Gallate, the Main Polyphenol in Green Tea, Inhibits Porcine Epidemic Diarrhea Virus In Vitro. Front. Pharmacol. 2021, 12, 628526. [Google Scholar] [CrossRef]
- Narayana, S.K.; Helbig, K.J.; McCartney, E.M.; Eyre, N.S.; Bull, R.A.; Eltahla, A.; Lloyd, A.R.; Beard, M.R. The Interferon-induced Transmembrane Proteins, IFITM1, IFITM2, and IFITM3 Inhibit Hepatitis C Virus Entry. J. Biol. Chem. 2015, 290, 25946–25959. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, F.; Karsten, C.B.; Gnirss, K.; Hoffmann, M.; Lu, K.; Takada, A.; Winkler, M.; Simmons, G.; Pohlmann, S. Interferon-Induced Transmembrane Protein-Mediated Inhibition of Host Cell Entry of Ebolaviruses. J. Infect. Dis. 2015, 212 (Suppl. S2), S210–S218. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Huang, I.C.; Farzan, M. IFITM proteins restrict antibody-dependent enhancement of dengue virus infection. PLoS ONE 2012, 7, e34508. [Google Scholar] [CrossRef]
- Desai, T.M.; Marin, M.; Chin, C.R.; Savidis, G.; Brass, A.L.; Melikyan, G.B. IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog. 2014, 10, e1004048. [Google Scholar] [CrossRef]
- Lu, G.; Ou, J.; Cai, S.; Lai, Z.; Zhong, L.; Yin, X.; Li, S. Canine Interferon-Inducible Transmembrane Protein Is a Host Restriction Factor That Potently Inhibits Replication of Emerging Canine Influenza Virus. Front. Immunol. 2021, 12, 710705. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Rice, C.M. Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 2015, 36, 124–138. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Acharya, B.; Gyeltshen, S.; Chaijaroenkul, W.; Na-Bangchang, K. Significance of Autophagy in Dengue Virus Infection: A Brief Review. Am. J. Trop. Med. Hyg. 2019, 100, 783–790. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Zhang, L.; Zhang, J.; Wang, J.; Zou, Y.; Wang, J. Berbamine hydrochloride inhibits bovine viral diarrhea virus replication via interfering in late-stage autophagy. Virus Res. 2022, 321, 198905. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.Y.; Wang, L.; Song, Y.Y.; Gu, J.; Hu, X.; Yuan, C.; Yang, M.; Pei, W.J.; Zhang, Y.; Gao, J.L. Sidt2 is a key protein in the autophagy-lysosomal degradation pathway and is essential for the maintenance of kidney structure and filtration function. Cell Death Dis. 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.; Kim, M.; Ahn, J.; Oh, H.; Lim, J.; Chae, W.; Yang, S.W.; Kim, M.S.; Yu, J.E.; Byun, S.; et al. Epigallocatechin-3-Gallate as a Novel Vaccine Adjuvant. Front. Immunol. 2021, 12, 769088. [Google Scholar] [CrossRef]
- Yu, P.; Fu, P.; Zeng, L.; Qi, Y.; Li, X.; Wang, Q.; Yang, G.; Li, H.; Wang, J.; Chu, B.; et al. EGCG Restricts PRRSV Proliferation by Disturbing Lipid Metabolism. Microbiol. Spectr. 2022, 10, e02276-21. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, J.; Wang, S.; Wang, J.; Wang, J.; Zhu, Y.; Wang, J. Gypenoside Inhibits Bovine Viral Diarrhea Virus Replication by Interfering with Viral Attachment and Internalization and Activating Apoptosis of Infected Cells. Viruses 2021, 13, 1810. [Google Scholar] [CrossRef]
- Sallman Almen, M.; Bringeland, N.; Fredriksson, R.; Schioth, H.B. The dispanins: A novel gene family of ancient origin that contains 14 human members. PLoS ONE 2012, 7, e31961. [Google Scholar] [CrossRef]
- Zhao, X.; Sehgal, M.; Hou, Z.; Cheng, J.; Shu, S.; Wu, S.; Guo, F.; Le Marchand, S.J.; Lin, H.; Chang, J.; et al. Identification of Residues Controlling Restriction versus Enhancing Activities of IFITM Proteins on Entry of Human Coronaviruses. J. Virol. 2018, 92, e01535-17. [Google Scholar] [CrossRef]
- Bailey, C.C.; Zhong, G.; Huang, I.C.; Farzan, M. IFITM-Family Proteins: The Cell’s First Line of Antiviral Defense. Annu. Rev. Virol. 2014, 1, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Teramoto, T.; Kulkarni, A.A.; Bhattacharjee, A.K.; Padmanabhan, R. Antiviral activities of selected antimalarials against dengue virus type 2 and Zika virus. Antivir. Res. 2017, 137, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Moncorge, O.; Bonaventure, B.; Pollpeter, D.; Lussignol, M.; Tauziet, M.; Apolonia, L.; Catanese, M.T.; Goujon, C.; Malim, M.H. The interferon-inducible isoform of NCOA7 inhibits endosome-mediated viral entry. Nat. Microbiol. 2018, 3, 1369–1376. [Google Scholar] [CrossRef]
- Rajput, M.K.S.; Abdelsalam, K.; Darweesh, M.F.; Braun, L.J.; Kerkvliet, J.; Hoppe, A.D.; Chase, C.C.L. Both cytopathic and non-cytopathic bovine viral diarrhea virus (BVDV) induced autophagy at a similar rate. Vet. Immunol. Immunopathol. 2017, 193–194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Ozog, S.; Torbett, B.E.; Compton, A.A. mTOR inhibitors lower an intrinsic barrier to virus infection mediated by IFITM3. Proc. Natl. Acad. Sci. USA 2018, 115, E10069–E10078. [Google Scholar] [CrossRef] [PubMed]
- McMichael, T.M.; Chemudupati, M.; Yount, J.S. A balancing act between IFITM3 and IRF3. Cell. Mol. Immunol. 2018, 15, 873–874. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, G.; Wang, J.; Zhang, J.; Chen, K.; Xiong, X.; Zhu, Y.; Xu, C.; Wang, J. Ethyl Gallate Inhibits Bovine Viral Diarrhea Virus by Promoting IFITM3 Expression, Lysosomal Acidification and Protease Activity. Int. J. Mol. Sci. 2023, 24, 8637. https://doi.org/10.3390/ijms24108637
Zhang L, Yang G, Wang J, Zhang J, Chen K, Xiong X, Zhu Y, Xu C, Wang J. Ethyl Gallate Inhibits Bovine Viral Diarrhea Virus by Promoting IFITM3 Expression, Lysosomal Acidification and Protease Activity. International Journal of Molecular Sciences. 2023; 24(10):8637. https://doi.org/10.3390/ijms24108637
Chicago/Turabian StyleZhang, Linlin, Guanghui Yang, Jun Wang, Jialu Zhang, Keyuan Chen, Xiaoran Xiong, Yaohong Zhu, Chuang Xu, and Jiufeng Wang. 2023. "Ethyl Gallate Inhibits Bovine Viral Diarrhea Virus by Promoting IFITM3 Expression, Lysosomal Acidification and Protease Activity" International Journal of Molecular Sciences 24, no. 10: 8637. https://doi.org/10.3390/ijms24108637




