Synonymous Variants of Uncertain Silence
Abstract
:1. Introduction
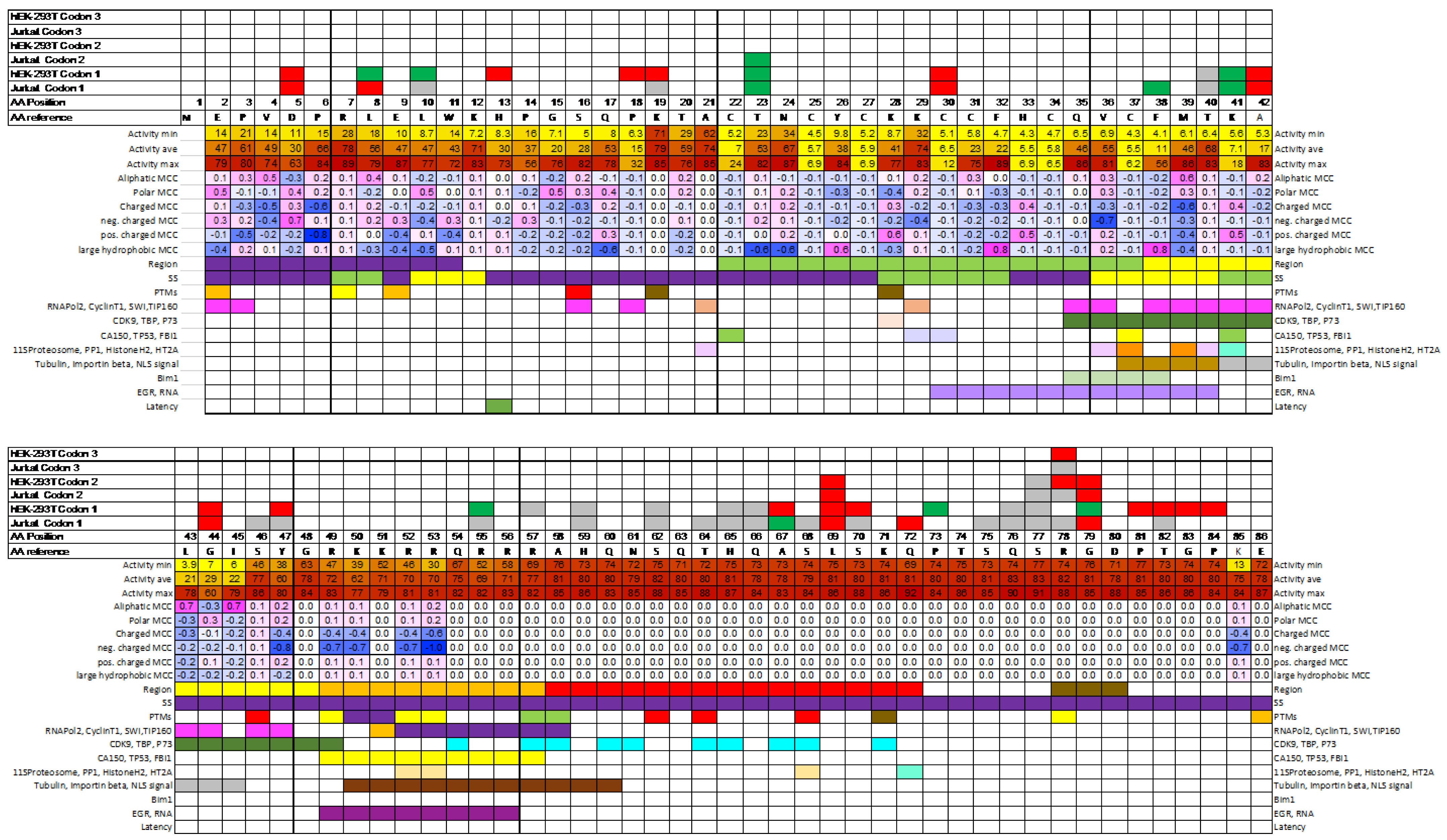
2. Results
3. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nirenberg, M.W.; Matthaei, J.H. The Dependence of Cell-Free Protein Synthesis in E. coli upon Naturally Occurring or Synthetic Polyribonucleotides. Proc. Natl. Acad. Sci. USA 1961, 47, 1588–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M. Preponderance of Synonymous Changes as Evidence for the Neutral Theory of Molecular Evolution. Nature 1977, 267, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.F.; Alonso Morales, L.A.; Kassen, R. Effects of Synonymous Mutations beyond Codon Bias: The Evidence for Adaptive Synonymous Substitutions from Microbial Evolution Experiments. Genome Biol. Evol. 2021, 13, evab141. [Google Scholar] [CrossRef]
- Bin, Y.; Wang, X.; Zhao, L.; Wen, P.; Xia, J. An Analysis of Mutational Signatures of Synonymous Mutations across 15 Cancer Types. BMC Med. Genet. 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.C.; Simhadri, V.L.; Iandoli, M.; Sauna, Z.E.; Kimchi-Sarfaty, C. Exposing Synonymous Mutations. Trends Genet. 2014, 30, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Zhao, F. Synonymous but Not Silent: The Codon Usage Code for Gene Expression and Protein Folding. Annu. Rev. Biochem. 2021, 90, 375–401. [Google Scholar] [CrossRef] [PubMed]
- Mankodi, A.; Ashizawa, T. Echo of Silence: Silent Mutations, RNA Splicing, and Neuromuscular Diseases. Neurology 2003, 61, 1330–1331. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Kimchi-Sarfaty, C. Understanding the Contribution of Synonymous Mutations to Human Disease. Nat. Rev. Genet. 2011, 12, 683–691. [Google Scholar] [CrossRef]
- Seton-Rogers, S. Silent Mutations Make Noise. Nat. Rev. Cancer 2022, 22, 257. [Google Scholar] [CrossRef]
- Shabalina, S.A.; Spiridonov, N.A.; Kashina, A. Sounds of Silence: Synonymous Nucleotides as a Key to Biological Regulation and Complexity. Nucleic Acids Res. 2013, 41, 2073–2094. [Google Scholar] [CrossRef] [Green Version]
- Lehner, B.; Crombie, C.; Tischler, J.; Fortunato, A.; Fraser, A.G. Systematic Mapping of Genetic Interactions in Caenorhabditis Elegans Identifies Common Modifiers of Diverse Signaling Pathways. Nat. Genet. 2006, 38, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Walsh, I.M.; Bowman, M.A.; Soto Santarriaga, I.F.; Rodriguez, A.; Clark, P.L. Synonymous Codon Substitutions Perturb Cotranslational Protein Folding in Vivo and Impair Cell Fitness. Proc. Natl. Acad. Sci. USA 2020, 117, 3528–3534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Y.; Sun, Y.; Wang, S.; Dong, M. Novel Homozygous Silent Mutation of ITGB3 Gene Caused Glanzmann Thrombasthenia. Front. Pediatr. 2022, 10, 1062900. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, S.; Li, C.; Zhang, J. Synonymous Mutations in Representative Yeast Genes Are Mostly Strongly Non-Neutral. Nature 2022, 606, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, S. Silence of the Mutations. J. Biosci. 2022, 47, 74. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Wang, Q.; Vitsios, D.; Burren, O.S.; Hu, F.; DiCarlo, J.E.; Kruglyak, L.; MacArthur, D.G.; Hurles, M.E.; Petrovski, S. A Minimal Role for Synonymous Variation in Human Disease. Am. J. Hum. Genet. 2022, 109, 2105–2109. [Google Scholar] [CrossRef]
- Kruglyak, L.; Beyer, A.; Bloom, J.S.; Grossbach, J.; Lieberman, T.D.; Mancuso, C.P.; Rich, M.S.; Sherlock, G.; Kaplan, C.D. Insufficient evidence for non-neutrality of synonymous mutations. Nature 2023, 616, E8–E9. [Google Scholar] [CrossRef]
- Giacoletto, C.J.; Schiller, M.R. The History and Conceptual Framework of Assays and Screens. BioEssays 2023, 45, 2200191. [Google Scholar] [CrossRef]
- Benjamin, R.; Giacoletto, C.J.; FitzHugh, Z.T.; Eames, D.; Buczek, L.; Wu, X.; Newsome, J.; Han, M.V.; Pearson, T.; Wei, Z.; et al. GigaAssay—An Adaptable High-Throughput Saturation Mutagenesis Assay Platform. Genomics 2022, 114, 110439. [Google Scholar] [CrossRef]
- Giacoletto, C.J.; Benjamin, R.; Deng, H.-W.; Rotter, J.I.; Schiller, M.R. Most Synonymous Allelic Variants in HIV Tat Are Not Silent. Genomics 2023, 115, 110603. [Google Scholar] [CrossRef]
- Cuevas, J.M.; Domingo-Calap, P.; Sanjuán, R. The Fitness Effects of Synonymous Mutations in DNA and RNA Viruses. Mol. Biol. Evol. 2012, 29, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan-Paiz, A.; Franco, S.; Martínez, M.A. Impact of Synonymous Genome Recoding on the HIV Life Cycle. Front. Microbiol. 2021, 12, 606087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Barr, I.; Guo, F.; Lee, C. Evidence of a Novel RNA Secondary Structurein the Coding Region of HIV-1 Pol Gene. RNA 2008, 14, 2478–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takata, M.A.; Soll, S.J.; Emery, A.; Blanco-Melo, D.; Swanstrom, R.; Bieniasz, P.D. Global Synonymous Mutagenesis Identifies Cis-Acting RNA Elements That Regulate HIV-1 Splicing and Replication. PLoS Pathog. 2018, 14, e1006824. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacoletto, C.J.; Rotter, J.I.; Grody, W.W.; Schiller, M.R. Synonymous Variants of Uncertain Silence. Int. J. Mol. Sci. 2023, 24, 10556. https://doi.org/10.3390/ijms241310556
Giacoletto CJ, Rotter JI, Grody WW, Schiller MR. Synonymous Variants of Uncertain Silence. International Journal of Molecular Sciences. 2023; 24(13):10556. https://doi.org/10.3390/ijms241310556
Chicago/Turabian StyleGiacoletto, Christopher J., Jerome I. Rotter, Wayne W. Grody, and Martin R. Schiller. 2023. "Synonymous Variants of Uncertain Silence" International Journal of Molecular Sciences 24, no. 13: 10556. https://doi.org/10.3390/ijms241310556




