Hypolipidemic and Anti-Inflammatory Effects of Curcuma longa-Derived Bisacurone in High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Results
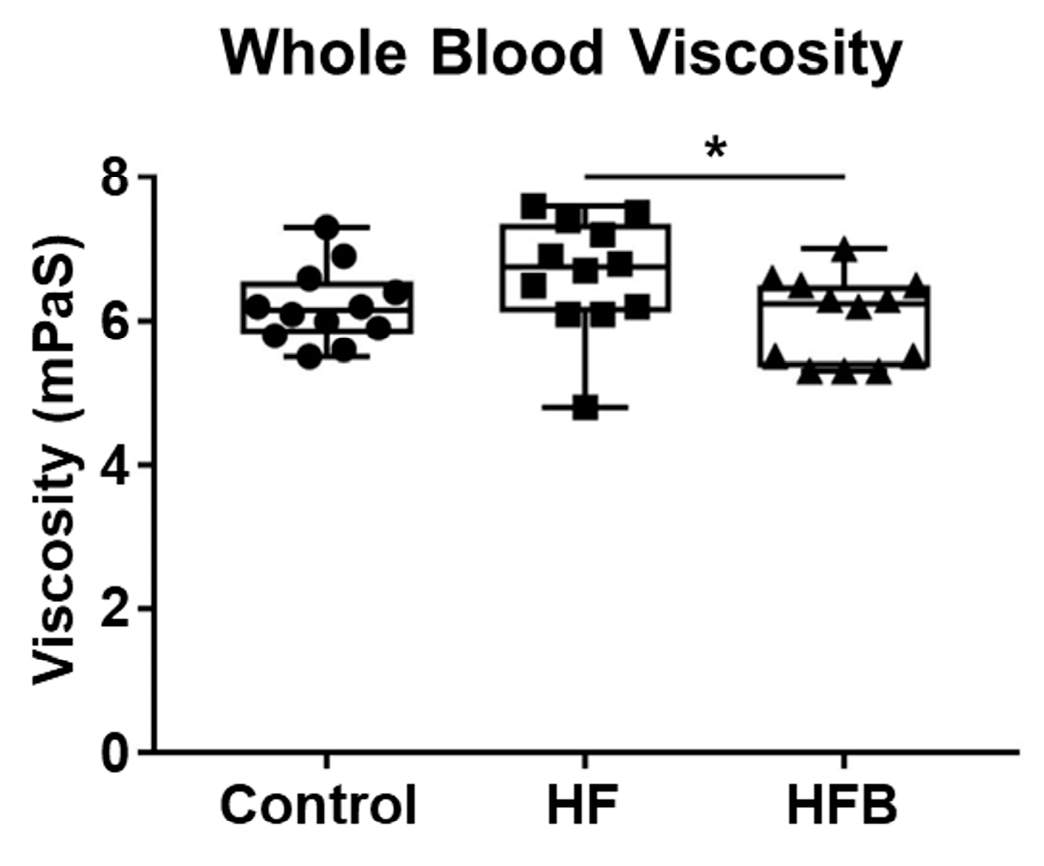
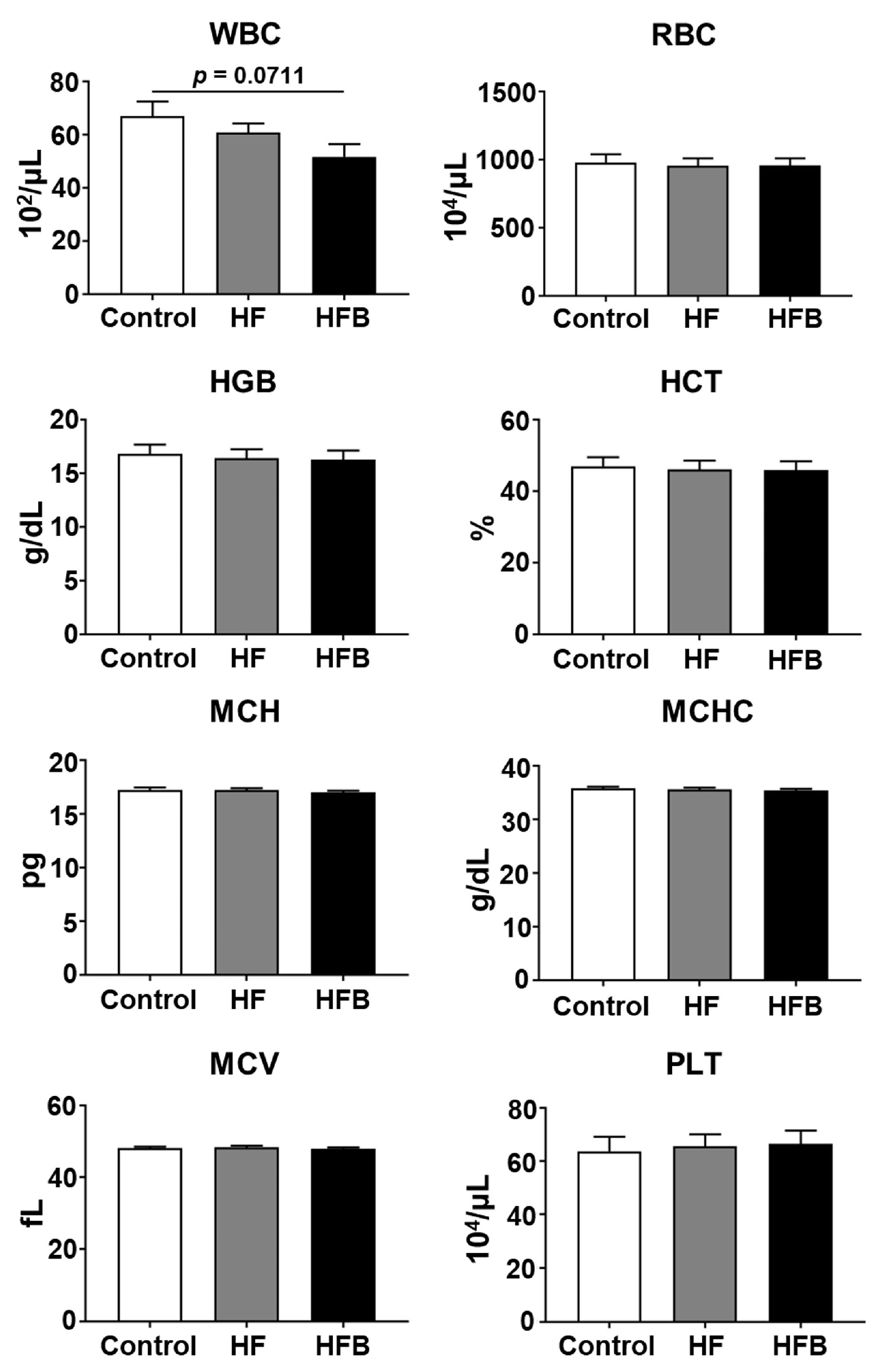
2.1. Bisacurone Reduces Serum Levels of Lipids and Blood Viscosity in HFD-Fed Mice
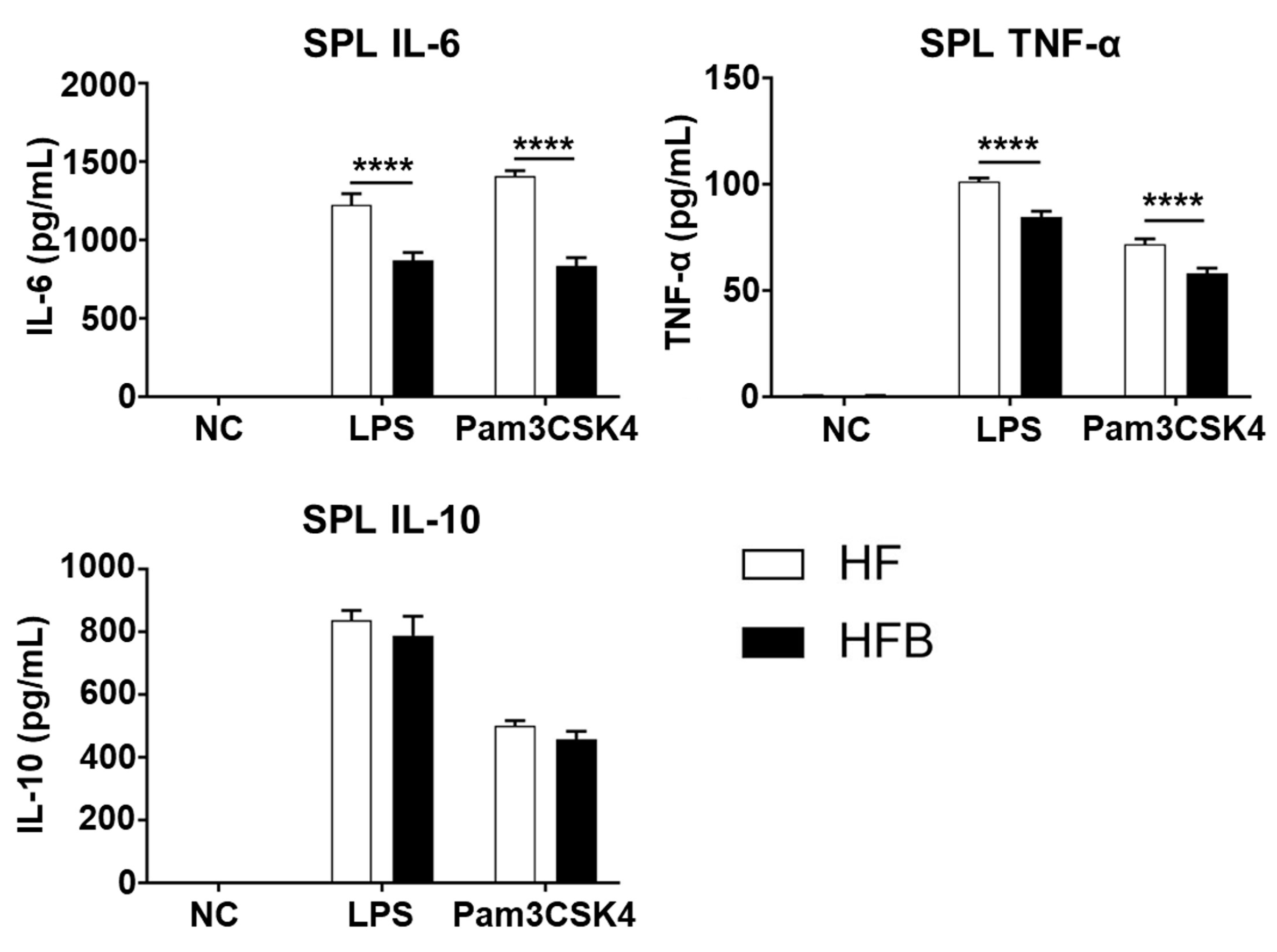
2.2. Bisacurone Reduces the Production of Pro-Inflammatory Cytokines in Spleens of HFD-Fed Mice
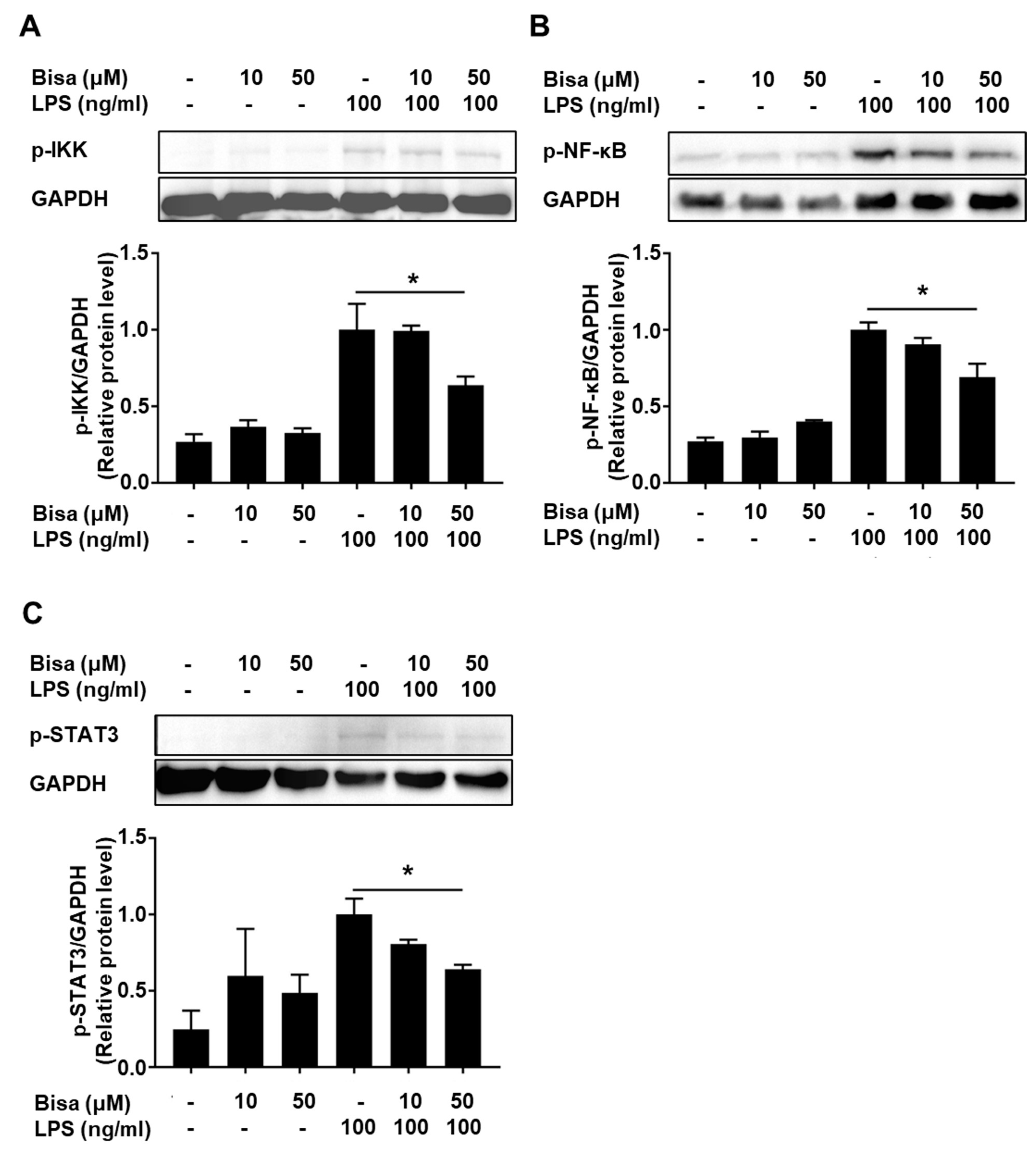
2.3. Bisacurone Inhibits Cytokine Production in RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Bisacurone Reduces Serum Levels of Lipids and Blood Viscosity in HFD-Fed Mice
4.2. Measurement of Blood Viscosity
4.3. Measurement of Serum Biochemical Parameters
4.4. Measurement of Hematological Parameters
4.5. Splenocyte Assay
4.6. FACS Analysis
4.7. Cellular Assay
4.8. Western Blot Analysis
4.9. DPPH Antioxidant Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or Protective Effects of Curcuma Longa (Turmeric) and Its Active Ingredient, Curcumin, against Natural and Chemical Toxicities: A Review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Srivastava, B.B.L.; Ripanda, A.S.; Mwanga, H.M. Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa). Compounds 2022, 2, 17. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B.B. Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.). Pharm. Crop. 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an Anti-Inflammatory Agent: Implications to Radiotherapy and Chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q.; et al. Curcumin Attenuates Collagen-Induced Rat Arthritis via Anti-Inflammatory and Apoptotic Effects. Int. Immunopharmacol. 2019, 72, 292–300. [Google Scholar] [CrossRef]
- Shu, H.-Z.; Peng, C.; Bu, L.; Guo, L.; Liu, F.; Xiong, L. Bisabolane-Type Sesquiterpenoids: Structural Diversity and Biological Activity. Phytochemistry 2021, 192, 112927. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-I.; Nizamutdinova, I.T.; Kim, Y.M.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Kim, Y.S.; Kang, K.M.; Chai, G.Y.; Chang, K.C.; et al. Bisacurone Inhibits Adhesion of Inflammatory Monocytes or Cancer Cells to Endothelial Cells through Down-Regulation of VCAM-1 Expression. Int. Immunopharmacol. 2008, 8, 1272–1281. [Google Scholar] [CrossRef]
- Ashida, H.; Tian, X.; Kitakaze, T.; Yamashita, Y. Bisacurone Suppresses Hepatic Lipid Accumulation through Inhibiting Lipogenesis and Promoting Lipolysis. J. Clin. Biochem. Nutr. 2020, 67, 43–52. [Google Scholar] [CrossRef]
- Uchio, R.; Higashi, Y.; Kohama, Y.; Kawasaki, K.; Hirao, T.; Muroyama, K.; Murosaki, S. A Hot Water Extract of Turmeric (Curcuma longa) Suppresses Acute Ethanol-Induced Liver Injury in Mice by Inhibiting Hepatic Oxidative Stress and Inflammatory Cytokine Production. J. Nutr. Sci. 2017, 6, e3. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.; Venkatakrishna, K.; Sundeep, K.; Vasavi, H.S.; Raj, A.; Chandrappa, S.; Shyamprasad, K. Turcuron: A Standardized Bisacurone-Rich Turmeric Rhizome Extract for the Prevention and Treatment of Hangover and Alcohol-Induced Liver Injury in Rats. Pharmacogn. Mag. 2020, 16, 263–271. [Google Scholar] [CrossRef]
- Gouthamchandra, K. Bioactive Compound Bisacurone in the Turmeric Extract (Turcuron) Prevents Non-Alcoholic Fatty Liver Disease by Reduction of Lipogenesis. Indian J. Pharm. Educ. Res. 2021, 55, s48–s55. [Google Scholar] [CrossRef]
- Uchio, R.; Muroyama, K.; Okuda-Hanafusa, C.; Kawasaki, K.; Yamamoto, Y.; Murosaki, S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Huang, F.; Ma, Y.; Tang, J. The Effect of High-Fat Diet and Exercise Intervention on the TNF-α Level in Rat Spleen. Front. Immunol. 2021, 12, 671167. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.N.O.; Santos-Eichler, R.A.; Dias, C.; Rodrigues, S.F.; Skiba, D.S.; Landgraf, R.G.; de Carvalho, M.H.C.; Guzik, T.; Fock, R.A.; Akamine, E.H. Immune Spleen Cells Attenuate the Inflammatory Profile of the Mesenteric Perivascular Adipose Tissue in Obese Mice. Sci. Rep. 2021, 11, 11153. [Google Scholar]
- Tanczos, B.; Somogyi, V.; Bombicz, M.; Juhasz, B.; Nemeth, N.; Deak, A. Changes of Hematological and Hemorheological Parameters in Rabbits with Hypercholesterolemia. Metabolites 2021, 11, 249. [Google Scholar] [CrossRef]
- Irace, C.; Scavelli, F.; Carallo, C.; Serra, R.; Gnasso, A. Plasma and Blood Viscosity in Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Fioravanti, M.; Pezza, N.; Locatelli, M.; Schifino, N.; Cerutti, N.; Severgnini, S.; Rondanelli, M.; Ferrari, E. Hyperviscosity and Microproteinuria in Central Obesity: Relevance to Cardiovascular Risk. Int. J. Obes. 1997, 21, 417–423. [Google Scholar] [CrossRef]
- Guiraudou, M.; Varlet-Marie, E.; Raynaud de Mauverger, E.; Brun, J.-F. Obesity-Related Increase in Whole Blood Viscosity Includes Different Profiles According to Fat Localization. Clin. Hemorheol. Microcirc. 2013, 55, 63–73. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Sig. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Olędzka, A.J.; Czerwińska, M.E. Role of Plant-Derived Compounds in the Molecular Pathways Related to Inflammation. Int. J. Mol. Sci. 2023, 24, 4666. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, A.; Domoradzki, T.; Pająk, B. Targeting the JAK2/STAT3 Pathway—Can We Compare It to the Two Faces of the God Janus? Int. J. Mol. Sci. 2020, 21, 8261. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Zucchi, A.; Claps, F.; Pastore, A.L.; Perotti, A.; Biagini, A.; Sallicandro, L.; Gentile, R.; Caglioti, C.; Palazzetti, F.; Fioretti, B. Focus on the Use of Resveratrol in Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 4562. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives-inter-laboratory evaluation study-. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- Ti, H.; Mai, Z.; Wang, Z.; Zhang, W.; Xiao, M.; Yang, Z.; Shaw, P. Bisabolane-Type Sesquiterpenoids from Curcuma Longa L. Exert Anti-Influenza and Anti-Inflammatory Activities through NF-ΚB/MAPK and RIG-1/STAT1/2 Signaling Pathways. Food Funct. 2021, 12, 6697–6711. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Killian, P.H.; Melchart, D. The Role of Curcumin in Prevention and Management of Metastatic Disease. Int. J. Mol. Sci. 2018, 19, 1716. [Google Scholar] [CrossRef]
- Khan, M.A.; Gahlot, S.; Majumdar, S. Oxidative Stress Induced by Curcumin Promotes the Death of Cutaneous T-Cell Lymphoma (HuT-78) by Disrupting the Function of Several Molecular Targets. Mol. Cancer Ther. 2012, 11, 1873–1883. [Google Scholar] [CrossRef]
- Carey, A.J.; Tan, C.K.; Ulett, G.C. Infection-Induced IL-10 and JAK-STAT. JAKSTAT 2012, 1, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Zeng, S.; Zhang, X.; Zhao, J.; Zhang, X.; Chen, X.; Yang, W.; Yang, Y.; Dong, Z.; et al. The Natural Polyphenol Curcumin Induces Apoptosis by Suppressing STAT3 Signaling in Esophageal Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 37, 303. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef]
- Yang, J.; Ran, Y.; Yang, Y.; Song, S.; Wu, Y.; Qi, Y.; Gao, Y.; Li, G. 4-Hydroxyisoleucine Alleviates Macrophage-Related Chronic Inflammation and Metabolic Syndrome in Mice Fed a High-Fat Diet. Front. Pharmacol. 2021, 11, 606514. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-Mediated Inflammation in Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef]
- Lackey, D.E.; Olefsky, J.M. Regulation of Metabolism by the Innate Immune System. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Fu, W.; Lee, Y.S.; Olefsky, J.M. The Role of Macrophages in Obesity-Associated Islet Inflammation and β-Cell Abnormalities. Nat. Rev. Endocrinol. 2020, 16, 81–90. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Z.; Zhang, L. Bisacurone Attenuates Diabetic Nephropathy by Ameliorating Oxidative Stress, Inflammation and Apoptosis in Rats. Hum. Exp. Toxicol. 2022, 41, 09603271221143713. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Ma, C.; Xing, S.; An, Y.; Feng, J.; Dang, H.; Huang, W.; Qiao, L.; Cheng, J.; Xie, L. White tea and its active polyphenols lower cholesterol through reduction of very-low-density lipoprotein production and induction of LDLR expression. Biomed. Pharmacother. 2020, 127, 110146. [Google Scholar] [CrossRef]
- Turpin, C.; Catan, A.; Meilhac, O.; Bourdon, E.; Canonne-Hergaux, F.; Rondeau, P. Erythrocytes: Central Actors in Multiple Scenes of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 5843. [Google Scholar] [CrossRef]
- Lam, N.Y.L.; Rainer, T.H.; Chiu, R.W.K.; Lo, Y.M.D. EDTA Is a Better Anticoagulant than Heparin or Citrate for Delayed Blood Processing for Plasma DNA Analysis. Clin. Chem. 2004, 50, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Rastogi, R.; Du, W.; Hüttemann, M.; Fite, A.; Franchi, L. STAT3 Tyrosine Phosphorylation Is Critical for Interleukin 1 Beta and Interleukin-6 Production in Response to Lipopolysaccharide and Live Bacteria. Mol. Immunol. 2009, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Miyazawa, T.; Abe, C.; Ueno, T.; Suzuki, M.; Mizukami, M.; Kurihara, K.; Toda, M. Hypolipidemic and Anti-Inflammatory Effects of Curcuma longa-Derived Bisacurone in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2023, 24, 9366. https://doi.org/10.3390/ijms24119366
He C, Miyazawa T, Abe C, Ueno T, Suzuki M, Mizukami M, Kurihara K, Toda M. Hypolipidemic and Anti-Inflammatory Effects of Curcuma longa-Derived Bisacurone in High-Fat Diet-Fed Mice. International Journal of Molecular Sciences. 2023; 24(11):9366. https://doi.org/10.3390/ijms24119366
Chicago/Turabian StyleHe, Chaoqi, Taiki Miyazawa, Chizumi Abe, Takahiro Ueno, Mikiko Suzuki, Masashi Mizukami, Kazue Kurihara, and Masako Toda. 2023. "Hypolipidemic and Anti-Inflammatory Effects of Curcuma longa-Derived Bisacurone in High-Fat Diet-Fed Mice" International Journal of Molecular Sciences 24, no. 11: 9366. https://doi.org/10.3390/ijms24119366





