CSF Markers of Oxidative Stress Are Associated with Brain Atrophy and Iron Accumulation in a 2-Year Longitudinal Cohort of Early MS
Abstract
:1. Introduction
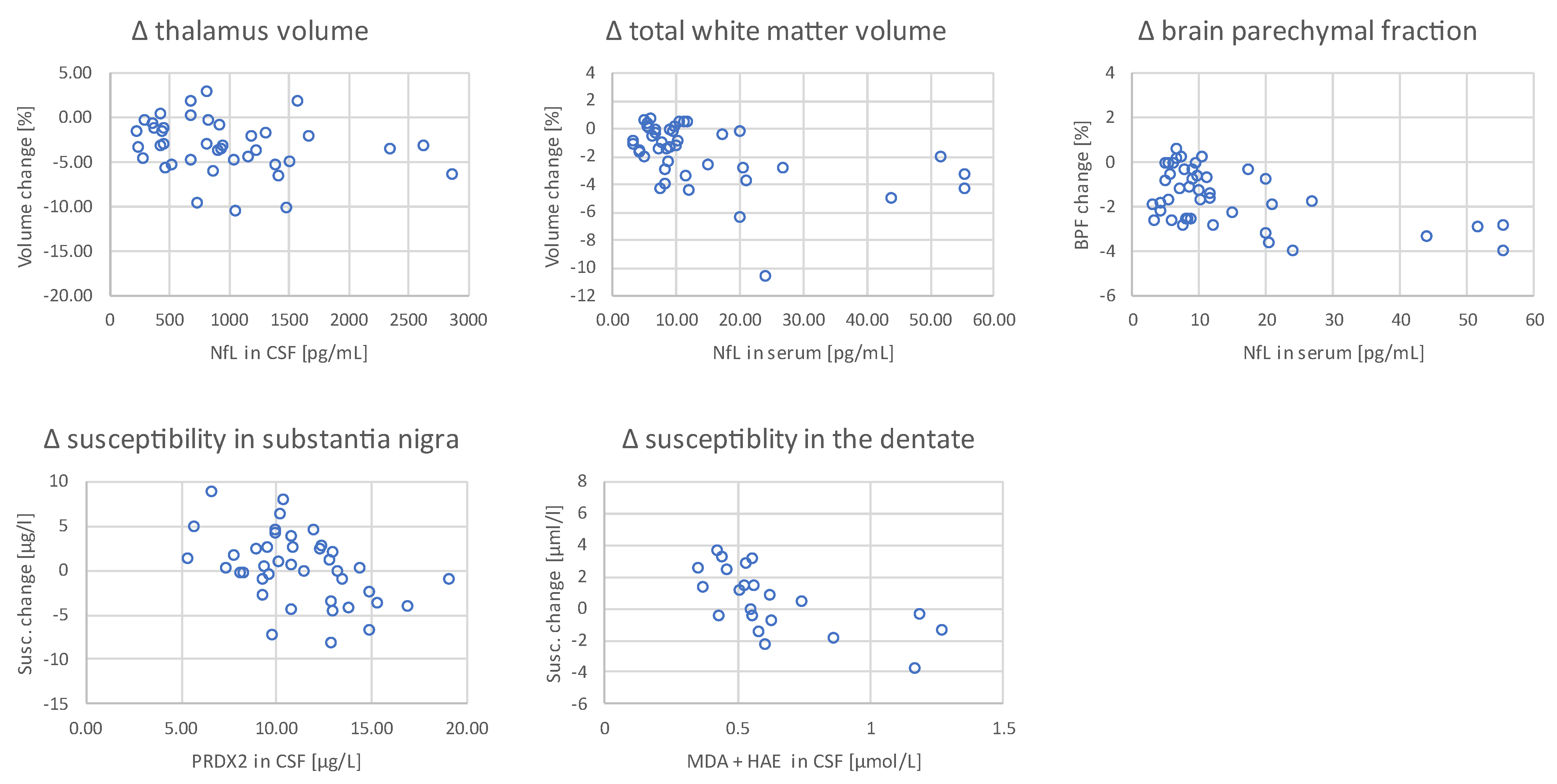
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Imaging Protocol
4.3. Image Processing
4.4. CSF and Serum Assays
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple Sclerosis: Clinical Aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Biernacki, T.; Kokas, Z.; Sandi, D.; Füvesi, J.; Fricska-Nagy, Z.; Faragó, P.; Kincses, T.Z.; Klivényi, P.; Bencsik, K.; Vécsei, L. Emerging Biomarkers of Multiple Sclerosis in the Blood and the CSF: A Focus on Neurofilaments and Therapeutic Considerations. Int. J. Mol. Sci. 2022, 23, 3383. [Google Scholar] [CrossRef]
- Burgetova, A.; Dusek, P.; Uher, T.; Vaneckova, M.; Vejrazka, M.; Burgetova, R.; Horakova, D.; Srpova, B.; Krasensky, J.; Lambert, L. Oxidative Stress Markers in Cerebrospinal Fluid of Newly Diagnosed Multiple Sclerosis Patients and Their Link to Iron Deposition and Atrophy. Diagnostics 2022, 12, 1365. [Google Scholar] [CrossRef]
- Uher, T.; Schaedelin, S.; Srpova, B.; Barro, C.; Bergsland, N.; Dwyer, M.; Tyblova, M.; Vodehnalova, K.; Benkert, P.; Oechtering, J.; et al. Monitoring of Radiologic Disease Activity by Serum Neurofilaments in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e714. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum Neurofilament Light Levels in Normal Aging and Their Association with Morphologic Brain Changes. Nat. Commun. 2020, 11, 812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-Y.; Gui, L.-N.; Liu, Y.-Y.; Shi, S.; Cheng, Y. Oxidative Stress Marker Aberrations in Multiple Sclerosis: A Meta-Analysis Study. Front. Neurosci. 2020, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Popescu, V.; Agosta, F.; Hulst, H.E.; Sluimer, I.C.; Knol, D.L.; Sormani, M.P.; Enzinger, C.; Ropele, S.; Alonso, J.; Sastre-Garriga, J. Brain Atrophy and Lesion Load Predict Long Term Disability in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Burgetova, A.; Seidl, Z.; Krasensky, J.; Horakova, D.; Vaneckova, M. Multiple Sclerosis and the Accumulation of Iron in the Basal Ganglia: Quantitative Assessment of Brain Iron Using MRI T2 Relaxometry. Eur. Neurol. 2010, 63, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pudlac, A.; Burgetova, A.; Dusek, P.; Nytrova, P.; Vaneckova, M.; Horakova, D.; Krasensky, J.; Lambert, L. Deep Gray Matter Iron Content in Neuromyelitis Optica and Multiple Sclerosis. BioMed Res. Int. 2020, 2020, 6492786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gauthier, S.A.; Gupta, A.; Comunale, J.; Chia-Yi Chiang, G.; Zhou, D.; Chen, W.; Giambrone, A.E.; Zhu, W.; Wang, Y. Longitudinal Change in Magnetic Susceptibility of New Enhanced Multiple Sclerosis (MS) Lesions Measured on Serial Quantitative Susceptibility Mapping (QSM). J. Magn. Reson. Imaging 2016, 44, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Schweser, F.; Hagemeier, J.; Dwyer, M.G.; Bergsland, N.; Hametner, S.; Weinstock-Guttman, B.; Zivadinov, R. Decreasing Brain Iron in Multiple Sclerosis: The Difference between Concentration and Content in Iron MRI. Hum. Brain Mapp. 2020, 42, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Brück, W.; Chard, D.; Fazekas, F.; Geurts, J.J.; Enzinger, C.; Hametner, S.; Kuhlmann, T.; Preziosa, P.; Rovira, À. Association between Pathological and MRI Findings in Multiple Sclerosis. Lancet Neurol. 2019, 18, 198–210. [Google Scholar] [CrossRef]
- Srpova, B.; Uher, T.; Hrnciarova, T.; Barro, C.; Andelova, M.; Michalak, Z.; Vaneckova, M.; Krasensky, J.; Noskova, L.; Havrdova, E.K. Serum Neurofilament Light Chain Reflects Inflammation-Driven Neurodegeneration and Predicts Delayed Brain Volume Loss in Early Stage of Multiple Sclerosis. Mult. Scler. J. 2021, 27, 52–60. [Google Scholar] [CrossRef]
- van Lierop, Z.Y.; Noteboom, S.; Steenwijk, M.D.; van Dam, M.; Toorop, A.A.; van Kempen, Z.L.; Moraal, B.; Barkhof, F.; Uitdehaag, B.M.; Schoonheim, M.M. Neurofilament-Light and Contactin-1 Association with Long-Term Brain Atrophy in Natalizumab-Treated Relapsing-Remitting Multiple Sclerosis. Mult. Scler. J. 2022, 28, 2231–2242. [Google Scholar] [CrossRef]
- Hänninen, K.; Viitala, M.; Paavilainen, T.; Karhu, J.O.; Rinne, J.; Koikkalainen, J.; Lötjönen, J.; Soilu-Hänninen, M. Thalamic Atrophy Predicts 5-Year Disability Progression in Multiple Sclerosis. Front. Neurol. 2020, 11, 606. [Google Scholar] [CrossRef]
- Bergsland, N.; Benedict, R.H.B.; Dwyer, M.G.; Fuchs, T.A.; Jakimovski, D.; Schweser, F.; Tavazzi, E.; Weinstock-Guttman, B.; Zivadinov, R. Thalamic Nuclei Volumes and Their Relationships to Neuroperformance in Multiple Sclerosis: A Cross-Sectional Structural MRI Study. J. Magn. Reson. Imaging JMRI 2021, 53, 731–739. [Google Scholar] [CrossRef]
- Rocca, M.A.; Valsasina, P.; Meani, A.; Gobbi, C.; Zecca, C.; Rovira, A.; Sastre-Garriga, J.; Kearney, H.; Ciccarelli, O.; Matthews, L.; et al. Association of Gray Matter Atrophy Patterns With Clinical Phenotype and Progression in Multiple Sclerosis. Neurology 2021, 96, e1561–e1573. [Google Scholar] [CrossRef]
- Steffen, F.; Uphaus, T.; Ripfel, N.; Fleischer, V.; Schraad, M.; Gonzalez-Escamilla, G.; Engel, S.; Groppa, S.; Zipp, F.; Bittner, S. Serum Neurofilament Identifies Patients with Multiple Sclerosis with Severe Focal Axonal Damage in a 6-Year Longitudinal Cohort. Neurol. Neuroimmunol. Neuroinflamm. 2022, 10, e200055. [Google Scholar] [CrossRef]
- Martin, S.-J.; McGlasson, S.; Hunt, D.; Overell, J. Cerebrospinal Fluid Neurofilament Light Chain in Multiple Sclerosis and Its Subtypes: A Meta-Analysis of Case–control Studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1059–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemssen, T.; Arnold, D.L.; Alvarez, E.; Cross, A.H.; Willi, R.; Li, B.; Kukkaro, P.; Kropshofer, H.; Ramanathan, K.; Merschhemke, M.; et al. Prognostic Value of Serum Neurofilament Light Chain for Disease Activity and Worsening in Patients with Relapsing Multiple Sclerosis: Results from the Phase 3 ASCLEPIOS I and II Trials. Front. Immunol. 2022, 13, 852563. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R.; Bergsland, N.; Jakimovski, D.; Weinstock-Guttman, B.; Benedict, R.H.B.; Riolo, J.; Silva, D.; Dwyer, M.G. Thalamic Atrophy Measured by Artificial Intelligence in a Multicentre Clinical Routine Real-World Study Is Associated with Disability Progression. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Bergsland, N.; Dwyer, M.G.; Ramasamy, D.P.; Ramanathan, M.; Weinstock-Guttman, B.; Zivadinov, R. Serum Neurofilament Light Chain Levels Are Associated with Lower Thalamic Perfusion in Multiple Sclerosis. Diagnostics 2020, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, F.; Hametner, S.; Yao, B.; van Gelderen, P.; Merkle, H.; Cantor, F.K.; Lassmann, H.; Duyn, J.H. Tracking Iron in Multiple Sclerosis: A Combined Imaging and Histopathological Study at 7 Tesla. Brain J. Neurol. 2011, 134, 3602–3615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagemeier, J.; Zivadinov, R.; Dwyer, M.G.; Polak, P.; Bergsland, N.; Weinstock-Guttman, B.; Zalis, J.; Deistung, A.; Reichenbach, J.R.; Schweser, F. Changes of Deep Gray Matter Magnetic Susceptibility over 2 Years in Multiple Sclerosis and Healthy Control Brain. NeuroImage Clin. 2017, 18, 1007–1016. [Google Scholar] [CrossRef]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple Sclerosis Deep Grey Matter: The Relation between Demyelination, Neurodegeneration, Inflammation and Iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langkammer, C.; Schweser, F.; Krebs, N.; Deistung, A.; Goessler, W.; Scheurer, E.; Sommer, K.; Reishofer, G.; Yen, K.; Fazekas, F.; et al. Quantitative Susceptibility Mapping (QSM) as a Means to Measure Brain Iron? A Post Mortem Validation Study. NeuroImage 2012, 62, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Burgetova, A.; Dusek, P.; Vaneckova, M.; Horakova, D.; Langkammer, C.; Krasensky, J.; Sobisek, L.; Matras, P.; Masek, M.; Seidl, Z. Thalamic Iron Differentiates Primary-Progressive and Relapsing-Remitting Multiple Sclerosis. AJNR Am. J. Neuroradiol. 2017, 38, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Langkammer, C.; Pichler, A.; Pinter, D.; Gattringer, T.; Bachmaier, G.; Ropele, S.; Fuchs, S.; Enzinger, C.; Fazekas, F. Dynamics of Brain Iron Levels in Multiple Sclerosis: A Longitudinal 3T MRI Study. Neurology 2015, 84, 2396–2402. [Google Scholar] [CrossRef]
- Voigt, D.; Scheidt, U.; Derfuss, T.; Brück, W.; Junker, A. Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. Int. J. Mol. Sci. 2017, 18, 760. [Google Scholar] [CrossRef] [Green Version]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative Damage in Multiple Sclerosis Lesions. Brain J. Neurol. 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [Green Version]
- Ghonimi, N.A.M.; Elsharkawi, K.A.; Khyal, D.S.M.; Abdelghani, A.A. Serum Malondialdehyde as a Lipid Peroxidation Marker in Multiple Sclerosis Patients and Its Relation to Disease Characteristics. Mult. Scler. Relat. Disord. 2021, 51, 102941. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Mori, M.; Masuda, H.; Ohtani, R.; Uchida, T.; Aoki, R.; Kuwabara, S. Peroxiredoxins Are Involved in the Pathogenesis of Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder. Clin. Exp. Immunol. 2020, 202, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Reeves, J.A.; Bergsland, N.; Dwyer, M.G.; Wilding, G.E.; Jakimovski, D.; Salman, F.; Sule, B.; Meineke, N.; Weinstock-Guttman, B.; Zivadinov, R.; et al. Susceptibility Networks Reveal Independent Patterns of Brain Iron Abnormalities in Multiple Sclerosis. NeuroImage 2022, 261, 119503. [Google Scholar] [CrossRef]
- Blazejewska, A.I.; Al-Radaideh, A.M.; Wharton, S.; Lim, S.Y.; Bowtell, R.W.; Constantinescu, C.S.; Gowland, P.A. Increase in the Iron Content of the Substantia Nigra and Red Nucleus in Multiple Sclerosis and Clinically Isolated Syndrome: A 7 Tesla MRI Study. J. Magn. Reson. Imaging 2015, 41, 1065–1070. [Google Scholar] [CrossRef]
- Moezzi, D.; Dong, Y.; Jain, R.W.; Lozinski, B.M.; Ghorbani, S.; D’Mello, C.; Wee Yong, V. Expression of Antioxidant Enzymes in Lesions of Multiple Sclerosis and Its Models. Sci. Rep. 2022, 12, 12761. [Google Scholar] [CrossRef]
- Singhal, T.; Cicero, S.; Pan, H.; Carter, K.; Dubey, S.; Chu, R.; Glanz, B.; Hurwitz, S.; Tauhid, S.; Park, M.-A.; et al. Regional Microglial Activation in the Substantia Nigra Is Linked with Fatigue in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e854. [Google Scholar] [CrossRef]
- Franceschi, L.D.; Bertoldi, M.; Falco, L.D.; Franco, S.S.; Ronzoni, L.; Turrini, F.; Colancecco, A.; Camaschella, C.; Cappellini, M.D.; Iolascon, A. Oxidative stress modulates heme synthesis and induces peroxiredoxin-2 as a novel cytoprotective response in β-thalassemic erythropoiesis. Haematologica 2011, 96, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Krata, N.; Foroncewicz, B.; Zagożdżon, R.; Moszczuk, B.; Zielenkiewicz, M.; Pączek, L.; Mucha, K. Peroxiredoxins as Markers of Oxidative Stress in IgA Nephropathy, Membranous Nephropathy and Lupus Nephritis. Arch. Immunol. Exp. 2021, 70, 3. [Google Scholar] [CrossRef]
- Albert, M.; Barrantes-Freer, A.; Lohrberg, M.; Antel, J.P.; Prineas, J.W.; Palkovits, M.; Wolff, J.R.; Brück, W.; Stadelmann, C. Synaptic Pathology in the Cerebellar Dentate Nucleus in Chronic Multiple Sclerosis. Brain Pathol. 2017, 27, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox Balance, Antioxidant Defense, and Oxidative Damage in the Hypothalamus and Cerebral Cortex of Rats with High Fat Diet-Induced Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6940515. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, G.; Bacchetti, T. Peroxidation of Lipoproteins in Multiple Sclerosis. J. Neurol. Sci. 2011, 311, 92–97. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Burgetova, R.; Dusek, P.; Burgetova, A.; Pudlac, A.; Vaneckova, M.; Horakova, D.; Krasensky, J.; Varga, Z.; Lambert, L. Age-Related Magnetic Susceptibility Changes in Deep Grey Matter and Cerebral Cortex of Normal Young and Middle-Aged Adults Depicted by Whole Brain Analysis. Quant. Imaging Med. Surg. 2021, 11, 3903919–3906919. [Google Scholar] [CrossRef]
- Acosta-Cabronero, J.; Milovic, C.; Mattern, H.; Tejos, C.; Speck, O.; Callaghan, M.F. A Robust Multi-Scale Approach to Quantitative Susceptibility Mapping. NeuroImage 2018, 183, 7–24. [Google Scholar] [CrossRef]
- Schmidt, P.; Gaser, C.; Arsic, M.; Buck, D.; Förschler, A.; Berthele, A.; Hoshi, M.; Ilg, R.; Schmid, V.J.; Zimmer, C.; et al. An Automated Tool for Detection of FLAIR-Hyperintense White-Matter Lesions in Multiple Sclerosis. NeuroImage 2012, 59, 3774–3783. [Google Scholar] [CrossRef]
- Mori, S.; Wu, D.; Ceritoglu, C.; Li, Y.; Kolasny, A.; Vaillant, M.A.; Faria, A.V.; Oishi, K.; Miller, M.I. MRICloud: Delivering High-Throughput MRI Neuroinformatics as Cloud-Based Software as a Service. Comput. Sci. Eng. 2016, 18, 21–35. [Google Scholar] [CrossRef]
- Ward, P.G.; Harding, I.H.; Close, T.G.; Corben, L.A.; Delatycki, M.B.; Storey, E.; Georgiou-Karistianis, N.; Egan, G.F. Longitudinal Evaluation of Iron Concentration and Atrophy in the Dentate Nuclei in Friedreich Ataxia. Mov. Disord. 2019, 34, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Torres, E.; Wiggermann, V.; Machan, L.; Sadovnick, A.D.; Li, D.K.; Traboulsee, A.; Hametner, S.; Rauscher, A. Increased Mean R2* in the Deep Gray Matter of Multiple Sclerosis Patients: Have We Been Measuring Atrophy? J. Magn. Reson. Imaging 2019, 50, 201–208. [Google Scholar] [CrossRef] [PubMed]

| MS Patients | Healthy Controls | p | |
|---|---|---|---|
| Number of subjects | 70 | 58 | |
| Sex [male/female] | 22 (31%) | 22 (38%) | 0.46 |
| Age [years] | 31 (IQR 26-41) | 38 (IQR 30–47) | 0.0020 |
| Time between first and second MRI [years] | 2.1 (IQR 2.0–2.2) | 4.1 (IQR 4.0–4.2) | <0.0001 |
| Freezer storage time of samples [years] | 1.5 (IQR 1.0–2.0) | - | - |
| CSF white blood cells/mm3 [n] | 17 (IQR 7–37) | - | - |
| CSF total protein [g/L] | 0.32 (IQR 0.24–0.43) | - | - |
| CSF albumin [mg/L] | 204.0 (IQR 152.5–267.5) | - | - |
| CSF IgG index [a.u.] | 0.9 (IQR 0.6–1.4) | - | - |
| CSF oligoclonal bands [n] | 15 (IQR 10–23) | - | - |
| CSF/serum albumin ratio [a.u.] | 4.6 (IQR 3.3–6.1) | - | - |
| Baseline | Follow-Up 2 Years | Change | ||||
|---|---|---|---|---|---|---|
| Mean | IQR | Mean | IQR | % | p Value | |
| Volume [cm3] | ||||||
| Caudate | 7.79 | 7.27 to 8.31 | 7.65 | 7.26 to 8.16 | −1.8 | <0.0001 |
| Putamen | 8.72 | 8.11 to 9.34 | 8.48 | 7.93 to 9.21 | −2.8 | 0.0038 |
| Globus pallidus | 4.24 | 4.03 to 4.57 | 4.32 | 4.01 to 4.62 | 1.9 | 0.428 |
| Thalamus | 11.5 | 10.6 to 12.2 | 10.9 | 10.4 to 11.9 | −5.2 | <0.0001 |
| Subthalamic nucleus | 0.339 | 0.31 to 0.38 | 0.34 | 0.31 to 0.38 | 0.3 | 0.315 |
| Substantia nigra | 1.33 | 1.2 to 1.45 | 1.27 | 1.21 to 1.38 | −4.5 | 0.0070 |
| Red nucleus | 0.615 | 0.58 to 0.65 | 0.61 | 0.57 to 0.65 | −0.8 | 0.392 |
| Dentate | 1.85 | 1.64 to 2.14 | 1.87 | 1.63 to 2.08 | 1.1 | 0.398 |
| Total grey matter | 651 | 614 to 693 | 643 | 615 to 673 | −1.2 | <0.0001 |
| Total white matter | 508 | 476 to 546 | 501 | 465 to 540 | −1.4 | <0.0001 |
| Brain parenchymal fraction [%] | 0.81 | 0.79 to 0.82 | 0.80 | 0.78 to 0.81 | −1.2 | <0.0001 |
| Susceptibility [ppb] | - | |||||
| Caudate | 20.5 | 17.9 to 24.8 | 21.7 | 19 to 25.6 | 5.9 | <0.0001 |
| Putamen | 20.3 | 15.7 to 23.3 | 21.2 | 17.1 to 24.8 | 4.4 | <0.0001 |
| Globus pallidus | 55.7 | 51.6 to 60.7 | 56.2 | 51.9 to 61.9 | 0.9 | 0.0012 |
| Thalamus | −2.15 | −3.23 to −0.77 | −3.00 | −3.89 to −1.42 | −39.5 | <0.0001 |
| Subthalamic nucleus | 38.7 | 34.2 to 43.5 | 38.7 | 35.3 to 44.2 | 0.0 | 0.497 |
| Substantia nigra | 49.7 | 45.1 to 55.2 | 49.4 | 44.9 to 55.7 | −0.6 | 0.249 |
| Red nucleus | 36.5 | 30.1 to 42.2 | 37.8 | 28.6 to 41.7 | 3.6 | 0.712 |
| Dentate | 33 | 28.1 to 39.3 | 33.1 | 30 to 40.4 | 0.3 | 0.0026 |
| Susceptibility mass [ppb·cm3] 1) | ||||||
| Caudate | 161 | 139 to 191 | 165 | 144 to 198 | 2.5 | 0.0085 |
| Putamen | 173 | 134 to 205 | 179 | 148 to 215 | 3.5 | <0.0001 |
| Globus pallidus | 237 | 211 to 274 | 241 | 215 to 284 | 1.7 | 0.0014 |
| Thalamus | 10.4 | −7.82 to 28.8 | 2.5 | −13.3 to 27.0 | −76.0 | 0.0003 |
| Subthalamic nucleus | 13.4 | 10.8 to 16.1 | 13.3 | 11.3 to 15.9 | −0.7 | 0.635 |
| Substantia nigra | 64.7 | 56.4 to 76.7 | 64.1 | 54.9 to 74.2 | −0.9 | 0.361 |
| Red nucleus | 22.7 | 17.9 to 26.7 | 23.3 | 17.3 to 26.1 | 2.6 | 0.409 |
| Dentate | 59 | 48.9 to 84.3 | 61.4 | 50.1 to 84.5 | 4.1 | 0.106 |
| Lesion load | - | |||||
| Lesion load [cm3] | 0.414 | 0.140 to 1.040 | 0.327 | 0.125 to 0.856 | −21.0 | 0.0553 |
| Lesion count | 5 | 2 to 11.8 | 5 | 3 to 10 | - | 0.666 |
| EDSS | ||||||
| EDSS | 2 | 1.5–2 | 1.5 | 1.5–2 | - | 0.911 |
| 8-OHDG | 8-IsoPG | NGAL | PRDX2 | MDA + HAE | NfL in CSF | NfL in Serum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | |
| Δ Volume [baseline—follow-up; cm3] | ||||||||||||||
| Caudate | 0.051 | 0.757 | 0.174 | 0.289 | −0.086 | 0.603 | 0.196 | 0.231 | 0.098 | 0.681 | 0.086 | 0.587 | 0.072 | 0.640 |
| Putamen | 0.091 | 0.580 | −0.076 | 0.644 | −0.099 | 0.548 | 0.204 | 0.213 | −0.080 | 0.738 | −0.005 | 0.975 | −0.099 | 0.517 |
| Globus pallidus | −0.190 | 0.247 | 0.199 | 0.226 | 0.190 | 0.247 | 0.038 | 0.819 | 0.330 | 0.155 | 0.199 | 0.206 | 0.184 | 0.225 |
| Thalamus | 0.055 | 0.741 | −0.161 | 0.327 | −0.046 | 0.781 | −0.054 | 0.746 | −0.075 | 0.752 | −0.411 | 0.007 | −0.371 | 0.012 |
| Subthalamic nucleus | −0.230 | 0.159 | −0.143 | 0.385 | 0.121 | 0.462 | −0.260 | 0.110 | −0.374 | 0.105 | 0.091 | 0.565 | 0.173 | 0.255 |
| Substantia nigra | −0.240 | 0.141 | −0.097 | 0.558 | 0.054 | 0.742 | −0.059 | 0.720 | −0.114 | 0.633 | −0.158 | 0.318 | −0.137 | 0.369 |
| Red nucleus | −0.123 | 0.456 | 0.132 | 0.424 | 0.193 | 0.238 | −0.129 | 0.435 | −0.255 | 0.278 | −0.054 | 0.734 | 0.077 | 0.613 |
| Dentate | 0.132 | 0.423 | −0.084 | 0.612 | 0.048 | 0.774 | −0.154 | 0.349 | 0.049 | 0.837 | 0.208 | 0.187 | 0.005 | 0.973 |
| Total grey matter | −0.076 | 0.644 | −0.095 | 0.565 | 0.061 | 0.712 | 0.185 | 0.259 | −0.293 | 0.211 | −0.001 | 0.995 | −0.052 | 0.734 |
| Total white matter | −0.082 | 0.621 | −0.271 | 0.095 | 0.112 | 0.497 | 0.011 | 0.949 | 0.107 | 0.654 | −0.399 | 0.009 | −0.456 | 0.002 |
| Brain parenchymal fraction | −0.066 | 0.688 | −0.222 | 0.175 | 0.079 | 0.634 | 0.196 | 0.233 | −0.109 | 0.647 | −0.308 | 0.047 | −0.355 | 0.017 |
| Δ Susceptibility [baseline—follow-up; ppb] 1) | ||||||||||||||
| Caudate | −0.058 | 0.724 | 0.191 | 0.245 | 0.009 | 0.957 | −0.139 | 0.399 | 0.077 | 0.747 | −0.110 | 0.489 | −0.064 | 0.678 |
| Putamen | −0.099 | 0.547 | 0.157 | 0.340 | −0.150 | 0.361 | 0.111 | 0.502 | −0.096 | 0.687 | −0.085 | 0.593 | −0.060 | 0.696 |
| Globus pallidus | −0.065 | 0.692 | −0.223 | 0.172 | −0.125 | 0.450 | −0.170 | 0.301 | −0.102 | 0.669 | 0.012 | 0.938 | −0.152 | 0.318 |
| Thalamus | −0.281 | 0.084 | 0.136 | 0.408 | −0.132 | 0.423 | 0.145 | 0.378 | 0.127 | 0.592 | −0.017 | 0.917 | −0.061 | 0.689 |
| Subthalamic nucleus | −0.056 | 0.734 | 0.049 | 0.768 | −0.020 | 0.902 | 0.059 | 0.719 | −0.014 | 0.953 | 0.158 | 0.316 | −0.139 | 0.362 |
| Substantia nigra | 0.138 | 0.401 | −0.308 | 0.056 | −0.116 | 0.481 | −0.407 | 0.010 | −0.088 | 0.713 | −0.089 | 0.576 | −0.180 | 0.237 |
| Red nucleus | −0.048 | 0.771 | 0.006 | 0.970 | −0.162 | 0.324 | 0.124 | 0.453 | 0.369 | 0.110 | −0.255 | 0.104 | −0.195 | 0.198 |
| Dentate | −0.075 | 0.648 | −0.315 | 0.051 | −0.159 | 0.334 | −0.266 | 0.102 | −0.559 | 0.010 | −0.155 | 0.326 | −0.156 | 0.306 |
| Δ Susceptibility mass [baseline—follow-up; ppb·cm3] 2) | ||||||||||||||
| Caudate | −0.031 | 0.850 | 0.225 | 0.169 | 0.015 | 0.926 | −0.030 | 0.855 | 0.168 | 0.479 | 0.009 | 0.952 | 0.006 | 0.971 |
| Putamen | 0.024 | 0.883 | 0.098 | 0.551 | −0.162 | 0.324 | 0.190 | 0.248 | −0.103 | 0.666 | −0.138 | 0.382 | −0.168 | 0.269 |
| Globus pallidus | −0.179 | 0.275 | 0.052 | 0.755 | 0.110 | 0.506 | −0.116 | 0.484 | 0.255 | 0.277 | 0.152 | 0.338 | 0.091 | 0.554 |
| Thalamus | −0.230 | 0.159 | 0.135 | 0.412 | −0.081 | 0.622 | 0.173 | 0.292 | 0.147 | 0.536 | 0.103 | 0.516 | −0.066 | 0.665 |
| Subthalamic nucleus | −0.224 | 0.170 | −0.111 | 0.502 | 0.039 | 0.814 | −0.205 | 0.211 | −0.403 | 0.078 | 0.092 | 0.561 | −0.039 | 0.799 |
| Substantia nigra | −0.089 | 0.590 | −0.198 | 0.228 | −0.026 | 0.876 | −0.307 | 0.057 | −0.202 | 0.392 | −0.181 | 0.250 | −0.183 | 0.228 |
| Red nucleus | −0.020 | 0.902 | 0.097 | 0.557 | 0.029 | 0.862 | 0.020 | 0.903 | 0.082 | 0.731 | −0.305 | 0.049 | −0.179 | 0.240 |
| Dentate | 0.022 | 0.892 | −0.318 | 0.049 | −0.109 | 0.510 | −0.331 | 0.040 | −0.451 | 0.046 | 0.012 | 0.940 | −0.048 | 0.754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgetova, A.; Dusek, P.; Uher, T.; Vaneckova, M.; Vejrazka, M.; Burgetova, R.; Horakova, D.; Srpova, B.; Kalousova, M.; Noskova, L.; et al. CSF Markers of Oxidative Stress Are Associated with Brain Atrophy and Iron Accumulation in a 2-Year Longitudinal Cohort of Early MS. Int. J. Mol. Sci. 2023, 24, 10048. https://doi.org/10.3390/ijms241210048
Burgetova A, Dusek P, Uher T, Vaneckova M, Vejrazka M, Burgetova R, Horakova D, Srpova B, Kalousova M, Noskova L, et al. CSF Markers of Oxidative Stress Are Associated with Brain Atrophy and Iron Accumulation in a 2-Year Longitudinal Cohort of Early MS. International Journal of Molecular Sciences. 2023; 24(12):10048. https://doi.org/10.3390/ijms241210048
Chicago/Turabian StyleBurgetova, Andrea, Petr Dusek, Tomas Uher, Manuela Vaneckova, Martin Vejrazka, Romana Burgetova, Dana Horakova, Barbora Srpova, Marta Kalousova, Libuse Noskova, and et al. 2023. "CSF Markers of Oxidative Stress Are Associated with Brain Atrophy and Iron Accumulation in a 2-Year Longitudinal Cohort of Early MS" International Journal of Molecular Sciences 24, no. 12: 10048. https://doi.org/10.3390/ijms241210048






