Excitation of a Single Compound by Light and Ultrasound Enhanced the Long-Term Cure of Mice Bearing Prostate Tumors
Abstract
:1. Introduction
2. Results and Discussions
Biological Studies
- (a)
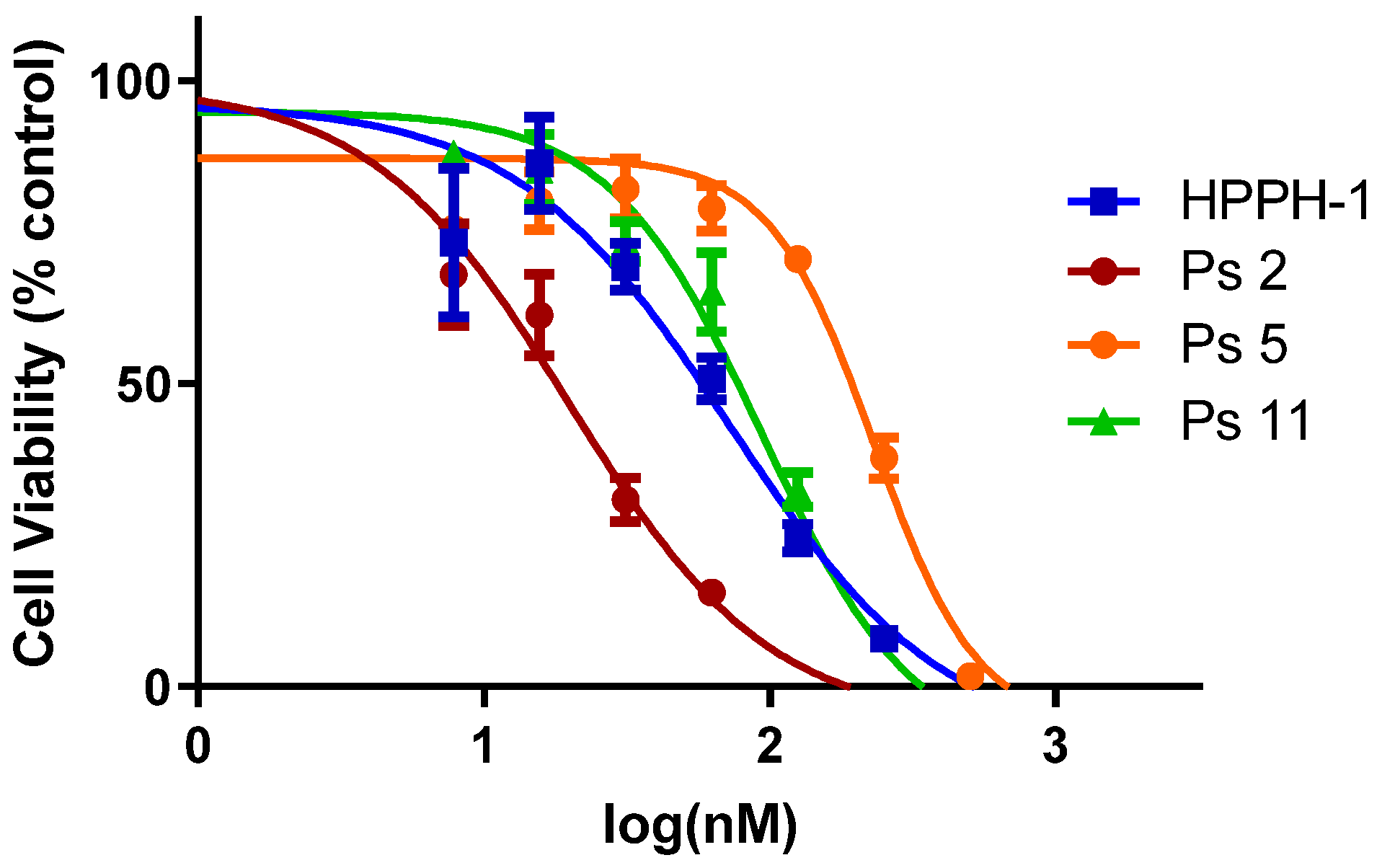
- In vitro photosensitizing efficacy: The in vitro data suggest that the addition of carbohydrate moieties to HPPH changes the biological activity in multiple ways; one of the most immediate and evident changes to the biological function of these derivatives is the change in overall lipophilicity. This in part governs the uptake, retention, and localization of the molecule which can substantially alter the PDT efficacy. To evaluate the in vitro efficacy of these compounds, the PSs were incubated in PC3 cells (known for overexpression of Gal-3) for 24 h and the photosensitizing efficacy of each PS was determined by the MTT assay. The IC50 values of HPPH were compared with a series of photosensitizers. The results summarized in Table 1 and Figure 2 indicate that among all the compounds, the HPPH conjugated with a b-galactose moiety at position 172- showed the best efficacy.
- (b)
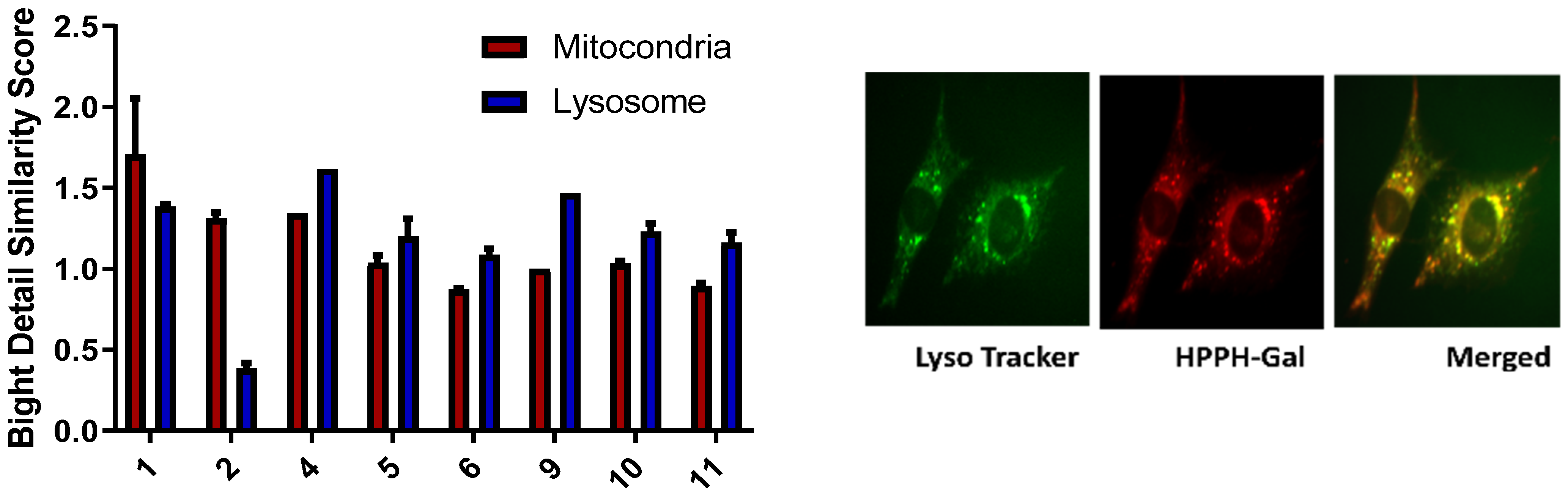
- Subcellular localization of HPPH and the corresponding carbohydrate analogs: The subcellular localization of a PS is an important property that is highly dependent on its chemical structure [41]. Previous studies have suggested that mitochondrial vs. lysosomal localization leads to two very different cell death pathways: localization of the PS in the mitochondria leads to cytochrome release and photoinduced necroptosis, while targeting the lysosome has been connected to autophagy [42]. To determine how galactose conjugation changes how HPPH localizes in the cell, the cells were visualized using an Amis Imagestream Mkit. HPPH has previously been shown to have a slight preference for mitochondrial localization in other cell lines, but in PC3 cell lines, there is no preference. The addition of a galactose moiety to HPPH either at the 17 or 20 position alters the localization dramatically. This observation is possibly due to Gal-3 receptor-mediated endocytosis and lysosomal localization. The subcellular localization of the representative galactose-conjugate 2 follows a similar distribution of HPPH, preferring the mitochondria over the lysosome. The mitochondrial bright detail similarity score observed from 2 was three times higher than the lysosomal detail similarity score. On the other hand, the HPPH mitochondrial bright detail similarity score was only 1.5 times greater than the lysosomal bright detail similarity score. Meanwhile, PS 11, the other galactose-conjugated photosensitizer which showed a high degree of PDT efficacy, slightly prefers the lysosome over the mitochondria (Figure 3). A similar trend was also observed in the other carbohydrate analogs.
- (c)
- Mechanisms of PDT and SDT in tumor cell kill: Sonodynamic therapy is a promising addition to photodynamic therapy which utilizes a different method of generating cell kill and subcellular damage [43]. Compound 2 was tested in the PC3 cells to determine the potential of PDT in combination with SDT. Both HPPH and compound 2 showed enhanced cell-killing efficacy when both treatment modalities are used in combination (Figure 4A,B). It is well established that PSs generate singlet oxygen species during exposure to an appropriate wavelength of light, which is responsible for inducing the cytotoxic effect. To determine if SDT is equally dependent on oxygen and utilizes singlet oxygen to induce cell kill, the reactive oxygen species (ROS) generated after exposing the compound used for PDT by US was identified in vitro using either carboxy-DCFDA (Figure 4C,D) or SOSG (singlet oxygen sensor green) for singlet oxygen (Figure 4E,F). Caboxy-DCFDA is a general indicator of ROS, while SOSG is a dye sensitive to only singlet oxygen [44]. The PC3 cells were then exposed to ultrasound and the fluorescence observed from the cells clearly indicates that the radical ions were formed by exciting the compound with ultrasound. The experimental result revealed that HPPH and compound 2 have similar efficacy when PDT was combined with SDT treatment.
- (d)
- SDT and hypoxia: The utility of SDT for treating cancer cells under a hypoxic environment was further confirmed. In brief, the PC3 cells were incubated with HPPH and PS 2 (1 μM). After 24 h incubation, some of the plates were placed in a hypoxic chamber at 1% O2 and 5% CO2 for 1 h to deplete the oxygen. The cells were then exposed to either light only or US only. At 24 h after exposure, the cells were counted using a trypan blue assay. As expected, light exposure (PDT) did not induce cell death due to the lack of oxygen needed to generate singlet oxygen, whereas US exposure produced significant cell kill mainly by radical ions (Type I reaction). In the control experiments, the cells were incubated with PS alone and were not exposed to light or US. The results depicted in Figure 4 show the advantage of SDT in treating hypoxic cells.
- (e)
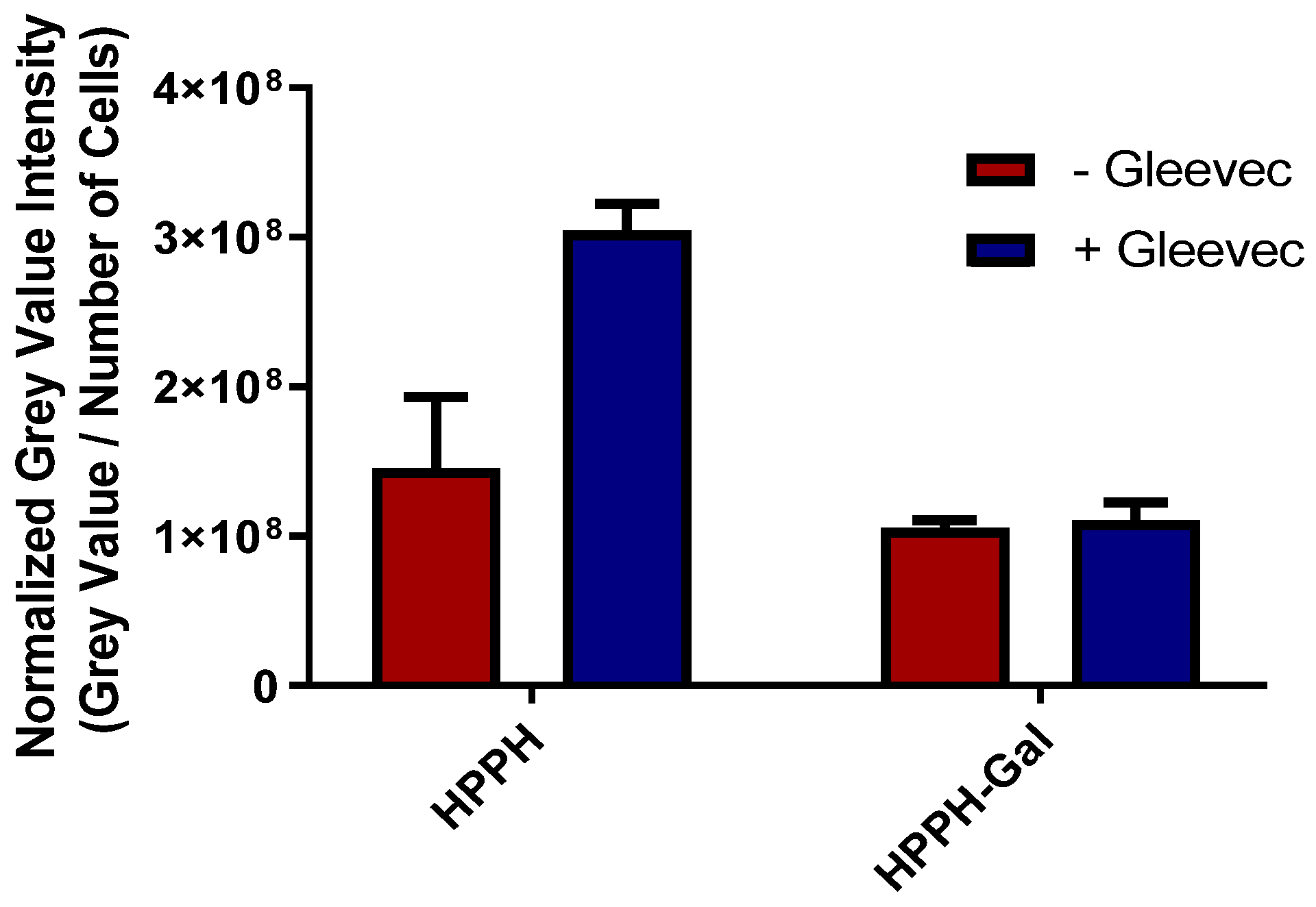
- In contrast to HPPH 1, HPPH–Gal 2 is not transported from PC3 cells by ABCG2: The ABCG2 protein is known to export many photosensitizers from tumor cells and has a significant negative impact on intracellular accumulation. Jonker et al. [45] showed that ABCG2 knock-out mice were photosensitive because of increased protoporphyrin IX (PP IX) levels. Robey et al. [46] reported that pheophorbide-a and its methyl ester analogs are a specific substrate for ABCG2. The results from our laboratory have shown that HPPH (hexyl ether derivative of pyropheophorbide-a) is a substrate of ABCG2, and the presence of a certain tyrosine kinase inhibitor (Gleevec) enhances its PDT efficacy [47]. We extended this approach in the PC3 tumor cell line and observed that Gleevec enhances the uptake of HPPH by inhibiting efflux from the cell. Interestingly, the addition of Gleevec did not have a significant impact on the accumulation of conjugate 2 (Figure 5), which suggests that HPPH–galactose conjugates are not a target of ABCG2 efflux, thus having higher clinical PDT efficacy [48].
- (f)
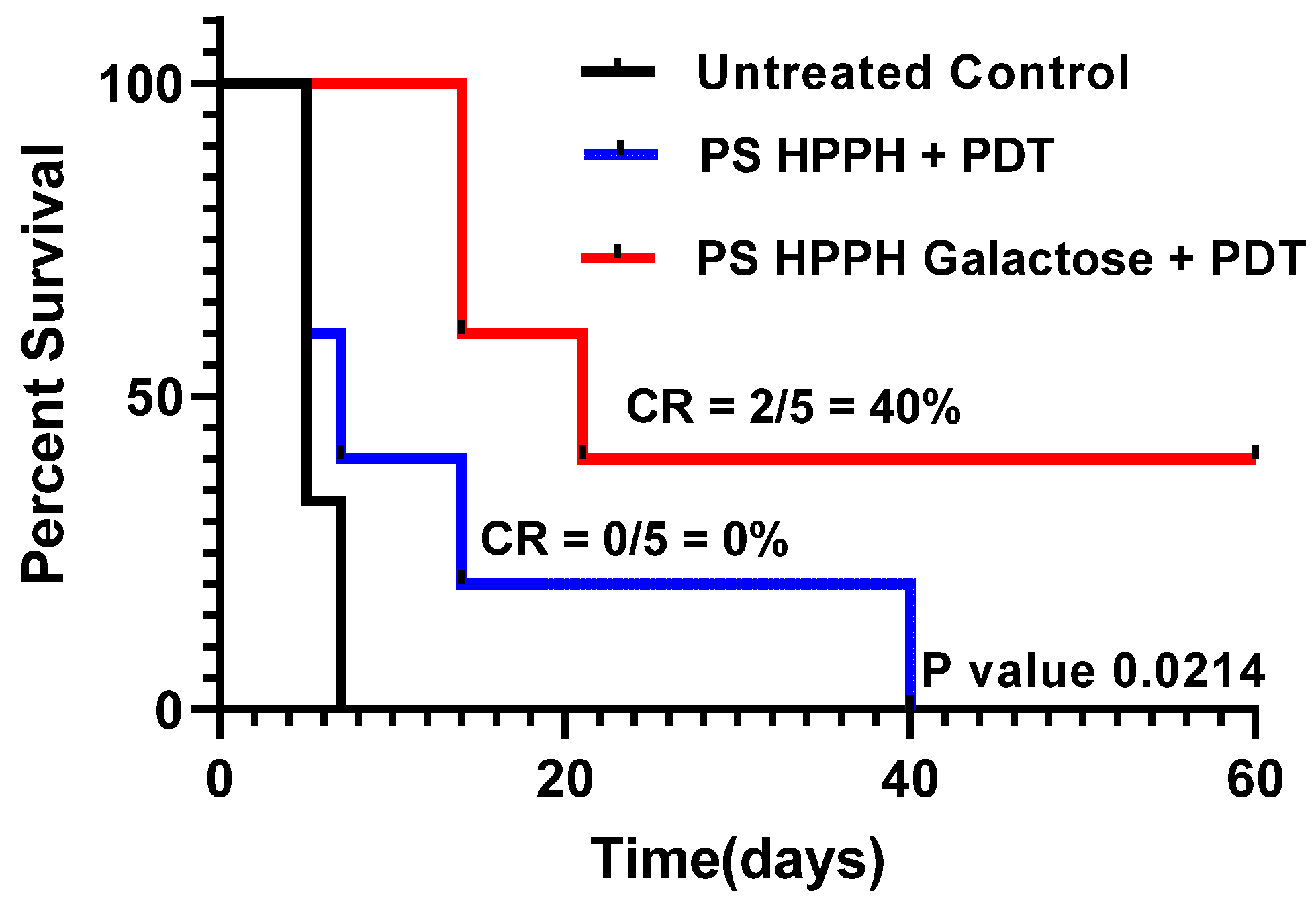
- Compared to HPPH 1, HPPH–Galactose 2 showed improved PDT efficacy in vivo: The PDT efficacy of both the PSs was investigated in SCID mice bearing PC3 tumor xenografts. When the tumor was around 200–250 mm3 in size, each PS was injected at a dose of 0.47 mmole/kg, and at 24 h post injection, the tumors were exposed to light (135 J/cm2; 75 mW/cm2) (two sets of experiments; five mice/group). Tumor growth was monitored daily. The results summarized in Figure 6 indicate that the tumor regrew in all mice treated with HPPH-PDT at day 60, and the mice were euthanized. Under similar treatment parameters, HPPG–Gal 2 showed improved long-term cure, and 2/5 mice were tumor-free on day 60. These results suggest that the presence of a galactose moiety at the appropriate position of the macrocycle and also the overall lipophilicity maintained by introducing a hexyl ether side chain at position 3 made a significant difference to the biological efficacy in vivo. This was a proof-of-principle study, and the treatment parameters are currently being optimized.
- (g)
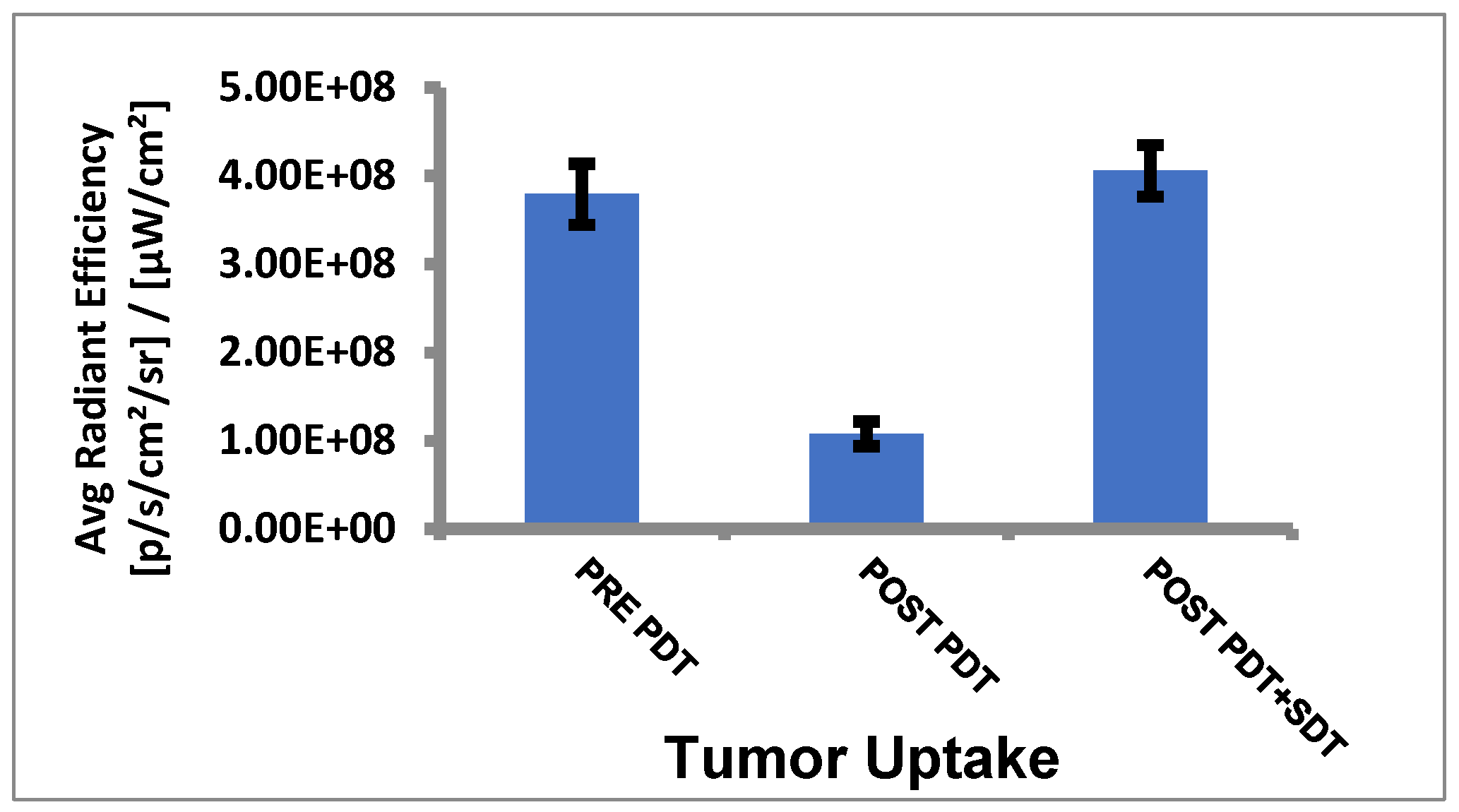
- Tumor uptake of HPPH before and after PDT/SDT in mice bearing PC3 tumors: The mice were imaged for HPPH, and uptake was determined by measuring the fluorescence intensity in the tumors by using an IVIS system (Perkin Elmer) at various time intervals after injecting the HPPH (dose: 0.47 μmole/kg) into the SCID mice bearing PC3 tumors (3 mice/group) at 24 h (pre PDT), post PDT, and post PDT–SDT treatment. Images (fluorescence) were collected at various time intervals, and the average radiant efficiency of HPPH in the tumors was determined. The results summarized in Figure 7 show a significant uptake of HPPH at 24 h post PS administration. After light treatment, a significant amount of HPPH photobleaches, resulting in reduced fluorescence intensity. However, after irradiating the tumor with ultrasound (US), the concentration of HPPH in the tumor increases, which was surprising and interesting as well. These results suggest that (a) exposure of tumors to US after light treatment further dilates the intratumoral blood vessels, which possibly allows more uptake of the PS from circulation, and subsequently enhances the fluorescence intensity of the PS in the tumor, and (b) US exposure did not photobleach the PS.
- (h)
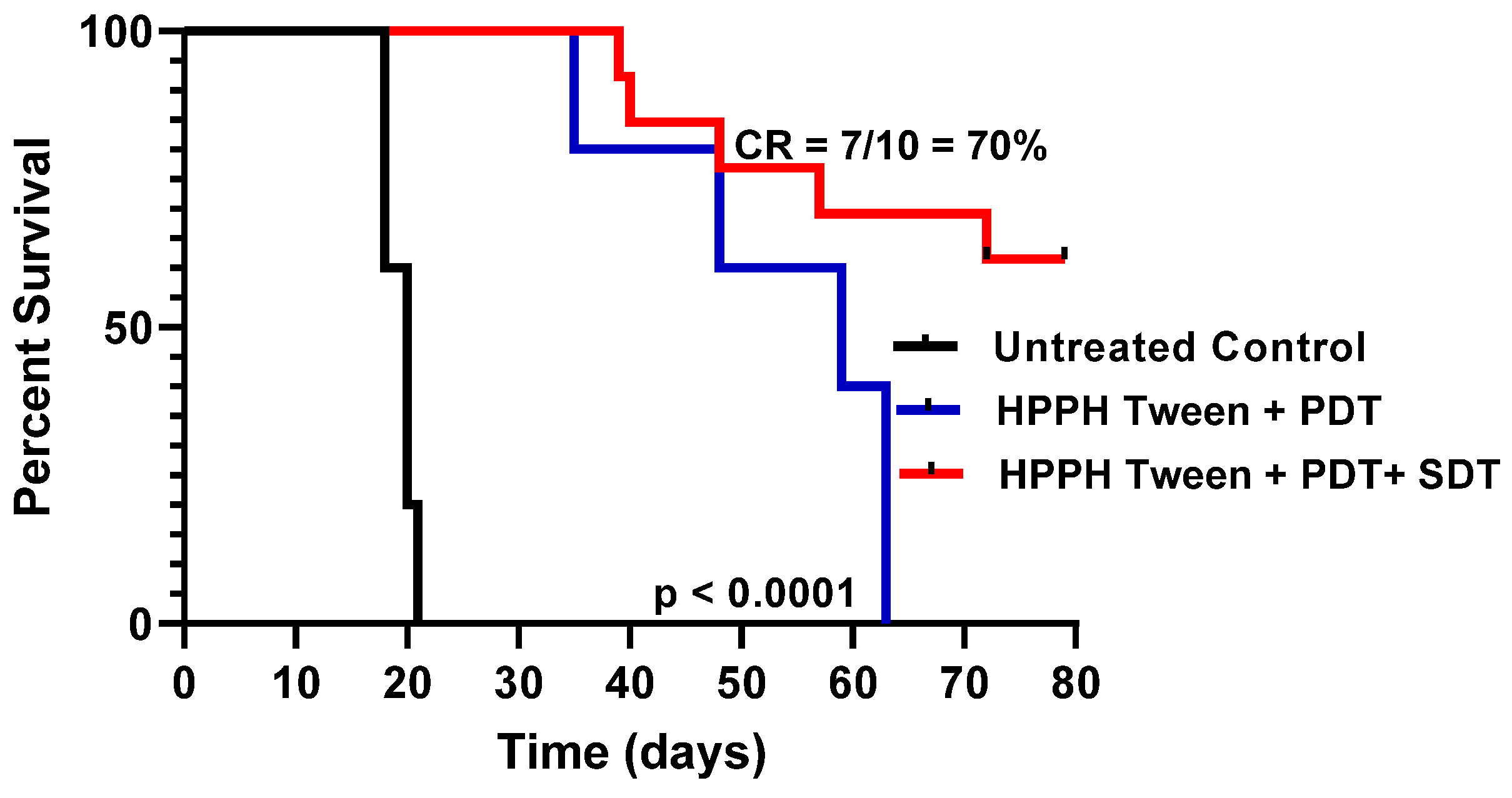
- PDT in combination with SDT showed enhanced PDT efficacy: HPPH 1 formulated in 1% Tween80 was intravenously injected into PC3 tumor-bearing mice at a dose of 0.47 μmol/kg. The drug dose (0.47 mmol/kg) was selected based on our previous study with HPPH in other tumor types, where it showed moderate long-term anticancer activity. For our initial study, PC3 tumors were implanted into SCID mice (5 mice/group), and when the tumor size was around 200–250 mm3, the PS was intravenously injected. At 24 h post injection, the tumors were exposed to light (135 J/cm2, 75 mW/cm2), and tumor growth was monitored. In another set of experiments, a group of SCID mice bearing PC-3 tumors were treated with light alone (control). After PDT treatment, these tumors were exposed to ultrasound (US dose: 2 W/cm2 for 30 min), and tumor regrowth was measured three times a week following the IACUC-approved animal protocol. Once the mice reached a tumor > 400 mm3, they were euthanized. The results illustrated in Figure 8 show a significantly improved long-term tumor cure of mice with a combination of PDT + SDT treatment over PDT alone.
- (i)
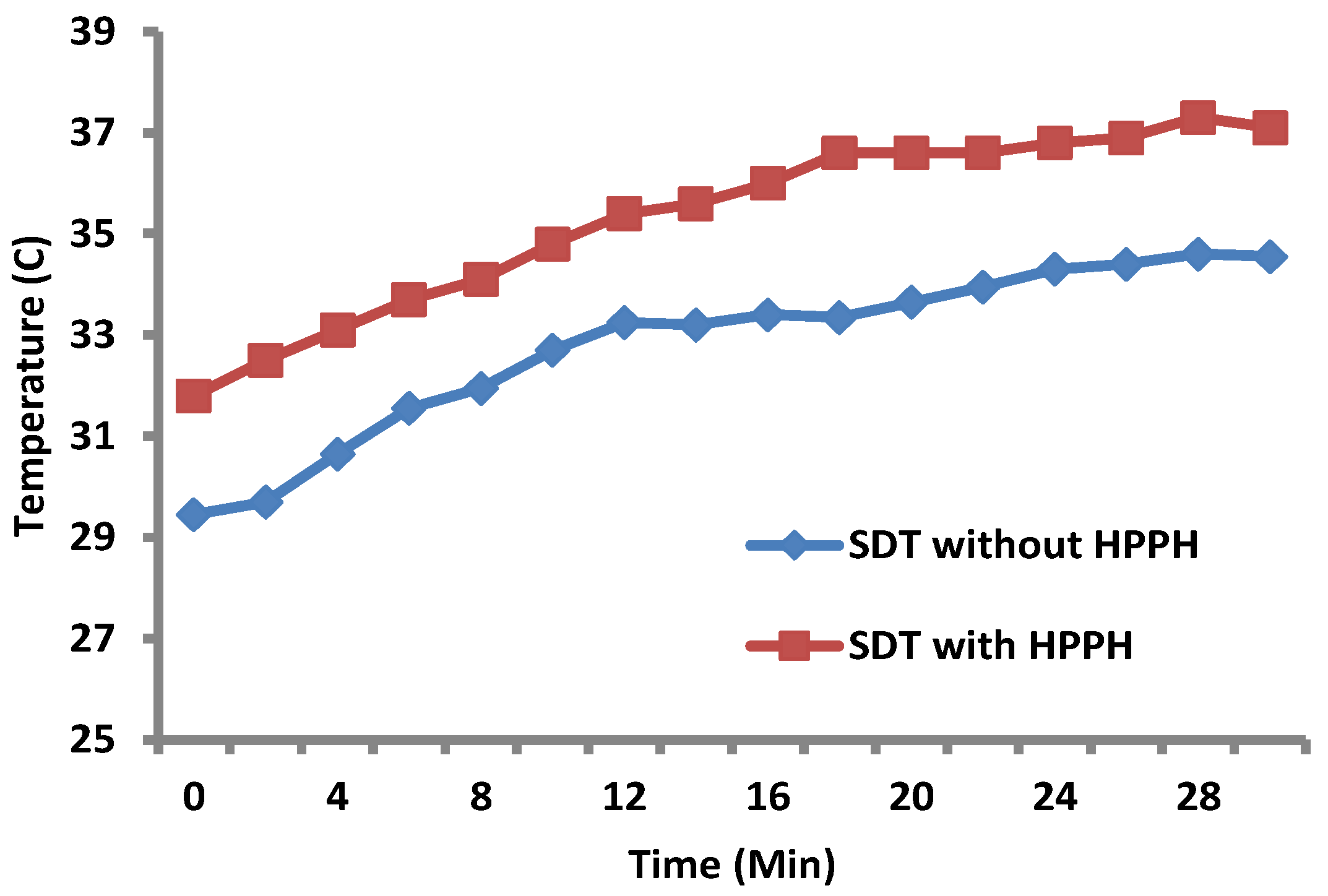
- Increase in tumor temperature (hyperthermia) during SDT: HPPH 1 at a dose of 0.47 µm/kg was injected into the tail vein of a PC3 = tumor-bearing mouse at 24 h prior to SDT. Sonodynamic therapy was carried out at the ultrasound dose of 3 MHz for 30 min with the temperature probe inserted directly into the tumor. In the control mice, the same procedure was followed except no HPPH in 1% Tween formulation was injected prior to SDT. The results shown in Figure 10 indicate an increase of 4–5 °C in tumor temperature on exposing the tumors with and without the presence of the PS (HPPH). The intra-tumoral temperature in mouse 1 (US alone) increased from 29 to 34 °C and in mouse 2 (US + HPPH), it increased from 31 to 37 °C.
3. Methods and Materials
3.1. Chemistry
3.2. In Vitro Cell Culture
3.3. In Vitro Viability Assay of Photodynamic Therapy in Combination with Sonodynamic Therapy
3.4. Intracellular Localization
3.5. Determination of Reactive Oxygen Species
3.6. Method for Tumor Implantation
3.7. Determination of In Vivo PDT and SDT Efficacy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hoffman, R.M.; Gilliand, F.D.; Ely, W.; Harlan, L.C.; Stephenson, J.L.; Albertson, P.C.; Hamilton, A.S.; Hunt, W.U.; Potosky, A.L. Racial and ethnic differences in advanced-stage prostate cancer: The Prostate cancer outcome study. J. Natl. Cancer Inst. 2001, 93, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Pietro, G.D.; Chomokur, G.; Kumar, N.B.; Davis, C.; Park, J.Y. Racial differences in the diagnosis and treatment of prostate cancer. Int. Neurourol. J. 2016, 20, S112–S119. [Google Scholar] [CrossRef] [Green Version]
- Lehto, U.-S.; Tenhola, H.; Taari, K.; Aromaa, A. Patients’ perceptions of the negative effects following different prostate cancer treatments and the impact on psychological well-being: A nationwide survey. Br. J. Cancer 2017, 116, 864–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, S.; Zhou, N.; Laughlin, M.M.; Rice, S.; Pillai, A.K.; Hao, G.; Dun, X. PSMA-targeting imaging and theranostic agents- current status and future perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic Therapy: A review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Tracy, E.C.; Bowman, M.; Pandey, R.K.; Baumann, H. Cell-specific retention and action of pheophorbidebased photosensitizers in human lung cancer cells. Photochem. Photobiol. 2019, 95, 846–885. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Cell death pathways associated with photodynamic therapy: An update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Coussios, C.C.; Farny, C.; ter Haar, G.; Roy, R.A. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int. J. Hyperth. 2007, 23, 105–120. [Google Scholar] [CrossRef]
- Choi, V.; Rajora, M.A.; Zheng, G. Activating drugs with sound: Mechanisms behind sonodynamic therapy and the role of nanomedicine. Bioconjug. Chem. 2020, 31, 967–989. [Google Scholar] [CrossRef]
- Sadanala, K.C.; Chaturvedi, P.K.; Seo, Y.M.; Kim, J.M.; Jo, Y.S.; Lee, Y.K.; Ahn, W.S. Sono-photodynamic combination therapy: A review on sensitizers. Anticancer Res. 2014, 34, 4657–4664. [Google Scholar]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Ahdoot, M.; Lebastchi, A.H.; Turkbey, B.; Wood, B.; Pinto, P.A. Contemporary treatments in prostate cancer focal therapy. Curr. Opin. Oncol. 2019, 31, 200–206. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Li, B. Photodynamic therapy for prostate cancer: A systematic review and meta-analysis. Prostate Int. 2019, 7, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, V.; Yang, X. Photodynamic therapy for prostate cancer: Recent advantages, challenges and opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, H.T.; Philippou, Y.; Magnussen, A.L.; Tullis, I.D.; Bridges, E.; Chatrian, A.; Lefebvre, J.; Tam, K.H.; Murphy, E.A.; Rittscher, J.; et al. Tumor irradiation combined with vascular-targeted photodynamic therapy enhances antitumor effects in pre-clinical prostate cancer. Br. J. Cancer 2021, 125, 534–546. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kitahara, S.; Kusuda, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Current landscape of sonodynamic therapy for cancer. Cancers 2021, 13, 6184. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.M.; Cacaccio, J.; Durrani, F.A.; Bshara, W.; Turowski, S.G.; Spernyak, J.A.; Pandey, R.K. Sonodynamic therapy in combination with photodynamic therapy shows enhanced long-term cure of brain tumor. Nat. Sci. Rep. 2020, 10, 21791. [Google Scholar] [CrossRef]
- Pandey, R.K.; Sumlin, A.B.; Constantine, S.; Aoudia, M.; Potter, W.R.; Bellnier, D.A.; Henderson, B.W.; Rodgers, M.A.; Smith, K.M.; Dougherty, T.J. Alkyl ether analogs of chlorophyll-a derivatives: Part-1. Synthesis, photophysical properties and photodynamic efficacy. Photochem. Photobiol. 1996, 64, 194–204. [Google Scholar] [CrossRef]
- Henderson, B.W.; Bellnier, D.A.; Greco, W.R.; Sharma, A.; Pandey, R.K.; Vaughan, L.A.; Weishaupt, K.R.; Dougherty, T.J. An in vivo quantitative structure-activity relationship for a congeneric series of Pyropheophorbide- derivatives as photosensitizers for photodynamic therapy. Cancer Res. 1997, 57, 4000–4007. [Google Scholar]
- Bellnier, D.A.; Greco, W.R.; Loewen, G.M.; Nava, H.; Oseroff, A.R.; Pandey, R.K.; Tsuchida, T.; Dougherty, T.J. Population pharmacokinetics of the photodynamic therapy agent 2-(1-hexyloxyethyl)-2-devinyl Pyropheophorbide-a in cancer patients. Cancer Res. 2003, 63, 1806–1813. [Google Scholar]
- Cacaccio, J.C.; Durrani, F.A.; Missert, J.R.; Pandey, R.K. Photodynamic therapy in combination with doxorubicin is superior to monotherapy for the treatment of lung cancer. Biomedicines 2022, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Nava, H.R.; Allamaneni, S.S.; Dougherty, T.J.; Cooper, M.T.; Tan, W.; Wilding, G.; Henderson, B.W. Photodynamic therapy using HPPH for the treatment of precancerous lesions associated with Barrett’s esophagus. Lasers Surg. Med. 2011, 43, 705–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigual, N.; Shafirstein, G.; Cooper, M.T.; Baumann, H.; Bellnier, D.A.; Sunar, U.; Tracy, E.C.; Rohrbach, D.J.; Wilding, G.; Tan, W.; et al. Photodynamic therapy with 3-(1′-hexyloxyethyl)Pyropheophorbide-a for cancer of the oral cavity. Clin. Cancer Res. 2013, 19, 6805–6813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivatsan, A.; Sen, A.; Cheruku, R.R.; Missert, J.R.; Durrani, F.A.; Guru, K.; Pandey, R.K. Whole body and local hyperthermia enhance the photosensitizing efficacy of 3-(1-hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH). Lasers Surg. Med. 2018, 50, 506–512. [Google Scholar] [CrossRef]
- Mei, X.; Ten Cate, R.; van Leeuwen, C.M.; Rodermond, H.M.; de Leeuw, L.; Dimitrakopoulou, D.; Stalpers, L.J.; Crezee, J.; Kok, H.P.; Franken, N.A.; et al. Radio-sensitization by hyperthermia: The effects of temperature, sequence, and time interval in cervical cell lines. Cancers 2020, 12, 582. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Alwjo, J.F.; Gomez, I.C.; Earl, J. Ultrasound in cancer therapy: A summary of their use and unexplored potential. Oncol. Rev. 2022, 16, 531. [Google Scholar]
- Cheruku, R.R.; Cacaccio, J.; Durrani, F.A.; Tabaczynski, W.A.; Watson, R.; Marko, A.J.; Kumar, R.; El-Khouly, M.E.; Fukuzumi, S.; Missert, J.R.; et al. Epidermal growth factor receptor-targeted multifunctional photosensitizers for bladder cancer imaging and therapy. J. Med. Chem. 2019, 62, 2587–2617. [Google Scholar] [CrossRef]
- Tracy, E.C.; Bowman, M.J.; Pandey, R.K.; Baumann, H. Tumor cell-specific retention of photosensitizers determines the outcome of photodynamic therapy for head and neck cancer. J. Photochem. Photobiol. B Biol. 2022, 234, 112513. [Google Scholar] [CrossRef]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-Da-Silva, L.C. Ligand targeted delivery of photosensitizers for cancer treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef]
- Laderach, D.J.; Gentilini, L.D.; Giribaldi, L.; Delgado, V.C.; Nugnes, L.; Croci, D.O.; Al Nakouzi, N.; Sacca, P.; Casas, G.; Mazza, O.; et al. A Unique Galectin Signature in Human Prostate Cancer Progression Suggests Galectin-1 as a Key Target for Treatment of Advanced Disease. Cancer Res. 2013, 73, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Marifio, K.V.; Cagnoni, A.J.; Croci, D.O.; Robinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316. [Google Scholar]
- Viguier, M.A.; Advedissian, T.; Detacour, D.; Poirier, F.; Deshayes, F. Galectins in epithelial functiona. Tissue Barriers 2014, 2, e29103. [Google Scholar] [CrossRef] [Green Version]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffer, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human Galectin-3 carbohydrate recognition domain at 2.1A0 Resolution. J. Bio. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.K.; Bold, B.; Lee, W.K.; Jeon, M.H.; An, K.H.; Jeong, S.Y.; Shim, Y.K. D-(+)-galactose-conjugated singled walled carbon nanotubes as new chemical probes for electrochemical biosensors for the cancer marker galection-3. Int. J. Mol. Sci. 2011, 12, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Subramanian, S.; Mallia, M.B.; Repaka, K.; Kaur, S.; Chandan, R.; Bhardwaj, P.; Dash, A.; Banerjee, R. Multifunctional core-shell glyconanoparticles for galectin-3 targeted, trigger-responsive combination chemotherapy. Biomacromolecules 2020, 21, 2645–2660. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Z.; He, S.; Fan, T.; Jin, Y.; Zhu, X.; Chen, C.; Zhang, Z.-r.; Huang, Y. Treatment of prostate carcinoma with (galectin-3)-targeted HPMA copolymer- (G3-C-12-5-Fluoracil conjugates. Biomaterials 2012, 33, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Zheng GGraham, A.; Shibata, M.; Missert, J.R.; Oseroff, A.R.; Dougherty, T.J.; Pandey, R.K. Synthesis of b-galactose conjugated chlorins derived by enyne-metathesis as galectin-specific photosensitizer for photodynamic therapy. J. Org. Chem. 2001, 66, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Morgan, J.; Pandey, S.K.; Chen, Y.; Tracy, E.; Baumann, H.; Missert, J.R.; Batt, C.; Jackson, J.; Bellnier, D.A.; et al. Conjugation of HPPH to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo. J. Med. Chem. 2009, 52, 4306–4318. [Google Scholar] [CrossRef] [Green Version]
- Dukh, M.; Cacaccio, J.; Durrani, F.A.; Kumar, I.; Watson, R.; Tabaczynski, W.A.; Joshi, P.; Missert, J.R.; Baumann, H.; Pandey, R.K. Impact of mono- and di-b-galactose moieties in in vitro/in vivo anticancer efficacy of Pyropheophorbide-carbohydrate conjugates by photodynamic therapy. Eur. J. Med. Chem. Rep. 2022, 5, 100047. [Google Scholar] [CrossRef] [PubMed]
- Tracy, E.C.; Joshi, P.; Dukh, M.; Durrani, F.A.; Pandey, R.K.; Baumann, H. Galactosyl, alkyl and acidic groups modify uptake and subcellular deposition of Pyropheophorbide-a by epithelial tumor cells and determine photosensitizing efficacy. J. Porphyr. Phthalocyanines 2023, in press. [Google Scholar] [CrossRef]
- Kessel, D. Correlation between subcellular localization and photodynamic therapy. J. Porphyr. Phthalocyanines 2004, 8, 1009–1014. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N. Initiation of autophagy by photodynamic therapy. Methods Enzymol. 2009, 453, 1–16. [Google Scholar] [PubMed] [Green Version]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar]
- Nath, P.; Hamadna, S.S.; Karamchand, L.; Foster, J.; Kopelman, R.; Amar, J.G.; Ray, A. Intracellular detection of singlet oxygen using fluorescent nanosensors. Analyst 2021, 146, 3933–3941. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; van der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plösch, T.; Kuipers, F.; Elferink, R.P.J.O.; Rosing, H.; et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef] [Green Version]
- Robey, R.W.; Streadman, K.; Polgar, D.; Morisaki, K.; Blayney, M.; Mistry, P.; Bates, S.E. Pheophorbide-a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004, 64, 1242. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.; Jackson, J.D.; Zheng, X.; Pandey, S.K.; Pandey, R.K. Subcellular affinity pf photosensitizers derived from chlorophyll-a: The ABCG 2transporter affects phototoxic response of side population stem cell like cancer cells to photodynamic therapy. Mol. Pharm. 2010, 7, 1789–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Baer, M.R.; Bowman, M.J.; Pera, P.; Zheng, X.; Morgan, J.; Pandey, R.A.; Oseroff, A.R. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin. Cancer Res. 2007, 13, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound | PC3 Cell Line (IC50 Value) | Log p |
|---|---|---|
| HPPH-1 | 54 ± 9 nM | 6.01 |
| 2 | 18 ± 3 nM | 5.04 |
| 3 | 85 ± 10 nM | 5.04 |
| 4 | 316 ± 32 nM | 4.53 |
| 5 | 156 ± 37 nM | 4.05 |
| 6 | 311 ± 54 nM | 2.14 |
| 9 | 101 ± 13 nM | 3.14 |
| 10 | 128 ± 14 nM | 3.14 |
| 11 | 71 ± 10 nM | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacaccio, J.; Durrani, F.A.; Kumar, I.; Dukh, M.; Camacho, S.; Fayazi, Z.; Sumlin, A.; Kauffman, E.; Guru, K.; Pandey, R.K. Excitation of a Single Compound by Light and Ultrasound Enhanced the Long-Term Cure of Mice Bearing Prostate Tumors. Int. J. Mol. Sci. 2023, 24, 10624. https://doi.org/10.3390/ijms241310624
Cacaccio J, Durrani FA, Kumar I, Dukh M, Camacho S, Fayazi Z, Sumlin A, Kauffman E, Guru K, Pandey RK. Excitation of a Single Compound by Light and Ultrasound Enhanced the Long-Term Cure of Mice Bearing Prostate Tumors. International Journal of Molecular Sciences. 2023; 24(13):10624. https://doi.org/10.3390/ijms241310624
Chicago/Turabian StyleCacaccio, Joseph, Farukh A. Durrani, Ishaan Kumar, Mykhaylo Dukh, Susan Camacho, Zahra Fayazi, Adam Sumlin, Eric Kauffman, Khurshid Guru, and Ravindra K. Pandey. 2023. "Excitation of a Single Compound by Light and Ultrasound Enhanced the Long-Term Cure of Mice Bearing Prostate Tumors" International Journal of Molecular Sciences 24, no. 13: 10624. https://doi.org/10.3390/ijms241310624




