Tissue-Specific Hormone Signalling and Defence Gene Induction in an In Vitro Assembly of the Rapeseed Verticillium Pathosystem
Abstract
:1. Introduction
2. Results
2.1. Analysis of V. longisporum Growth in Rapeseed
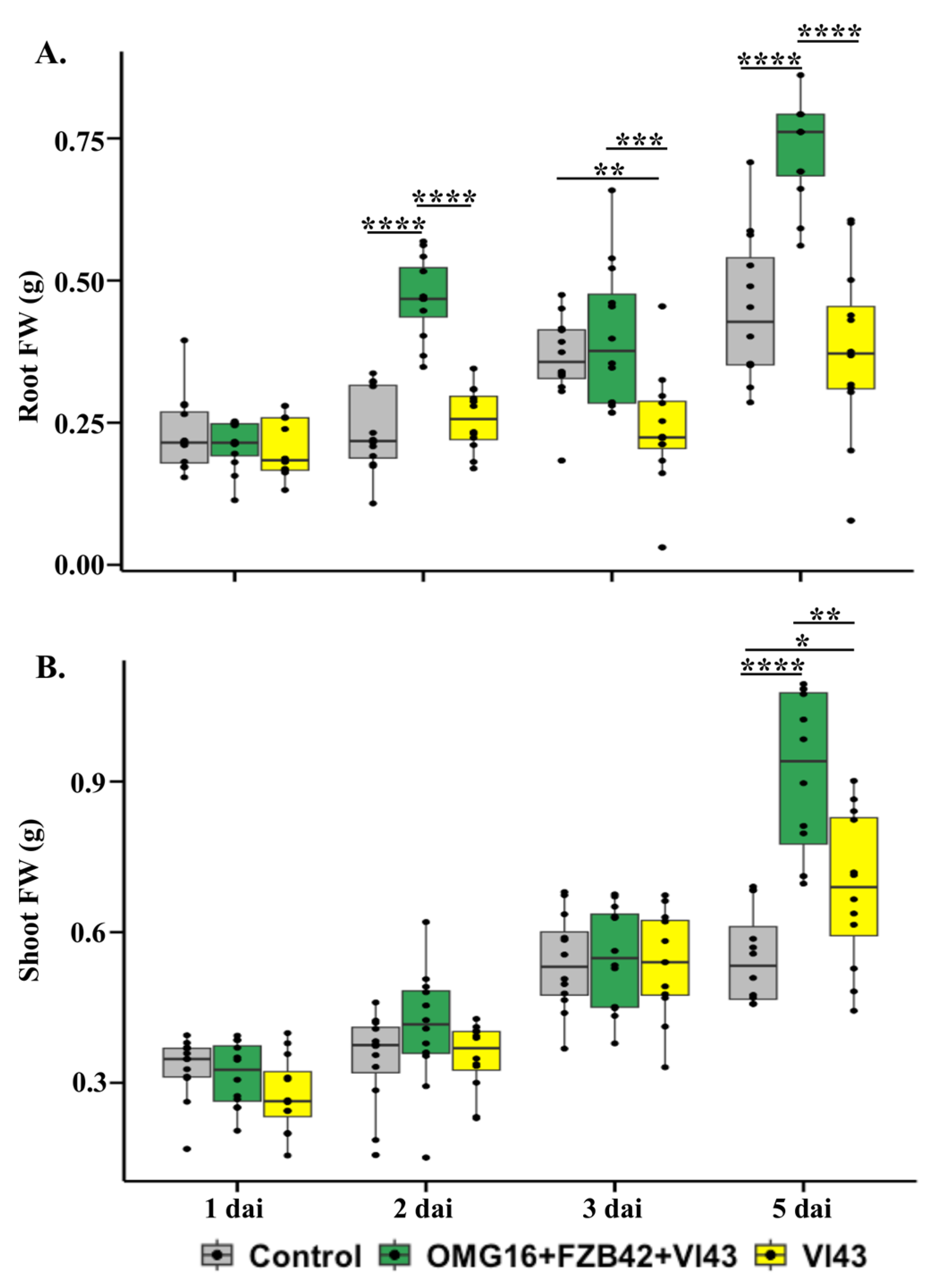
2.2. Effects of Priming on Rapeseed Growth Performance
2.3. Effects of Priming on V. longisporum 43 Root Infection
2.4. Enhanced Rapeseed Defence upon Priming
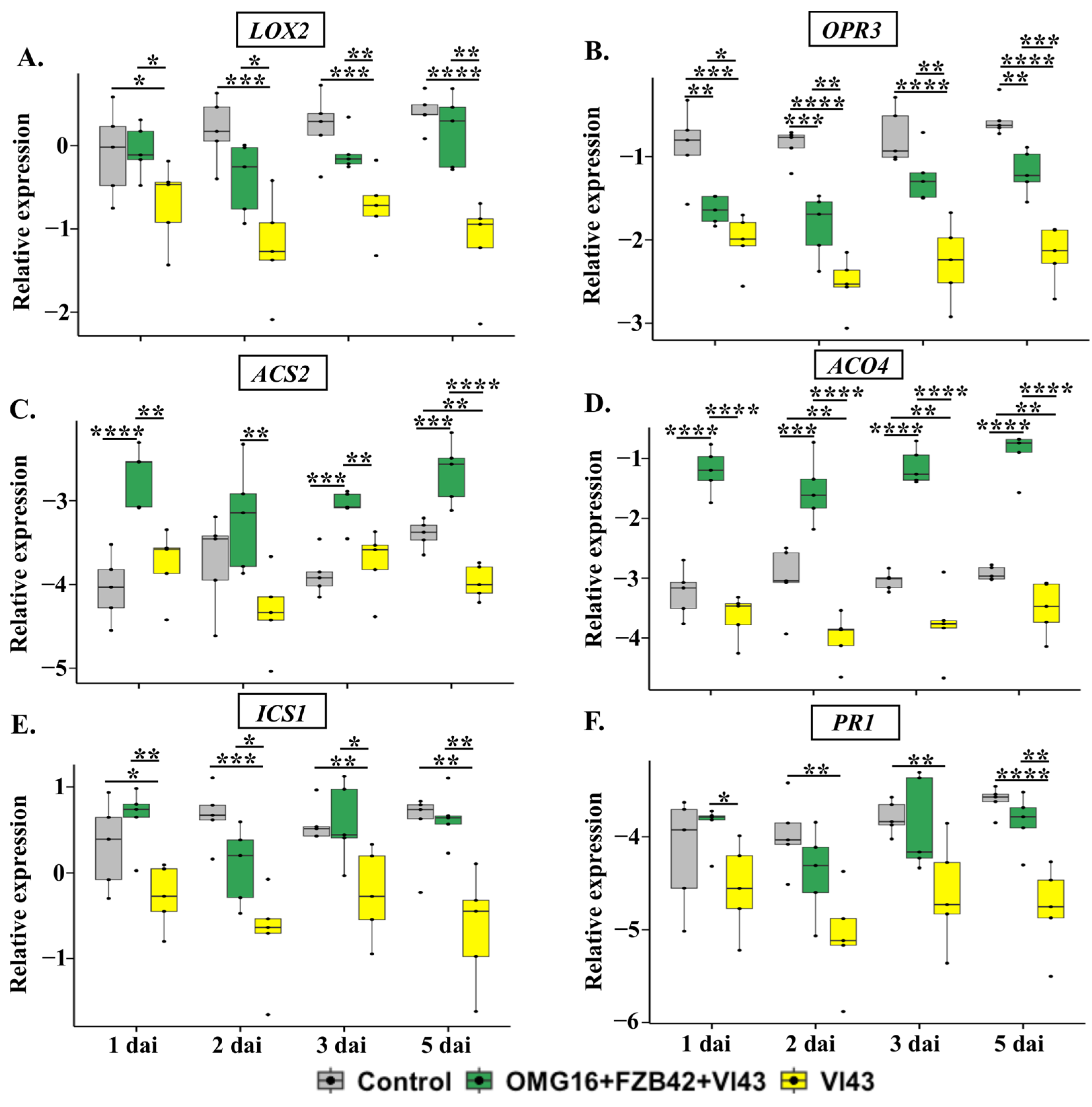
2.4.1. The Expression of Defence-Related Rapeseed Genes in Roots
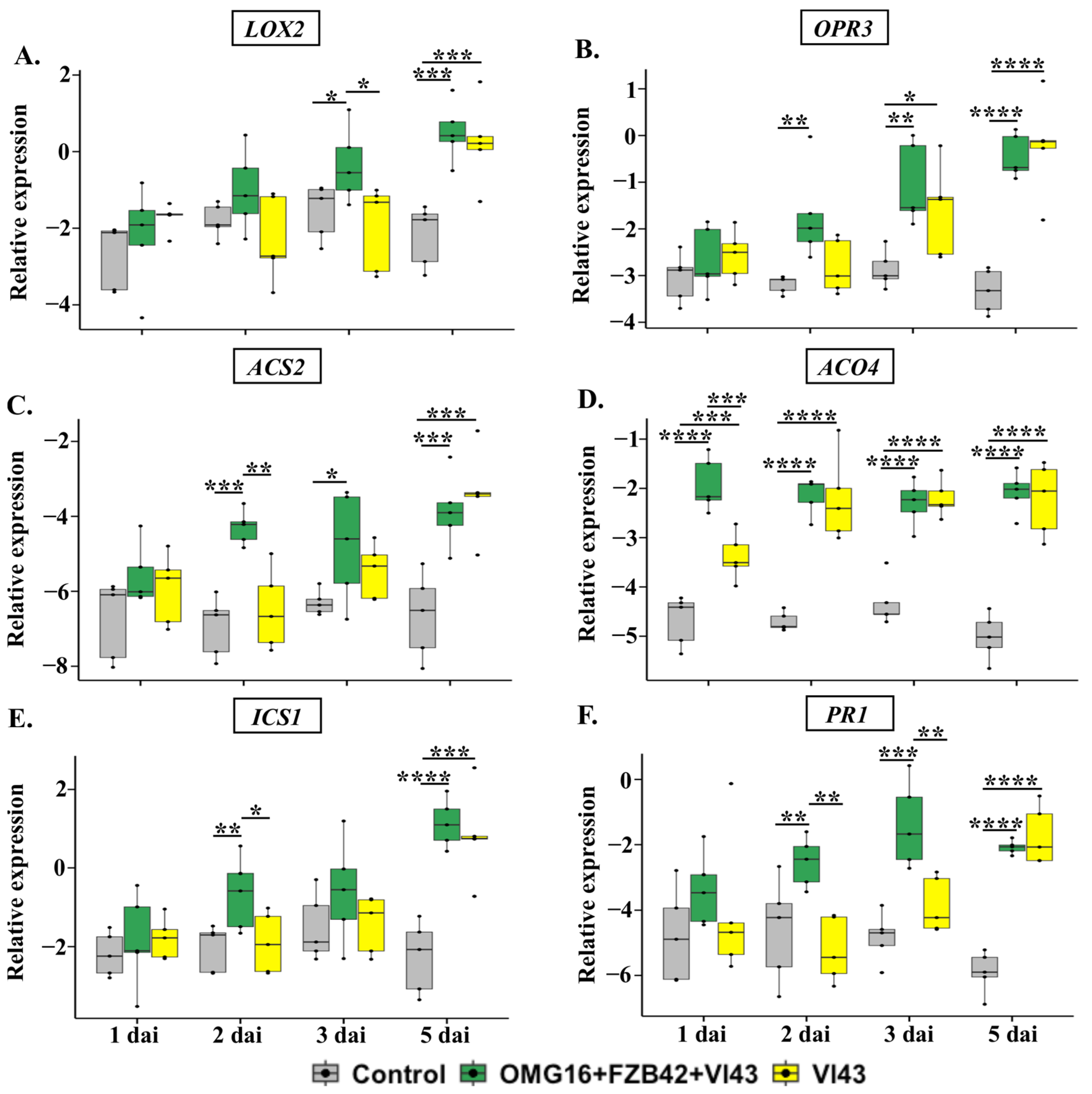
2.4.2. The Expression of Defence-Related Genes in Stems
2.4.3. The Expression of Defence-Related Genes in Leaves
2.5. Determination of Stress-Related Phytohormonal Homeostasis
2.5.1. Phytohormone Contents in Root Tissue
2.5.2. Phytohormone Contents in Shoot Tissues
3. Discussion
3.1. Microscopic Observation of V. longisporum Growth in Rapeseed Leaves
3.2. Priming Effects on Plant Growth
3.3. Priming Effects on Reducing Root Infection by Vl43
3.4. Priming Effects on Rapeseed-Enhanced Defence Activation after Vl43 Pathogen Challenge
3.4.1. Priming Effects on Roots
3.4.2. Priming Effects on Stems
3.4.3. Priming Effects on Leaves
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Microbial Priming and Pathogen Infection
4.3. Microscopic Analysis of Vl43-Infected Rapeseed Leaf Tissue
4.4. Absolute Quantification of Vl43 DNA in Root Samples via qPCR
4.5. Gene Expression Studies with Total RNA Extraction, cDNA Synthesis and qRT-PCR
4.6. Determination of Phytohormones Using UHPLC-MS Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, M.; Kühn, A.; Harting, R.; Maurus, I.; Nagel, A.; Starke, J.; Kusch, H.; Valerius, O.; Feussner, K.; Feussner, I.; et al. Verticillium longisporum Elicits Media-Dependent Secretome Responses With Capacity to Distinguish Between Plant-Related Environments. Front. Microbiol. 2020, 11, 1876. [Google Scholar] [CrossRef] [PubMed]
- Novakazi, F.; Inderbitzin, P.; Sandoya, G.; Hayes, R.J.; von Tiedemann, A.; Subbarao, K.V. The Three Lineages of the Diploid Hybrid Verticillium longisporum Differ in Virulence and Pathogenicity. Phytopathology 2015, 105, 662–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harting, R.; Starke, J.; Kusch, H.; Pöggeler, S.; Maurus, I.; Schlüter, R.; Landesfeind, M.; Bulla, I.; Nowrousian, M.; de Jonge, R.; et al. A 20-kb lineage-specific genomic region tames virulence in pathogenic amphidiploid Verticillium longisporum. Mol. Plant Pathol. 2021, 22, 939–953. [Google Scholar] [CrossRef]
- Depotter, J.R.; Deketelaere, S.; Inderbitzin, P.; Tiedemann, A.V.; Höfte, M.; Subbarao, K.V.; Wood, T.A.; Thomma, B.P. Verticillium longisporum, the invisible threat to oilseed rape and other brassicaceous plant hosts. Mol. Plant Pathol. 2016, 17, 1004–1016. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.T.; Harting, R.; Shalaby, S.; Kenerley, C.M.; Braus, G.H.; Horwitz, B.A. Adhesion as a Focus in Trichoderma-Root Interactions. J. Fungi 2022, 8, 372. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; Von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Kamble, A.; Koopmann, B.; Von Tiedemann, A. Induced resistance to Verticillium longisporum in Brassica napus by β-aminobutyric acid. Plant Pathol. 2013, 62, 552–561. [Google Scholar] [CrossRef]
- Heale, J.B.; Karapapa, V.K. The Verticillium threat to Canada’s major oilseed crop: Canola. Can. J. Plant Pathol. 1999, 21, 1–7. [Google Scholar] [CrossRef]
- Sarenqimuge, S.; Rahman, S.; Wang, Y.; von Tiedemann, A. Dormancy and germination of microsclerotia of Verticillium longisporum are regulated by soil bacteria and soil moisture levels but not by nutrients. Front. Microbiol. 2022, 13, 979218. [Google Scholar] [CrossRef]
- Dunker, S.; Keunecke, H.; Steinbach, P.; Von Tiedemann, A. Impact of Verticillium longisporum on yield and morphology of winter oilseed rape (Brassica napus) in relation to systemic spread in the plant. J. Phytopathol. 2008, 156, 698–707. [Google Scholar] [CrossRef]
- Wang, Y.; Strelkov, S.E.; Hwang, S.F. Blackleg Yield Losses and Interactions with Verticillium Stripe in Canola (Brassica napus) in Canada. Plants 2023, 12, 434. [Google Scholar] [CrossRef]
- Yadeta, K.A.; Hanemian, M.; Smit, P.; Hiemstra, J.A.; Pereira, A.; Marco, Y.; Thomma, B.P. The Arabidopsis thaliana DNA-binding protein AHL19 mediates Verticillium wilt resistance. Mol. Plant Microbe Interact. 2011, 24, 1582–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadeta, K.A.; Valkenburg, D.J.; Hanemian, M.; Marco, Y.; Thomma, B.P. The Brassicaceae-specific EWR1 gene provides resistance to vascular wilt pathogens. PLoS ONE 2014, 9, e88230. [Google Scholar] [CrossRef] [Green Version]
- Obermeier, C.; Hossain, M.A.; Snowdon, R.; Knüfer, J.; von Tiedemann, A.; Friedt, W. Genetic analysis of phenylpropanoid metabolites associated with resistance against Verticillium longisporum in Brassica napus. Mol. Breed. 2013, 31, 347–361. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.; Li, X.; Ma, L.; Gu, Q.; Wu, H.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X. Stomatal Closure and SA-, JA/ET-Signaling Pathways Are Essential for Bacillus amyloliquefaciens FZB42 to Restrict Leaf Disease Caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Alkooranee, J.T.; Aledan, T.R.; Ali, A.K.; Lu, G.; Zhang, X.; Wu, J.; Fu, C.; Li, M. Detecting the Hormonal Pathways in Oilseed Rape behind Induced Systemic Resistance by Trichoderma harzianum TH12 to Sclerotinia sclerotiorum. PLoS ONE 2017, 12, e0168850. [Google Scholar] [CrossRef] [Green Version]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—A review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, V.B.; Meyer, T.; Arias, A.A.; Ongena, M.; Oni, F.E.; Höfte, M. Bacillus Cyclic Lipopeptides Iturin and Fengycin Control Rice Blast Caused by Pyricularia oryzae in Potting and Acid Sulfate Soils by Direct Antagonism and Induced Systemic Resistance. Microorganisms 2021, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Koopmann, B.; von Tiedemann, A. Role of Salicylic Acid and Components of the Phenylpropanoid Pathway in Basal and Cultivar-Related Resistance of Oilseed Rape (Brassica napus) to Verticillium longisporum. Plants 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar] [CrossRef]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous monitoring of chemical signals in plants under stress. Nat. Rev. Chem. 2022, 7, 7–25. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, M.; Sharma, A.; Sharma, V. Insights into Plant Beneficial Microorganism-Triggered Induced Systemic Resistance. Plant Stress 2023, 7, 100140. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.; Van Wees, S.; Ton, J.; Van Pelt, J.; Van Loon, L. Signalling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biology 2002, 4, 535–544. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; van Wees, S.C.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, L.C.; Bakker, P.A.; Pieterse, C.M. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, N.; Chen, J.Y.; Subbarao, K.V.; Klosterman, S.J. Hormone Signaling and Its Interplay With Development and Defense Responses in Verticillium-Plant Interactions. Front. Plant Sci. 2020, 11, 584997. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Timmermann, T.; González, B.; Ruz, G.A. Reconstruction of a gene regulatory network of the induced systemic resistance defense response in Arabidopsis using boolean networks. BMC Bioinform. 2020, 21, 142. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Marwal, A. Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress 2022, 5, 100103. [Google Scholar] [CrossRef]
- He, X.; Jiang, J.; Wang, C.Q.; Dehesh, K. ORA59 and EIN3 interaction couples jasmonate-ethylene synergistic action to antagonistic salicylic acid regulation of PDF expression. J. Integr. Plant Biol. 2017, 59, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillmer, R.A.; Tsuda, K.; Rallapalli, G.; Asai, S.; Truman, W.; Papke, M.D.; Sakakibara, H.; Jones, J.D.G.; Myers, C.L.; Katagiri, F. The highly buffered Arabidopsis immune signaling network conceals the functions of its components. PLoS Genet. 2017, 13, e1006639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Tsuda, K.; Igarashi, D.; Hillmer, R.A.; Sakakibara, H.; Myers, C.L.; Katagiri, F. Mechanisms underlying robustness and tunability in a plant immune signaling network. Cell Host Microbe 2014, 15, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Mine, A.; Nobori, T.; Salazar-Rondon, M.C.; Winkelmüller, T.M.; Anver, S.; Becker, D.; Tsuda, K. An incoherent feed-forward loop mediates robustness and tunability in a plant immune network. EMBO Rep. 2017, 18, 464–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, K.; Mine, A.; Bethke, G.; Igarashi, D.; Botanga, C.J.; Tsuda, Y.; Glazebrook, J.; Sato, M.; Katagiri, F. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet. 2013, 9, e1004015. [Google Scholar] [CrossRef]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network properties of robust immunity in plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef] [Green Version]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Xu, J.; Höfte, M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014, 5, 611. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Berens, M.L.; Tsuda, K.; Argueso, C.T. Towards engineering of hormonal crosstalk in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, F.B.; Moradtalab, N.; Goertz, S.; Rietz, S.; Dietel, K.; Rozhon, W.; Humbeck, K.; Geistlinger, J.; Neumann, G.; Schellenberg, I. Synergistic Effects of a Root-Endophytic Trichoderma Fungus and Bacillus on Early Root Colonization and Defense Activation Against Verticillium longisporum in Rapeseed. Mol. Plant Microbe Interact. 2022, 35, 380–392. [Google Scholar] [CrossRef]
- Zheng, X.; Lopisso, D.T.; Eseola, A.B.; Koopmann, B.; von Tiedemann, A. Potential for Seed Transmission of Verticillium longisporum in Oilseed Rape (Brassica napus). Plant Dis. 2019, 103, 1843–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mpanga, I.K.; Gomez-Genao, N.; Moradtalab, N.; Wanke, D.; Chrobaczek, V.; Ahmed, A.; Windisch, S.; Geistlinger, J.; Hafiz, F.B.; Walker, F.J.; et al. The role of N form supply for PGPM-host plant interactions in maize. J. Plant Nutr. Soil Sci. 2019, 182, 908–920. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Avilés, M.; Trillas, I. Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017, 5, 1005–1026. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Uresti-Rivera, E.E.; Castro-Longoria, E.; Herrera-Estrella, A.; Casas-Flores, S. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Wang, R.; Chen, D.; Khan, R.A.A.; Cui, J.; Hou, J.; Liu, T. A novel Trichoderma asperellum strain DQ-1 promotes tomato growth and induces resistance to gray mold caused by Botrytis cinerea. FEMS Microbiol. Lett. 2021, 368, fnab140. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Behboudi, K.; Ahmadzadeh, M.; Javan-Nikkhah, M.; Zamioudis, C.; Pieterse, C.M.; Bakker, P.A. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol. Control. 2013, 65, 14–23. [Google Scholar] [CrossRef]
- Ralhan, A.; Schöttle, S.; Thurow, C.; Iven, T.; Feussner, I.; Polle, A.; Gatz, C. The vascular pathogen Verticillium longisporum requires a jasmonic acid-independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol. 2012, 159, 1192–1203. [Google Scholar] [CrossRef] [Green Version]
- Johansson, A.; Staal, J.; Dixelius, C. Early responses in the Arabidopsis-Verticillium longisporum pathosystem are dependent on NDR1, JA- and ET-associated signals via cytosolic NPR1 and RFO1. Mol. Plant Microbe Interact. 2006, 19, 958–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, P.; Narasimhan, M.L.; Stevenson, R.A.; Zhu, J.K.; Weller, S.C.; Subbarao, K.V.; Bressan, R.A. Identification of a locus controlling Verticillium disease symptom response in Arabidopsis thaliana. Plant J. 2003, 35, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Behrens, F.H.; Schenke, D.; Hossain, R.; Ye, W.; Schemmel, M.; Bergmann, T.; Häder, C.; Zhao, Y.; Ladewig, L.; Zhu, W.; et al. Suppression of abscisic acid biosynthesis at the early infection stage of Verticillium longisporum in oilseed rape (Brassica napus). Mol. Plant Pathol. 2019, 20, 1645–1661. [Google Scholar] [CrossRef]
- Ratzinger, A.; Riediger, N.; von Tiedemann, A.; Karlovsky, P. Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum. J. Plant Res. 2009, 122, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.M.; Lian, Q.G.; Chen, J.; Jia, R.M.; Zong, Z.F.; Ma, Q.; Wang, Y. The Improved Biocontrol Agent, F1-35, Protects Watermelon against Fusarium Wilt by Triggering Jasmonic Acid and Ethylene Pathways. Microorganisms 2022, 10, 1710. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Li, Y.; Wang, X.; Chen, J. Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the Biocontrol of Wheat Head Blight. J. Fungi 2022, 8, 1250. [Google Scholar] [CrossRef]
- Sun, K.; Xie, X.-G.; Lu, F.; Zhang, F.-M.; Zhang, W.; He, W.; Dai, C.-C. Peanut preinoculation with a root endophyte induces plant resistance to soil-borne pathogen Fusarium oxysporum via activation of salicylic acid-dependent signaling. Plant Soil 2021, 460, 297–312. [Google Scholar] [CrossRef]
- Sarosh, B.R.; Danielsson, J.; Meijer, J. Transcript profiling of oilseed rape (Brassica napus) primed for biocontrol differentiate genes involved in microbial interactions with beneficial Bacillus amyloliquefaciens from pathogenic Botrytis cinerea. Plant Mol. Biol. 2009, 70, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Gkizi, D.; Lehmann, S.; L’Haridon, F.; Serrano, M.; Paplomatas, E.J.; Métraux, J.P.; Tjamos, S.E. The Innate Immune Signaling System as a Regulator of Disease Resistance and Induced Systemic Resistance Activity Against Verticillium dahliae. Mol. Plant-Microbe Interact. 2016, 29, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morán-Diez, M.E.; Carrero-Carrón, I.; Rubio, M.B.; Jiménez-Díaz, R.M.; Monte, E.; Hermosa, R. Transcriptomic Analysis of Trichoderma atroviride Overgrowing Plant-Wilting Verticillium dahliae Reveals the Role of a New M14 Metallocarboxypeptidase CPA1 in Biocontrol. Front. Microbiol. 2019, 10, 1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef] [Green Version]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon Improves Chilling Tolerance During Early Growth of Maize by Effects on Micronutrient Homeostasis and Hormonal Balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]




| Treatment | Vl43 Infection | OMG16-FZB42 Priming + Vl43 Infection |
|---|---|---|
| Days after Infection (dai) | ng Vl43 DNA from 15 ng Root DNA | ng Vl43 DNA from 15 ng Root DNA |
| 1 | 40.8 (±4.8) | 2.1 (±0.3) **** |
| 2 | 111.4 (±21.7) | 3.5 (±1.0) *** |
| 3 | 229.5 (±33.8) | 1.4 (±0.7) **** |
| 5 | 271.7 (±15.8) | 15.2 (±9.5) **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafiz, F.B.; Geistlinger, J.; Al Mamun, A.; Schellenberg, I.; Neumann, G.; Rozhon, W. Tissue-Specific Hormone Signalling and Defence Gene Induction in an In Vitro Assembly of the Rapeseed Verticillium Pathosystem. Int. J. Mol. Sci. 2023, 24, 10489. https://doi.org/10.3390/ijms241310489
Hafiz FB, Geistlinger J, Al Mamun A, Schellenberg I, Neumann G, Rozhon W. Tissue-Specific Hormone Signalling and Defence Gene Induction in an In Vitro Assembly of the Rapeseed Verticillium Pathosystem. International Journal of Molecular Sciences. 2023; 24(13):10489. https://doi.org/10.3390/ijms241310489
Chicago/Turabian StyleHafiz, Fatema Binte, Joerg Geistlinger, Abdullah Al Mamun, Ingo Schellenberg, Günter Neumann, and Wilfried Rozhon. 2023. "Tissue-Specific Hormone Signalling and Defence Gene Induction in an In Vitro Assembly of the Rapeseed Verticillium Pathosystem" International Journal of Molecular Sciences 24, no. 13: 10489. https://doi.org/10.3390/ijms241310489
APA StyleHafiz, F. B., Geistlinger, J., Al Mamun, A., Schellenberg, I., Neumann, G., & Rozhon, W. (2023). Tissue-Specific Hormone Signalling and Defence Gene Induction in an In Vitro Assembly of the Rapeseed Verticillium Pathosystem. International Journal of Molecular Sciences, 24(13), 10489. https://doi.org/10.3390/ijms241310489







