Biochemical Targets and Molecular Mechanism of Matrine against Aging
Abstract
:1. Introduction
2. Results
2.1. Analysis of MAT and Collection of Its Potential Targets
2.2. Collection of Cellular Aging-Related Genes
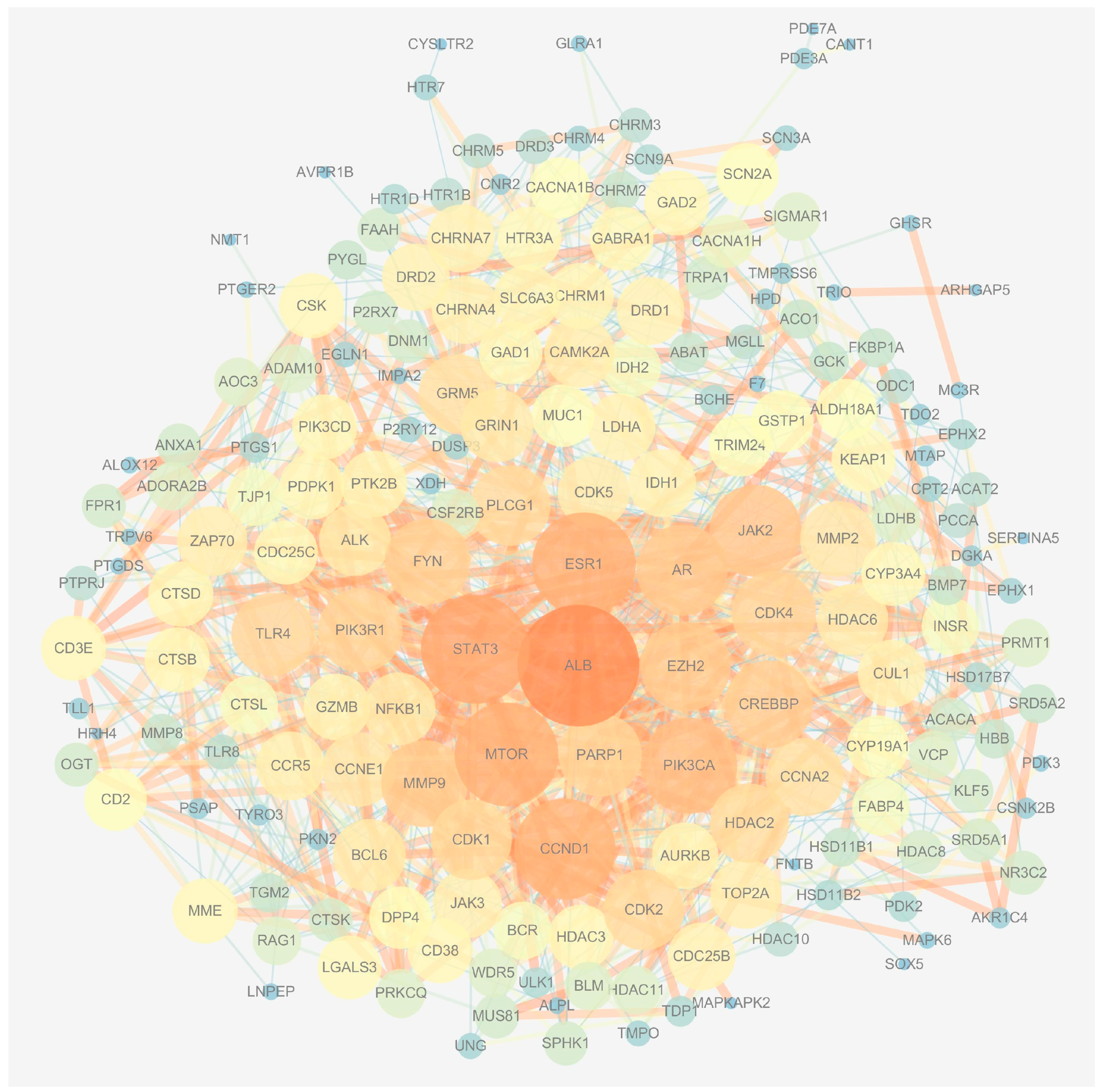
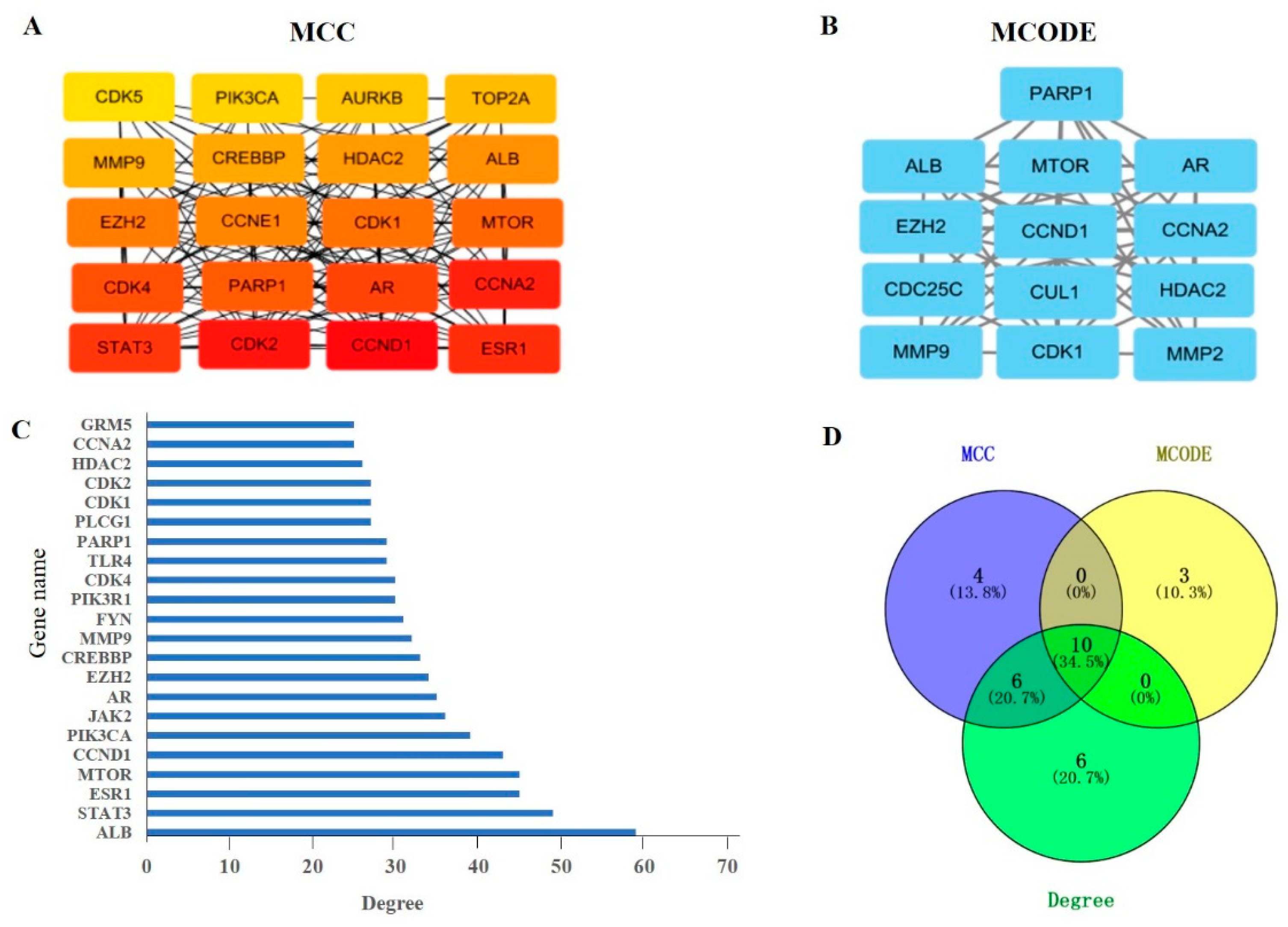
2.3. Protein–Protein Interaction Network Analysis
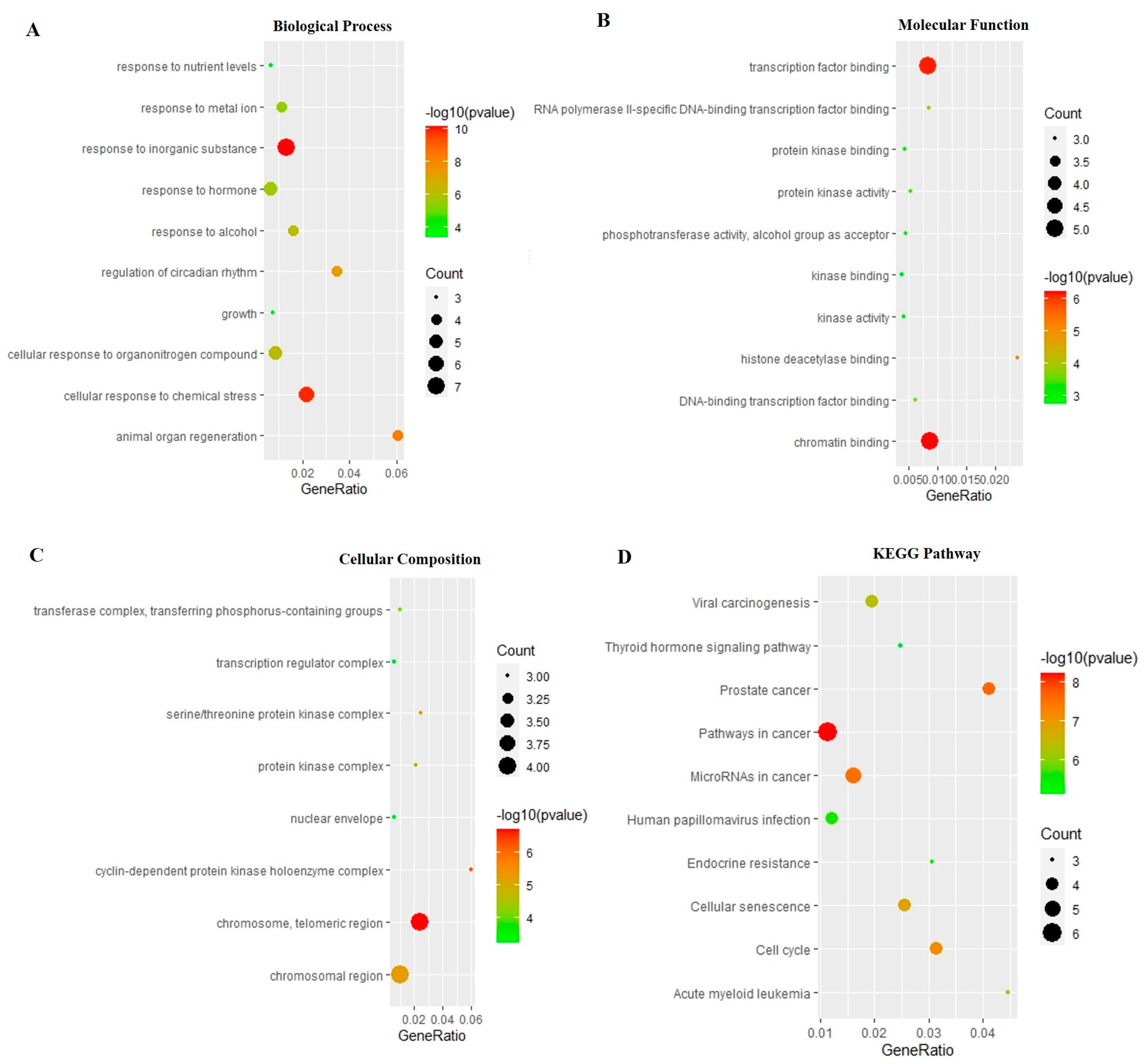
2.4. Biological Processes and Pathway Enrichment Analysis of the Key Genes
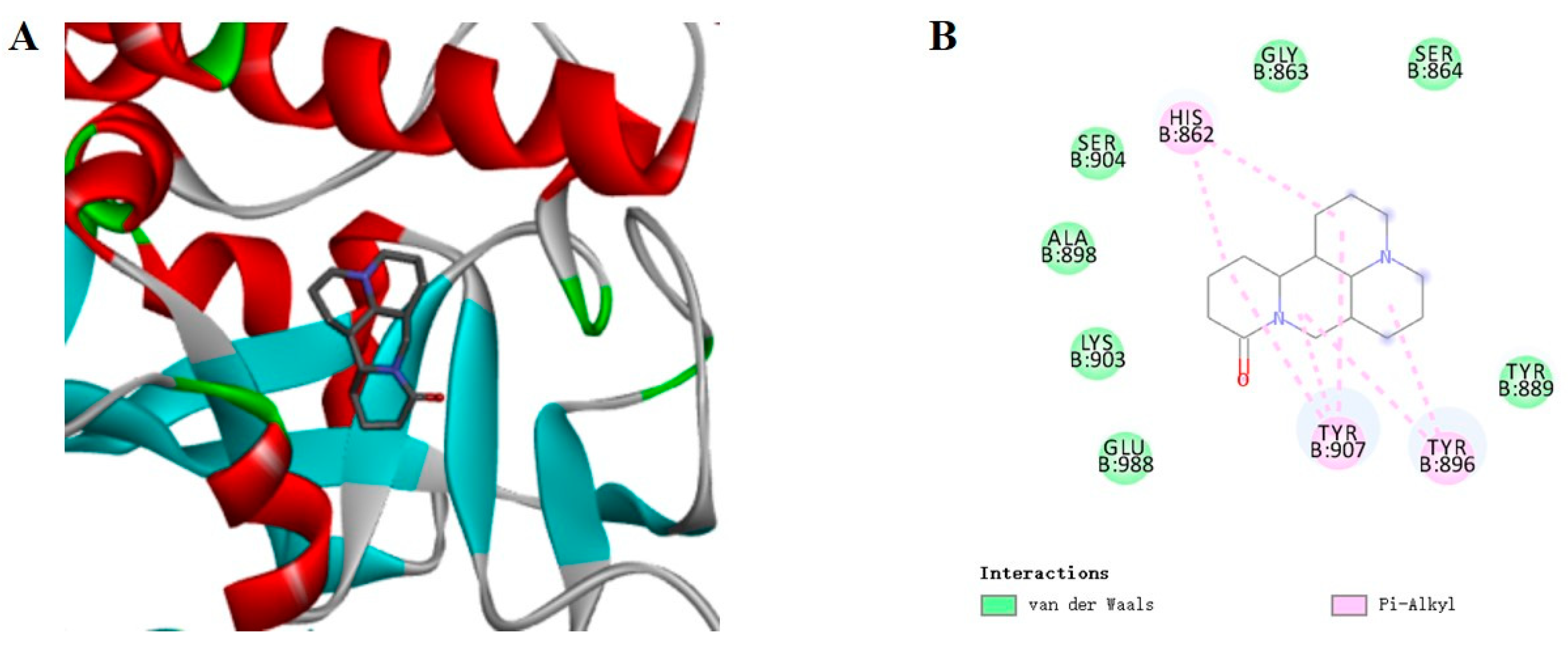
2.5. Molecular Docking Analysis of PARP1
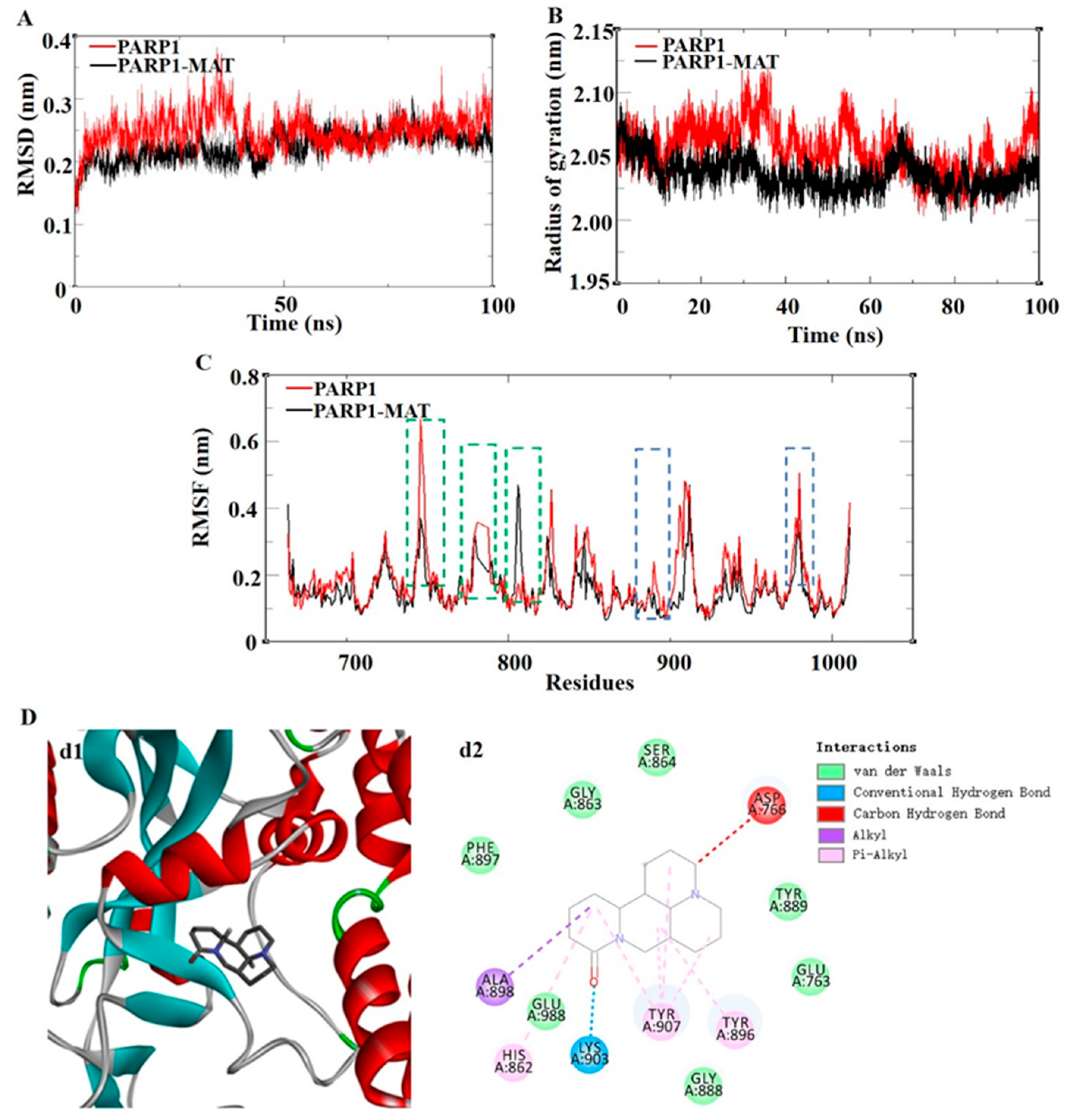
2.6. Molecular Dynamics Simulation of the PARP1-MAT Complex
2.7. The Calculation of Binding-Free Energy
2.8. MAT Effect on the NAD+ Level in the Liver of d-gal-Induced Aging Mice
3. Discussion
4. Materials and Methods
4.1. Clustering of Matrine- and Aging-Related Target Genes
4.2. The Protein–Protein Interaction Network Map Construction of Potential Target Genes of Matrine against Aging
4.3. GO and KEGG Pathway Enrichment Analysis
4.4. Molecular Docking
4.5. Molecular Dynamics Simulations
4.6. Binding-Free Energy Calculation
4.7. Animals and Drug Administration
4.8. NAD+ Analysis
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, E.; Walker, R. Global Ageing: Successes, Challenges and Opportunities. Br. J. Hosp. Med. 2020, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora Flavescens Ait.: Traditional Usage, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; You, Z.; Chen, P.; Chen, C.; Chen, F.; Shen, J.; Xu, H. Matrine Exerts Antidepressant-like Effects on Mice: Role of the Hippocampal PI3K/Akt/MTOR Signaling. Int. J. Neuropsychopharmacol. 2018, 21, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Sun, P.; Lv, H.; Sun, Y.; Guo, J.; Wang, Z.; Luo, T.; Wang, S.; Li, H. Matrine Displayed Antiviral Activity in Porcine Alveolar Macrophages Co-Infected by Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2. Sci. Rep. 2016, 6, 24401. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Hao, X.; Yao, H.; Ge, K.; Ma, L.; Ma, W. Matrine Inhibits Mycelia Growth of Botryosphaeria Dothidea by Affecting Membrane Permeability. J. For. Res. 2019, 30, 1105–1113. [Google Scholar] [CrossRef]
- Shi, J.; Han, G.; Wang, J.; Han, X.; Zhao, M.; Duan, X.; Mi, L.; Li, N.; Yin, X.; Shi, H.; et al. Matrine Promotes Hepatic Oval Cells Differentiation into Hepatocytes and Alleviates Liver Injury by Suppression of Notch Signalling Pathway. Life Sci. 2020, 261, 118354. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, Q.; Dong, H.; Ge, Z. Matrine Improves Diabetic Cardiomyopathy through TGF-β-Induced Protein Kinase RNA-like Endoplasmic Reticulum Kinase Signaling Pathway. J. Cell. Biochem. 2019, 120, 13573–13582. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Cao, J.; Yao, S. Matrine Promotes Liver Cancer Cell Apoptosis by Inhibiting Mitophagy and PINK1/Parkin Pathways. Cell Stress Chaperones 2018, 23, 1295–1309. [Google Scholar] [CrossRef]
- Li, P.; Lei, J.; Hu, G.; Chen, X.; Liu, Z.; Yang, J. Matrine Mediates Inflammatory Response via Gut Microbiota in TNBS-Induced Murine Colitis. Front. Physiol. 2019, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Bai, Y.; Zhao, R.; Guo, Z.; Su, X.; Li, P.; Yang, P. Neuroprotective Effects of Matrine on Scopolamine-Induced Amnesia via Inhibition of AChE/BuChE and Oxidative Stress. Metab. Brain Dis. 2019, 34, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Tang, Z.; Xu, J.; Ma, M.; Pan, S.; Qiu, C.; Guan, G.; Wang, J. Matrine Attenuates Cardiac Fibrosis by Affecting ATF6 Signaling Pathway in Diabetic Cardiomyopathy. Eur. J. Pharmacol. 2017, 804, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Yang, P.; Zhao, R.; Bai, Y.; Guo, Z. Matrine Attenuates D-Galactose-Induced Aging-Related Behavior in Mice via Inhibition of Cellular Senescence and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 7108604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [Green Version]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [Green Version]
- Salmaso, V.; Moro, S. Bridging Molecular Docking to Molecular Dynamics in Exploring Ligand-Protein Recognition Process: An Overview. Front. Pharmacol. 2018, 9, 923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Zhang, Y.L.; Ma, Y.H.; Yu, H.Y.; Zhao, X.Z.; Zhang, L.H.; Ge, S.Q.; Zhang, G.W. A Network Pharmacology Approach to Investigate the Mechanism of Shuxuening Injection in the Treatment of Ischemic Stroke. J. Ethnopharmacol. 2020, 257, 112891. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Lu, S.; Ostrovsky, J.; McCormack, S.E.; Falk, M.J.; Grant, S.F.A. PARP-1 Inhibition Rescues Short Lifespan in Hyperglycemic, C. Elegans And Improves GLP-1 Secretion in Human Cells. Aging Dis. 2018, 9, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.F.; Fan, S.H.; Zheng, Y.L.; Lu, J.; Wu, D.M.; Shan, Q.; Hu, B. Troxerutin improves hepatic lipid homeostasis by restoring NAD+-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem. Pharmacol. 2014, 91, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, S.; Zhuang, Y.; Xie, F.; Wang, R.; Kong, X.; Zhang, Q.; Feng, Y.; Gao, H.; Kong, X.; et al. Muscle PARP1 inhibition extends lifespan through AMPKα PARylation and activation in Drosophila. Proc. Natl. Acad. Sci. USA 2023, 120, e2213857120. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as regulator of lifespan, aging and cellular senescence. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Xu, S.; Wu, W.; Huang, H.; Huang, R.; Xie, L.; Su, A.; Liu, S.; Zheng, R.; Yuan, Y.; Zheng, H.L.; et al. The p53/miRNAs/Ccna2 pathway serves as a novel regulator of cellular senescence: Complement of the canonical p53/p21 pathway. Aging Cell 2019, 18, e12918. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The role of cellular senescence in cardiac disease: Basic biology and clinical relevance. Nat. Rev. Cardiol. 2022, 19, 250–264. [Google Scholar] [CrossRef]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem. Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox Regulation of SIRT1 in Inflammation and Cellular Senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, L.; Ding, Z.; Luo, Q.; Ju, Y.; Song, G. Exogenous NAD+ Postpones the D-Gal-Induced Senescence of Bone Marrow-Derived Mesenchymal Stem Cells via Sirt1 Signaling. Antioxidants 2021, 10, 254. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD+/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, E.F.; Scheibye-Knudsen, M.; Brace, L.E.; Kassahun, H.; Sengupta, T.; Nilsen, H.; Mitchell, J.R.; Croteau, D.L.; Bohr, V.A. Defective Mitophagy in XPA via PARP-1 Hyperactivation and NAD+/SIRT1 Reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK Activation Protects Cells from Oxidative Stress-Induced Senescence via Autophagic Flux Restoration and Intracellular NAD+ Elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Poljak, A.; Grant, R. Age Related Changes in NAD+ Metabolism Oxidative Stress and Sirt1 Activity in Wistar Rats. PLoS ONE 2011, 6, e19194. [Google Scholar] [CrossRef] [Green Version]
- Pirinen, E.; Cantó, C.; Jo, Y.S.; Morato, L.; Zhang, H.; Menzies, K.J.; Williams, E.G.; Mouchiroud, L.; Moullan, N.; Hagberg, C.; et al. Pharmacological Inhibition of Poly(ADP-Ribose) Polymerases Improves Fitness and Mitochondrial Function in Skeletal Muscle. Cell Metab. 2014, 19, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Gariani, K.; Ryu, D.; Menzies, K.J.; Yi, H.S.; Stein, S.; Zhang, H.; Perino, A.; Lemos, V.; Katsyuba, E.; Jha, P.; et al. Inhibiting Poly ADP-Ribosylation Increases Fatty Acid Oxidation and Protects against Fatty Liver Disease. J. Hepatol. 2017, 66, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmasry, G.F.; Aly, E.E.; Awadallah, F.M.; El-Moghazy, S.M. Design and Synthesis of Novel PARP-1 Inhibitors Based on Pyridopyridazinone Scaffold. Bioorg. Chem. 2019, 87, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, S.; Chen, T.; Niu, M.M. Structure-Based Pharmacophore Modeling, Virtual Screening, Molecular Docking and Biological Evaluation for Identification of Potential Poly (ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors. Molecules 2019, 24, 4258. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yao, S. JNK-Bcl-2/Bcl-xL-Bax/Bak Pathway Mediates the Crosstalk between Matrine-Induced Autophagy and Apoptosis via Interplay with Beclin 1. Int. J. Mol. Sci. 2015, 16, 25744–25758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.-Z.; Xu, H.-X.; Tian, S.-J.; Pui-Hay But, P. Determination of Quinolizidine Alkaloids in Traditional Chinese Herbal Drugs by Nonaqueous Capillary Electrophoresis. J. Chromatogr. A 1999, 857, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems Approaches and Polypharmacology for Drug Discovery from Herbal Medicines: An Example Using Licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper Server: A Web Server for Potential Drug Target Identification Using Pharmacophore Mapping Approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on Drug Classification and Target Prediction. Nucleic Acids Res. 2014, 42, W726–W731. [Google Scholar] [CrossRef]
- Harel, A.; Inger, A.; Stelzer, G.; Strichman-Almashanu, L.; Dalah, I.; Safran, M.; Lancet, D. GIFtS: Annotation Landscape Analysis with GeneCards. BMC Bioinform. 2009, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhang, Y.; Wang, N. Systematic Identification of Key Extracellular Proteins as the Potential Biomarkers in Lupus Nephritis. Front. Immunol. 2022, 13, 915784. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.J.; Tu, S.; Zhang, Z.H.; Meng, F.H. Identification of Novel Src Inhibitors: Pharmacophore-Based Virtual Screening, Molecular Docking and Molecular Dynamics Simulations. Molecules 2020, 25, 4094. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Han, L.; An, X.; Kong, R.; Gao, Z.; Yang, Y.; Wang, X.; Zhang, P.; Ding, Q.; Wu, H.; et al. In Silico Network Pharmacology and in Vivo Analysis of Berberine-Related Mechanisms against Type 2 Diabetes Mellitus and Its Complications. J. Ethnopharmacol. 2021, 276, 114180. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Lynn, A. G-Mmpbsa-A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]


| Name | MW | AlogP | TPSA | HL | DL | OB (%) | BBB |
|---|---|---|---|---|---|---|---|
| MAT | 248.41 | 1.42 | 23.55 | 6.69 | 0.25 | 63.77 | 1.52 |
| van der Waal Energy (kcal/mol) | Electrostattic Energy (kcal/mol) | Polar Solvation Energy (kcal/mol) | SASA Energy (kcal/mol) | Binding Energy (kcal/mol) |
|---|---|---|---|---|
| −34.246 +/− 2.307 | −11.513 +/− 2.152 | −32.912 +/− 3.022 | −3.117 +/− 0.146 | −15.963 +/− 2.784 |
| Name | TYR907 | TYR896 | GLU988 | TYR889 | GLU763 | ASP899 | ALA898 | PHE897 |
|---|---|---|---|---|---|---|---|---|
| Energy (kJ/mol) | −10.965 | −7.3233 | −5.4298 | −5.3366 | −2.9389 | −1.8721 | −1.2314 | −0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Zhang, Y.; Li, Y.; Yang, P.; Sun, Y. Biochemical Targets and Molecular Mechanism of Matrine against Aging. Int. J. Mol. Sci. 2023, 24, 10098. https://doi.org/10.3390/ijms241210098
Sun K, Zhang Y, Li Y, Yang P, Sun Y. Biochemical Targets and Molecular Mechanism of Matrine against Aging. International Journal of Molecular Sciences. 2023; 24(12):10098. https://doi.org/10.3390/ijms241210098
Chicago/Turabian StyleSun, Kaiyue, Yingzi Zhang, Yingliang Li, Pengyu Yang, and Yingting Sun. 2023. "Biochemical Targets and Molecular Mechanism of Matrine against Aging" International Journal of Molecular Sciences 24, no. 12: 10098. https://doi.org/10.3390/ijms241210098





