Phylogenetic and Evolutionary Comparison of Mitogenomes Reveal Adaptive Radiation of Lampriform Fishes
Abstract
:1. Introduction
2. Results
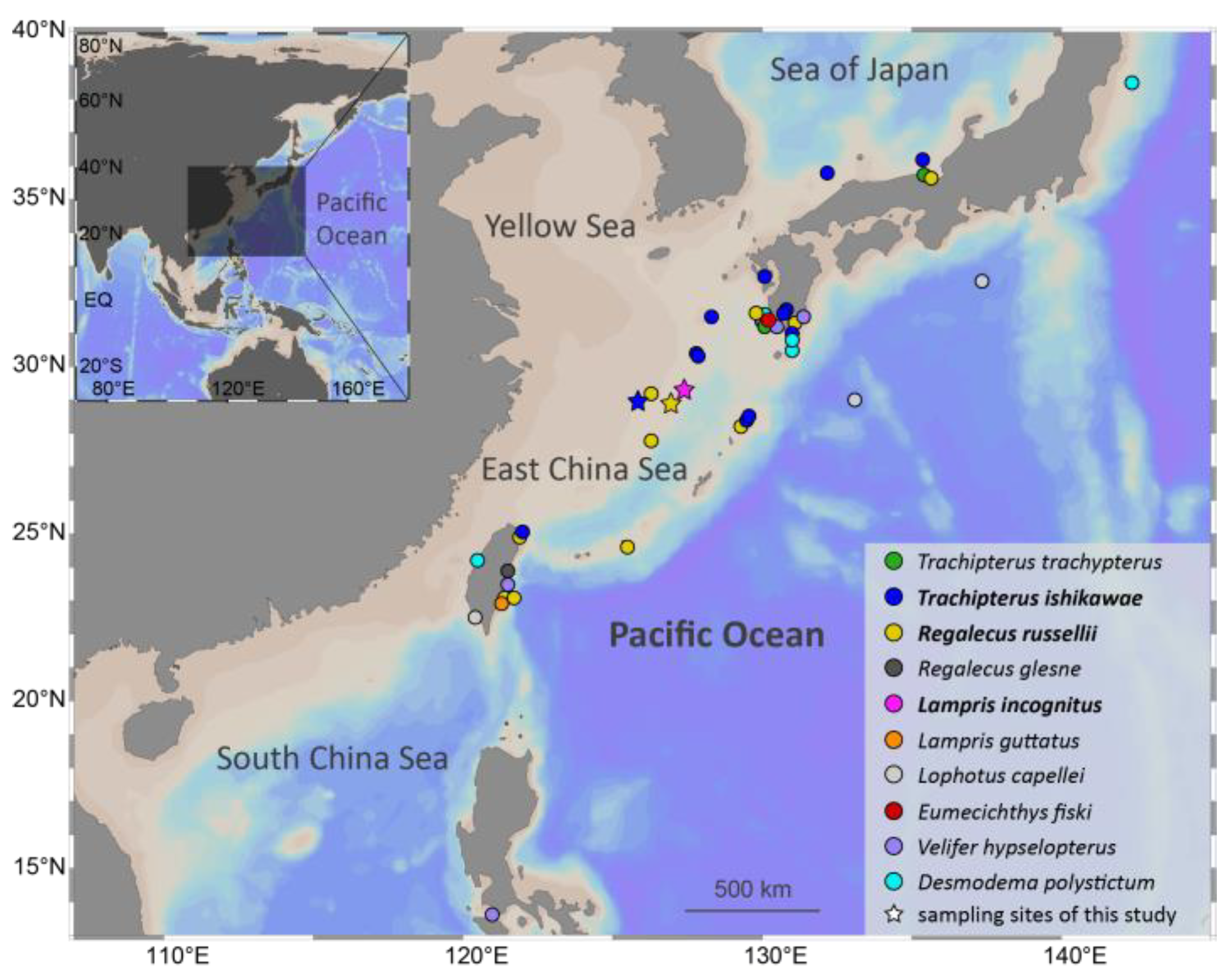
2.1. Phylogeny of Lampriformes
2.2. Mitogenome Organization and tRNA Loss of Lampriformes
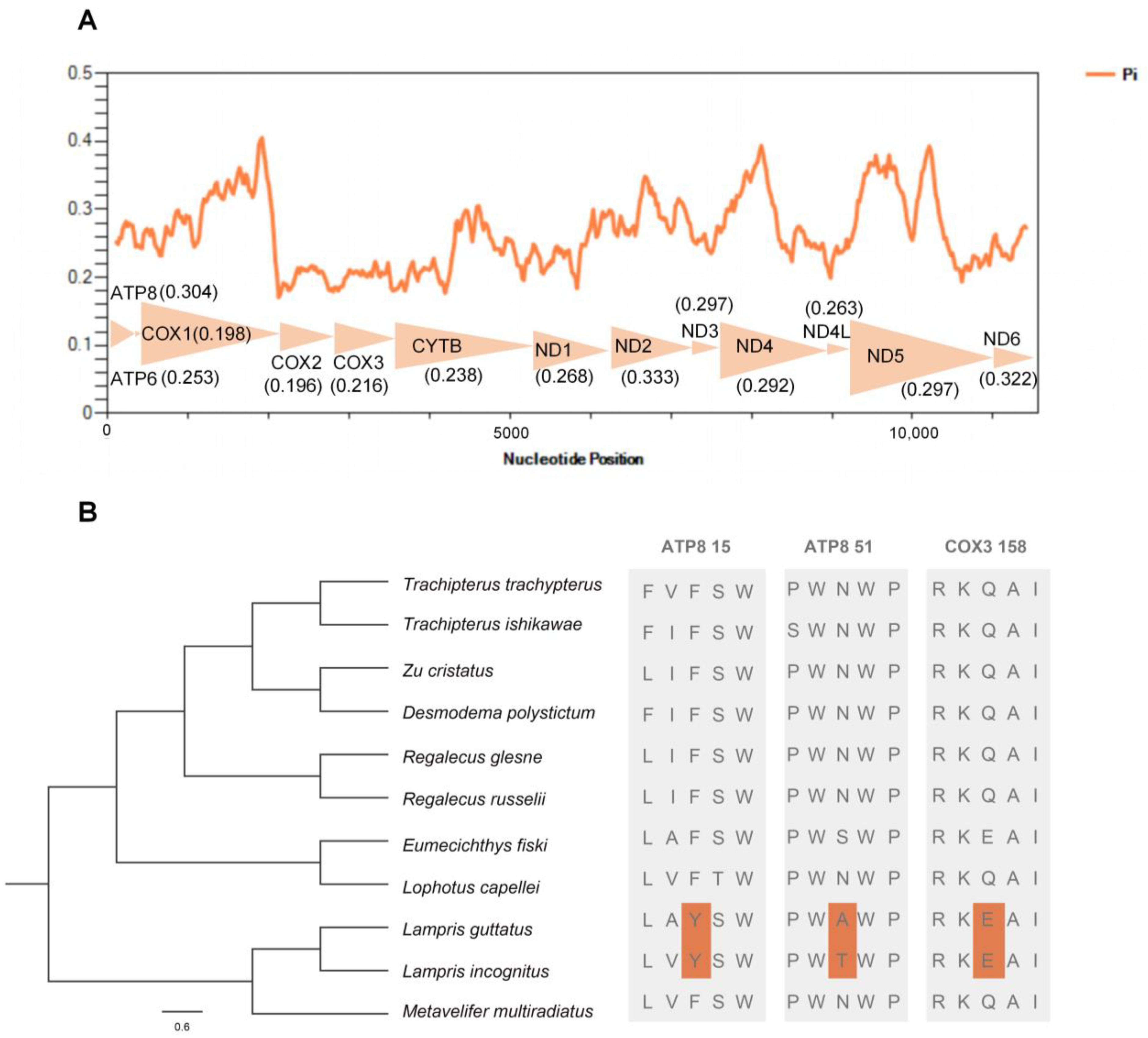
2.3. Nucleotide Diversity and Positive Selection Analysis
3. Discussion
3.1. Lampriformes Phylogeny
3.2. Mitogenomic Basis of Adaptive Radiation of Lampriformes
3.3. Adaptive Evolution of Mitochondrial Genes in Whole-Body Endothermic Opah
4. Materials and Methods
4.1. Sample Acquisition, Tissue Collection, and DNA Extraction
4.2. Library Construction and High-Throughput Sequencing
4.3. Sequence Assembly and Annotation
4.4. Phylogenetic Analyses
4.5. Bioinformatics Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhang, Y.H.; Zhang, H.X.; Qin, G.; Lin, Q. Complete mitochondrial genomes of eight seahorses and pipefishes (Syngnathiformes: Syngnathidae): Insight into the adaptive radiation of syngnathid fishes. BMC Evol. Biol. 2019, 19, 119. [Google Scholar] [CrossRef]
- Jia, W.Z.; Yan, H.B.; Guo, A.J.; Zhu, X.Q.; Wang, Y.C.; Shi, W.G.; Chen, H.T.; Zhan, F.; Zhang, S.H.; Fu, B.Q.; et al. Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: Additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genom. 2010, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Liu, F.F.; Chiba, H.; Yuan, X.Q. The mitochondrial genomes of three skippers: Insights into the evolution of the family Hesperiidae (Lepidoptera). Genomics 2020, 112, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Shi, W.; Si, L.Z.; Kong, X.Y. Rearrangement of mitochondrial genome in fishes. Dongwuxue Yanjiu 2013, 34, 666–673. [Google Scholar] [PubMed]
- Zhang, K.; Zhu, K.H.; Liu, Y.F.; Zhang, H.; Gong, L.; Jiang, L.H.; Liu, L.Q.; Lu, Z.M.; Liu, B.J. Novel gene rearrangement in the mitochondrial genome of Muraenesox cinereus and the phylogenetic relationship of Anguilliformes. Sci. Rep. 2021, 11, 2411. [Google Scholar] [CrossRef]
- Dorner, M.; Altmann, M.; Paabo, S.; Morl, M. Evidence for import of a lysyl-tRNA into marsupial mitochondria. Mol. Biol. Cell 2001, 12, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.L.; Nie, L.W.; Pu, Y.G. Complete mitochondrial genome of Chinese big-headed turtle, Platysternon megacephalum, with a novel gene organization in vertebrate mtDNA. Gene 2006, 380, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Salinas, T.; Duchene, A.M.; Marechal-Drouard, L. Recent advances in tRNA mitochondrial import. Trends Biochem. Sci. 2008, 33, 320–329. [Google Scholar] [CrossRef]
- Nicholls, D.G. Power, sex, suicide—Mitochondria and the meaning of life. Science 2006, 311, 1869. [Google Scholar] [CrossRef]
- da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 2008, 9, 119. [Google Scholar] [CrossRef]
- Ballard, J.W.; Melvin, R.G. Linking the mitochondrial genotype to the organismal phenotype. Mol. Ecol. 2010, 19, 1523–1539. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Shi, P.; Sun, Y.B.; Zhang, Y.P. Relaxation of selective constraints on avian mitochondrial DNA following the degeneration of flight ability. Genome Res. 2009, 19, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Wegner, N.C.; Snodgrass, O.E.; Dewar, H.; Hyde, J.R. Animal physiology. Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science 2015, 348, 786–789. [Google Scholar] [CrossRef]
- Olney, J.E.; Johnson, G.D.; Baldwin, C.C. Phylogeny of lampridiform fishes. Bull. Mar. Sci. 1993, 52, 137–169. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley and Sons: New York, NY, USA, 2017; pp. 280–284. [Google Scholar] [CrossRef]
- Van Der Laan, R.; Eschmeyer, W.N.; Fricke, R. Family-group names of Recent fishes. Zootaxa 2014, 3882, 1–230. [Google Scholar] [CrossRef]
- Angulo, A.; Lopez-Sanchez, M.I. New records of lampriform fishes (Teleostei: Lampriformes) from the Pacific coast of lower Central America, with comments on the diversity, taxonomy and distribution of the Lampriformes in the eastern Pacific Ocean. Zootaxa 2017, 4236, 573–591. [Google Scholar] [CrossRef]
- Miya, M.; Holcroft, N.I.; Satoh, T.P.; Yamaguchi, M.; Nishida, M.; Wiley, E.O. Mitochondrial genome and a nuclear gene indicate a novel phylogenetic position of deep-sea tube-eye fish (Stylephoridae). Ichthyol. Res. 2007, 54, 323–332. [Google Scholar] [CrossRef]
- Near, T.J.; Dornburg, A.; Eytan, R.I.; Keck, B.P.; Smith, W.L.; Kuhn, K.L.; Moore, J.A.; Price, S.A.; Burbrink, F.T.; Friedman, M.; et al. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc. Natl. Acad. Sci. USA 2013, 110, 12738–12743. [Google Scholar] [CrossRef]
- Hughes, L.C.; Orti, G.; Huang, Y.; Sun, Y.; Baldwin, C.C.; Thompson, A.W.; Arcila, D.; Betancur-R, R.; Li, C.H.; Becker, L.; et al. Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc. Natl. Acad. Sci. USA 2018, 115, 6249–6254. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Faircloth, B.C.; Harrington, R.C.; Sorenson, L.; Friedman, M.; Thacker, C.E.; Oliveros, C.H.; Cerny, D.; Near, T.J. Explosive diversification of marine fishes at the Cretaceous-Palaeogene boundary. Nat. Ecol. Evol. 2018, 2, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Near, T.J.; Eytan, R.I.; Dornburg, A.; Kuhn, K.L.; Moore, J.A.; Davis, M.P.; Wainwright, P.C.; Friedman, M.; Smith, W.L. Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl. Acad. Sci. USA 2012, 109, 13698–13703. [Google Scholar] [CrossRef]
- Betancur, R.R.; Broughton, R.E.; Wiley, E.O.; Carpenter, K.; Lopez, J.A.; Li, C.; Holcroft, N.I.; Arcila, D.; Sanciangco, M.; Cureton Ii, J.C.; et al. The tree of life and a new classification of bony fishes. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Ghezelayagh, A.; Harrington, R.C.; Burress, E.D.; Campbell, M.A.; Buckner, J.C.; Chakrabarty, P.; Glass, J.R.; McCraney, W.T.; Unmack, P.J.; Thacker, C.E.; et al. Prolonged morphological expansion of spiny-rayed fishes following the end-Cretaceous. Nat. Ecol. Evol. 2022, 6, 1211–1220. [Google Scholar] [CrossRef]
- Poulsen, J.Y.; Byrkjedal, I.; Willassen, E.; Rees, D.; Takeshima, H.; Satoh, T.P.; Shinohara, G.; Nishida, M.; Miya, M. Mitogenomic sequences and evidence from unique gene rearrangements corroborate evolutionary relationships of myctophiformes (Neoteleostei). BMC Evol. Biol. 2013, 13, 1–22. [Google Scholar] [CrossRef]
- Chen, W.J.; Bonillo, C.; Lecointre, G. Repeatability of clades as a criterion of reliability: A case study for molecular phylogeny of Acanthomorpha (Teleostei) with larger number of taxa. Mol. Phylogenet. Evol. 2003, 26, 262–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Santini, F.; Carnevale, G.; Chen, J.N.; Liu, S.H.; Lavoue, S.; Mayden, R.L. New insights on early evolution of spiny-rayed fishes (Teleostei: Acanthomorpha). Front. Mar. Sci. 2014, 1, 53. [Google Scholar] [CrossRef]
- Albano, M.; D’Iglio, C.; Spano, N.; Fernandes, J.M.D.; Savoca, S.; Capillo, G. Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy. Biology 2022, 11, 1534. [Google Scholar] [CrossRef]
- Claverie, T.; Wainwright, P.C. A Morphospace for Reef Fishes: Elongation Is the Dominant Axis of Body Shape Evolution. PLoS ONE 2014, 9, e112732. [Google Scholar] [CrossRef]
- Zhang, J.F.; Nie, L.W.; Wang, Y.; Hu, L.L. The complete mitochondrial genome of the large-headed frog, Limnonectes bannaensis (Amphibia: Anura), and a novel gene organization in the vertebrate mtDNA (vol 442, pg 119, 2009). Gene 2009, 446, 50. [Google Scholar] [CrossRef]
- Dowling, D.K.; Friberg, U.; Lindell, J. Evolutionary implications of non-neutral mitochondrial genetic variation. Trends Ecol. Evol. 2008, 23, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.W.O.; Whitlock, M.C. The incomplete natural history of mitochondria. Mol. Ecol. 2004, 13, 729–744. [Google Scholar] [CrossRef]
- Foote, A.D.; Morin, P.A.; Durban, J.W.; Pitman, R.L.; Wade, P.; Willerslev, E.; Gilbert, M.T.P.; da Fonseca, R.R. Positive selection on the killer whale mitogenome. Biol. Lett. 2011, 7, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Dolla, A.; Blanchard, L.; Guerlesquin, F.; Bruschi, M. The Protein Moiety Modulates the Redox Potential in Cytochromes-C. Biochimie 1994, 76, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Becker, D.F. Characterization of a bifunctional PutA homologue from Bradyrhizobium japonicum and identification of an active site residue that modulates proline reduction of the flavin adenine dinucleotide cofactor. Biochemistry 2005, 44, 9130–9139. [Google Scholar] [CrossRef]
- Park, J.S.; Ohmura, T.; Katayama, A.; Sagara, M.; Niki, K.; Cusanovich, M.A.; Akutsu, H. A comparison of the redox potentials of cytochrome c (3) from Desulfovibrio vulgaris Hildenborough with those from Desulfovibrio vulgaris Miyazaki F—Effects of amino acid substitutions on the redox potentials. J. Electroanal. Chem. 1997, 438, 231–236. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, Z. DAMBE: Software package for data analysis in molecular biology and evolution. J. Hered. 2001, 92, 371–373. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchene, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kuhnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Allen, J.M.; LaFrance, R.; Folk, R.A.; Johnson, K.P.; Guralnick, R.P. aTRAM 2.0: An Improved, Flexible Locus Assembler for NGS Data. Evol. Bioinform. 2018, 14, 1176934318774546. [Google Scholar] [CrossRef]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]



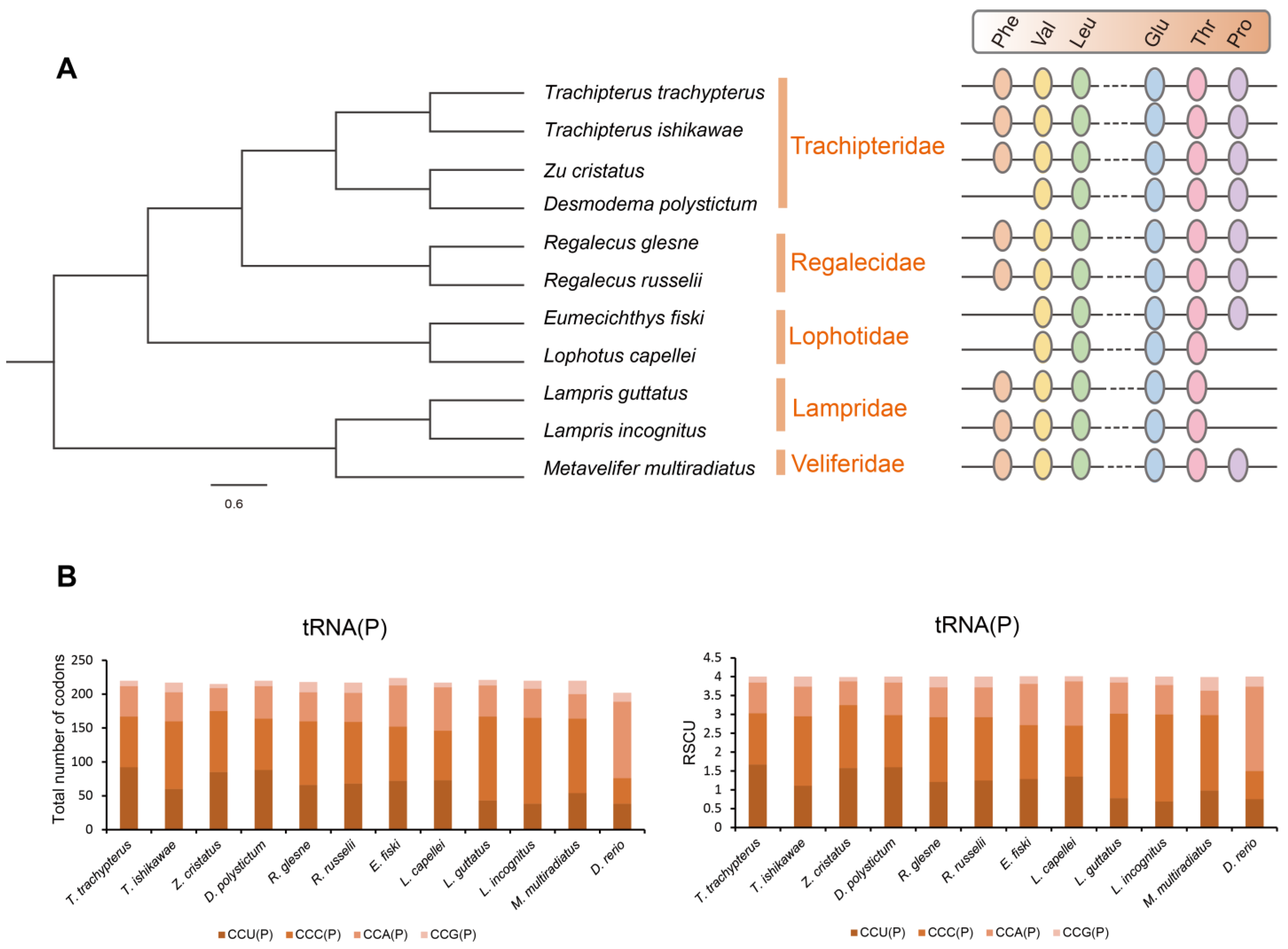
| Taxon | Species | Number | Lost (−) tRNAs | Accession |
|---|---|---|---|---|
| Chionodraco myersi | 21 | −E | NC_010689 | |
| Lampris incognitus | 21 | −P | OP979106 | |
| Osteichthyes | Lampris guttatus | 21 | −P | NC_003165 |
| Desmodema ploystictum | 21 | −F | AP012969 | |
| Eumecichthys fiski | 21 | −F | AP012970 | |
| Lophotus capellei | 20 | −(F and P) | AP012971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-f.; Yu, H.-y.; Ma, S.-b.; Lin, Q.; Wang, D.-z.; Wang, X. Phylogenetic and Evolutionary Comparison of Mitogenomes Reveal Adaptive Radiation of Lampriform Fishes. Int. J. Mol. Sci. 2023, 24, 8756. https://doi.org/10.3390/ijms24108756
Wang J-f, Yu H-y, Ma S-b, Lin Q, Wang D-z, Wang X. Phylogenetic and Evolutionary Comparison of Mitogenomes Reveal Adaptive Radiation of Lampriform Fishes. International Journal of Molecular Sciences. 2023; 24(10):8756. https://doi.org/10.3390/ijms24108756
Chicago/Turabian StyleWang, Jin-fang, Hai-yan Yu, Shao-bo Ma, Qiang Lin, Da-zhi Wang, and Xin Wang. 2023. "Phylogenetic and Evolutionary Comparison of Mitogenomes Reveal Adaptive Radiation of Lampriform Fishes" International Journal of Molecular Sciences 24, no. 10: 8756. https://doi.org/10.3390/ijms24108756





