Identification of a Gene Signature Predicting (Nano)Particle-Induced Adverse Lung Outcome in Rats
Abstract
:1. Introduction
2. Results
2.1. Pulmonary Exposure to Crystalline Silica, Titanium Dioxide, Carbon Black, and Carbon Nanotube-Induced Inflammation and Cytotoxicity
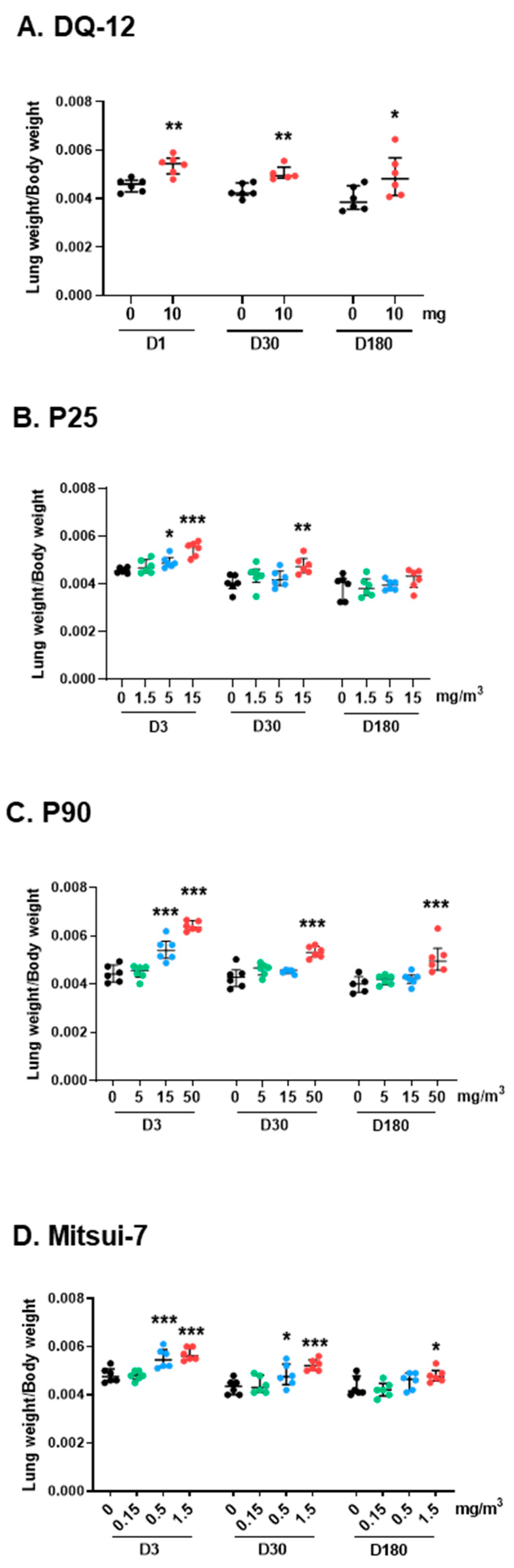
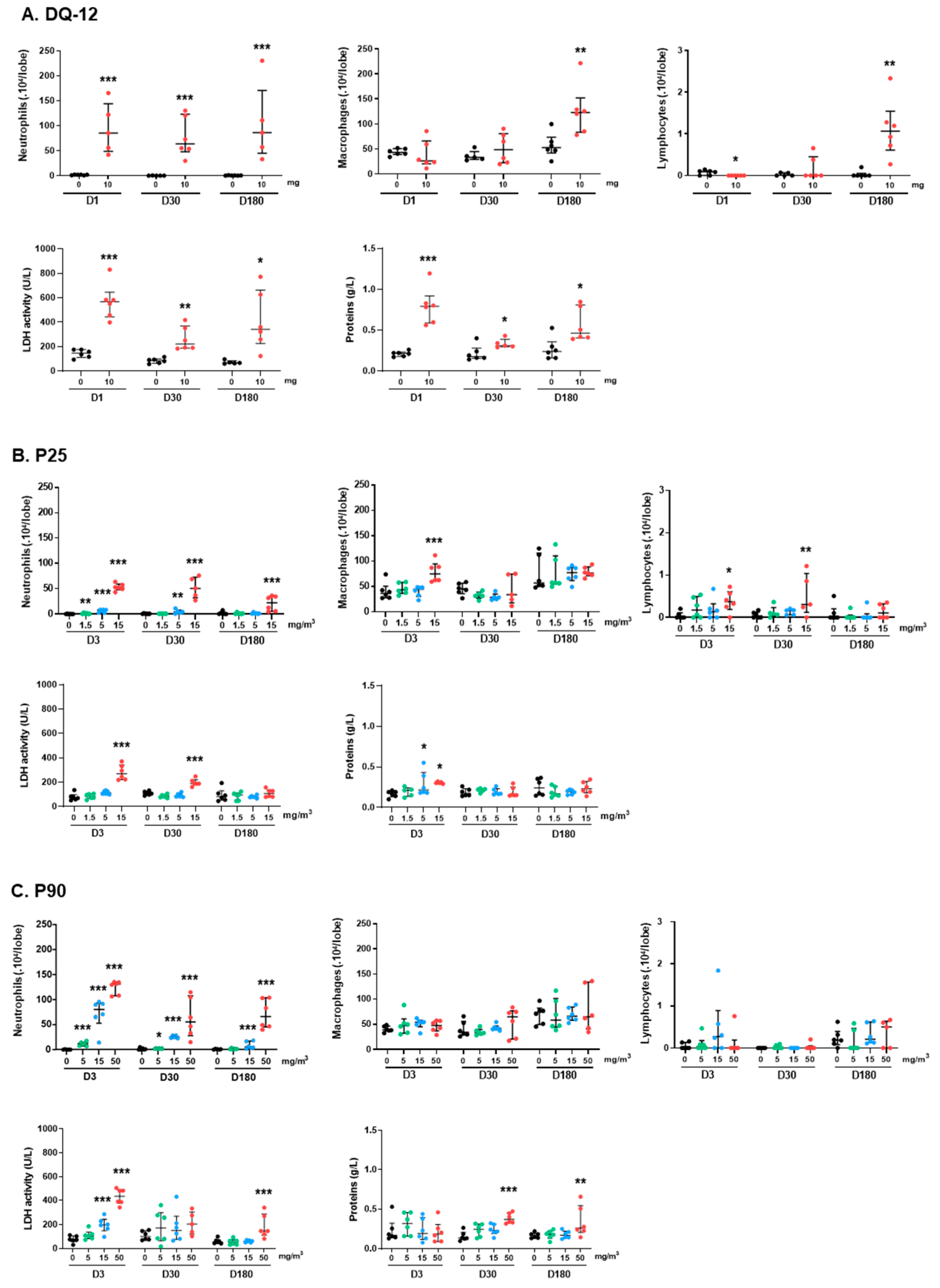
2.1.1. Lung Biometry, Bronchoalveolar Lavage Cytology, and Biochemistry
2.1.2. Cytokines and Genotoxicity
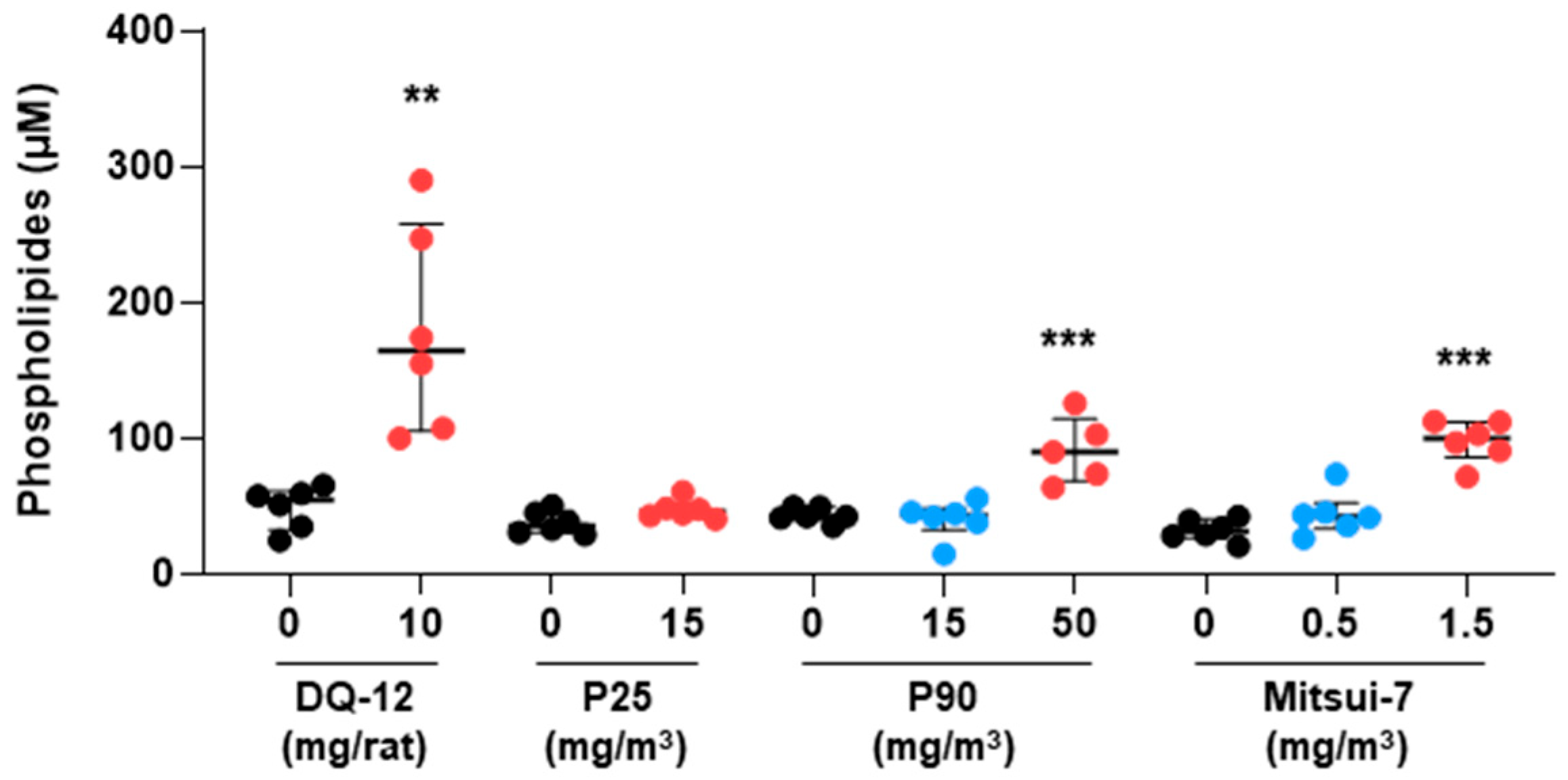
2.2. Histopathology and Phospholipids
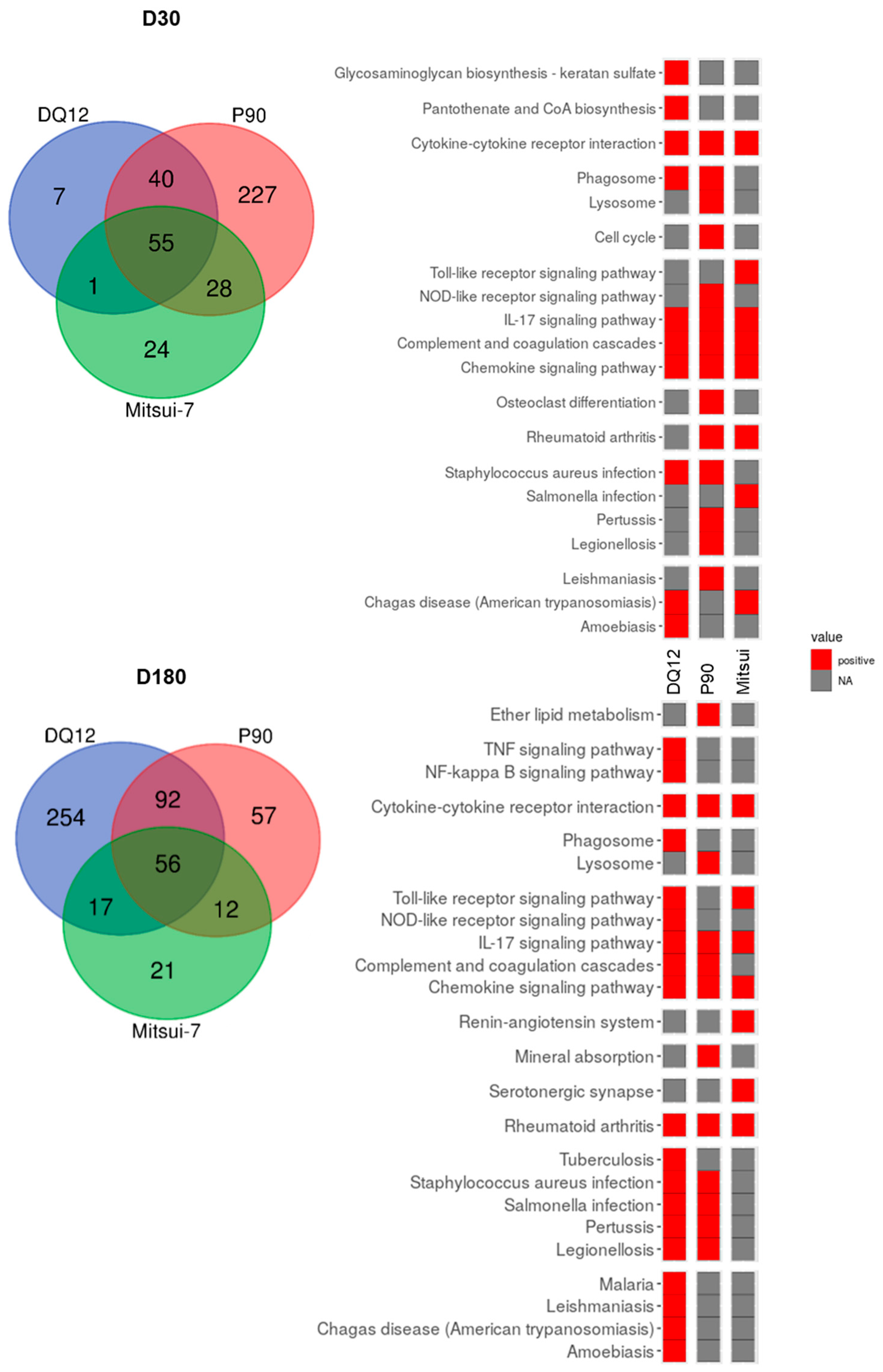
2.3. Exposure to Particles Modulated the Gene Expression Profile in Lung Tissue
2.4. Gene Expression Signature Predictive of Adverse Outcomes
3. Discussion
3.1. Conventional Approach
3.2. Molecular Approach
4. Materials and Methods
4.1. Particles
4.2. Experimental Design
4.3. Necropsy and Tissue Sampling
4.4. Cytokine/Chemokine Detection
4.5. Histological Analysis
4.6. Phospholipid Content Analysis
4.7. Transcriptomic Analysis of Lung Tissue
4.8. Differential Gene Expression Analysis
4.9. Statistical Analysis
4.10. Supplementary Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Huang, Y.; Qiu, Z.; Xu, Z.; Li, D.; Chen, L.; Jiang, J.; Gao, L. Vitamin B2 functionalized iron oxide nanozymes for mouth ulcer healing. Sci. China Life Sci. 2020, 63, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Wang, J.; Bai, R.; Yang, R.; Liu, J.; Tang, J.; Liu, Y.; Li, J.; Chai, Z.; Chen, C. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Metallomics 2015, 7, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Jiang, X.; Foldbjerg, R.; Miclaus, T.; Wang, L.; Singh, R.; Hayashi, Y.; Sutherland, D.; Chen, C.; Autrup, H.; Beer, C. Multi-platform genotoxicity analysis of silver nanoparticles in the model cell line CHO-K1. Toxicol. Lett. 2013, 222, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, Titanium dioxide and Talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93. [Google Scholar]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- ES-OSHA. Workplace exposure to nanoparticles. Eur. Risk Obs.-Litt. Rev. 2009, 2. Available online: https://osha.europa.eu/en/publications/workplace-exposure-nanoparticles (accessed on 19 October 2022).
- Oberbek, P.; Kozikowski, P.; Czarnecka, K.; Sobiech, P.; Jakubiak, S.; Jankowski, T. Inhalation exposure to various nanoparticles in work envrionments—contextual information and results measurements. J. Nanopart. Res. 2019, 21, 222. [Google Scholar] [CrossRef] [Green Version]
- Ribalta, C.; Viana, M.; Lopez-Lilao, A.; Estupina, S.; Minguillon, M.C.; Mendoza, J.; Diaz, J.; Dahmann, D.; Monfort, E. On the Relationship between Exposure to Particles and Dustiness during Handling of Powders in Industrial Settings. Ann. Work. Expo Health 2019, 63, 107–123. [Google Scholar] [CrossRef]
- Damoiseaux, R.; George, S.; Li, M.; Pokhrel, S.; Ji, Z.; France, B.; Xia, T.; Suarez, E.; Rallo, R.; Madler, L.; et al. No time to lose--high throughput screening to assess nanomaterial safety. Nanoscale 2011, 3, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Annangi, B.; Rubio, L.; Marcos, R.; Dorn, M.; Merker, C.; Estrela-Lopis, I.; Cimpan, M.R.; Ibrahim, M.; Cimpan, E.; et al. High throughput toxicity screening and intracellular detection of nanomaterials. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2017, 9, e1413. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; van den Brule, S.; Nymark, P.; Gate, L.; Seidel, C.; Valentino, S.; Zhernovkov, V.; Hogh Danielsen, P.; De Vizcaya, A.; Wolff, H.; et al. Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part Fibre Toxicol. 2020, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Yokohira, M.; Kuno, T.; Yamakawa, K.; Hosokawa, K.; Matsuda, Y.; Hashimoto, N.; Suzuki, S.; Saoo, K.; Imaida, K. Lung toxicity of 16 fine particles on intratracheal instillation in a bioassay model using f344 male rats. Toxicol. Pathol. 2008, 36, 620–631. [Google Scholar] [CrossRef] [Green Version]
- Ernst, H.; Rittinghausen, S.; Bartsch, W.; Creutzenberg, O.; Dasenbrock, C.; Gorlitz, B.D.; Hecht, M.; Kairies, U.; Muhle, H.; Muller, M.; et al. Pulmonary inflammation in rats after intratracheal instillation of quartz, amorphous SiO2, carbon black, and coal dust and the influence of poly-2-vinylpyridine-N-oxide (PVNO). Exp. Toxicol. Pathol. 2002, 54, 109–126. [Google Scholar] [CrossRef]
- Creutzenberg, O.; Hansen, T.; Ernst, H.; Muhle, H.; Oberdorster, G.; Hamilton, R. Toxicity of a quartz with occluded surfaces in a 90-day intratracheal instillation study in rats. Inhal. Toxicol. 2008, 20, 995–1008. [Google Scholar] [CrossRef]
- Ziemann, C.; Escrig, A.; Bonvicini, G.; Ibanez, M.J.; Monfort, E.; Salomoni, A.; Creutzenberg, O. Organosilane-Based Coating of Quartz Species from the Traditional Ceramics Industry: Evidence of Hazard Reduction Using In Vitro and In Vivo Tests. Ann. Work Expo Health 2017, 61, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, T.; Ikegami, M.; Cherniack, R.M.; Mason, R.J. Increased surface tension of the lung and surfactant in bleomycin-induced pulmonary fibrosis in rats. Am. J. Respir. Crit Care Med. 1996, 154 Pt 1, 1002–1005. [Google Scholar] [CrossRef]
- Kuroda, K.; Morimoto, Y.; Ogami, A.; Oyabu, T.; Nagatomo, H.; Hirohashi, M.; Yamato, H.; Nagafuchi, Y.; Tanaka, I. Phospholipid concentration in lung lavage fluid as biomarker for pulmonary fibrosis. Inhal. Toxicol. 2006, 18, 389–393. [Google Scholar] [CrossRef]
- Joseph, P. Transcriptomics in toxicology. Food Chem. Toxicol. 2017, 109 Pt 1, 650–662. [Google Scholar] [CrossRef]
- Rahman, L.; Williams, A.; Gelda, K.; Nikota, J.; Wu, D.; Vogel, U.; Halappanavar, S. 21st Century Tools for Nanotoxicology: Transcriptomic Biomarker Panel and Precision-Cut Lung Slice Organ Mimic System for the Assessment of Nanomaterial-Induced Lung Fibrosis. Small 2020, 16, e2000272. [Google Scholar] [CrossRef] [PubMed]
- Jagiello, K.; Halappanavar, S.; Rybinska-Fryca, A.; Willliams, A.; Vogel, U.; Puzyn, T. Transcriptomics-Based and AOP-Informed Structure-Activity Relationships to Predict Pulmonary Pathology Induced by Multiwalled Carbon Nanotubes. Small 2021, 17, e2003465. [Google Scholar] [CrossRef]
- Cosnier, F.; Seidel, C.; Valentino, S.A.; Schmid, O.; Bau, S.; Vogel, U.; Devoy, J.; Gaté, L. Retained particle surface area dose drives inflammation in rat lungs following acute, subacute, and subchronic inhalation of nanomaterials. Part. Fibre Toxicol. 2021, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Cabello, N.; Mishra, V.; Sinha, U.; DiAngelo, S.L.; Chroneos, Z.C.; Ekpa, N.A.; Cooper, T.K.; Caruso, C.R.; Silveyra, P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1150–L1163. [Google Scholar] [CrossRef] [PubMed]
- Friemann, J.; Pott, F.; Wilk, K.B.; Ahrens, O.; Rosenbruch, M.; Hilscher, W.; Schlipkoter, H.W. Pulmonary alveolar proteinosis in rats after administration of quartz: Its possible role in morphogenesis of lung cancer. J. Cancer Res. Clin. Oncol. 1994, 120, 348–353. [Google Scholar] [CrossRef]
- OCDE. Test No. 412: Subacute Inhalation Toxicity: 28-Day Study; OCDE: Paris, France, 2018. [Google Scholar]
- McKinney, W.; Chen, B.; Frazer, D. Computer controlled multi-walled carbon nanotube inhalation exposure system. Inhal. Toxicol. 2009, 21, 1053–1061. [Google Scholar] [CrossRef]
- Gate, L.; Knudsen, K.B.; Seidel, C.; Berthing, T.; Chezeau, L.; Jacobsen, N.R.; Valentino, S.; Wallin, H.; Bau, S.; Wolff, H.; et al. Pulmonary toxicity of two different multi-walled carbon nanotubes in rat: Comparison between intratracheal instillation and inhalation exposure. Toxicol. Appl. Pharmacol. 2019, 375, 17–31. [Google Scholar] [CrossRef]
- Dazon, C.; Witschger, O.; Bau, S.; Payet, R.; Beugnon, K.; Petit, G.; Garin, T.; Martinon, L. Dustiness of 14 carbon nanotubes using the vortex shaker method. J. Phys. Conf. Ser. 2017, 838, 012005. [Google Scholar] [CrossRef]
- Zhou, L.; Li, P.; Zhang, M.; Han, B.; Chu, C.; Su, X.; Li, B.; Kang, H.; Ning, J.; Zhang, B.; et al. Carbon black nanoparticles induce pulmonary fibrosis through NLRP3 inflammasome pathway modulated by miR-96 targeted FOXO3a. Chemosphere 2020, 241, 125075. [Google Scholar] [CrossRef]
- Li, W.; Pauluhn, J. Re-defining kinetic lung overload: Time for new paradigms. Toxicol. Lett. 2018, 295, 212–219. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Berthing, T.; Jackson, P.; Poulsen, S.S.; Mortensen, A.; Jacobsen, N.R.; Skaug, V.; Szarek, J.; Hougaard, K.S.; Wolff, H.; et al. Physicochemical predictors of Multi-Walled Carbon Nanotube-induced pulmonary histopathology and toxicity one year after pulmonary deposition of 11 different Multi-Walled Carbon Nanotubes in mice. Basic Clin. Pharmacol. Toxicol. 2019, 124, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Bo, C.; Zhang, J.; Sai, L.; Du, Z.; Yu, G.; Li, C.; Li, M.; Peng, C.; Jia, Q.; Shao, H. Integrative transcriptomic and proteomic analysis reveals mechanisms of silica-induced pulmonary fibrosis in rats. BMC Pulm. Med. 2022, 22, 13. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Li, T.; Jin, F.; Li, Y.; Xu, H.; Yang, F. Transcriptomic analysis identifies upregulation of secreted phosphoprotein 1 in silicotic rats. Exp. Ther. Med. 2021, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, R.; Umbright, C.; Roberts, J.R.; Cumpston, A.; McKinney, W.; Chen, B.T.; Frazer, D.; Li, S.; Kashon, M.; Joseph, P. Molecular insights into the progression of crystalline silica-induced pulmonary toxicity in rats. J. Appl. Toxicol. 2013, 33, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Umbright, C.; Sellamuthu, R.; Roberts, J.R.; Young, S.H.; Richardson, D.; Schwegler-Berry, D.; McKinney, W.; Chen, B.; Gu, J.K.; Kashon, M.; et al. Pulmonary toxicity and global gene expression changes in response to sub-chronic inhalation exposure to crystalline silica in rats. J. Toxicol. Environ. Health A 2017, 80, 1349–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, Z.; Lv, R.; Li, X.; Xing, Y.; Gao, Y.; Lv, D.; Si, Y.; Wang, J.; Li, J.; et al. Transcriptome profile analysis reveals a silica-induced immune response and fibrosis in a silicosis rat model. Toxicol. Lett. 2020, 333, 42–48. [Google Scholar] [CrossRef]
- Pang, J.; Luo, Y.; Wei, D.; Cao, Z.; Qi, X.; Song, M.; Liu, Y.; Li, Z.; Zhang, J.; Li, B.; et al. Comparative Transcriptome Analyses Reveal a Transcriptional Landscape of Human Silicosis Lungs and Provide Potential Strategies for Silicosis Treatment. Front. Genet. 2021, 12, 652901. [Google Scholar] [CrossRef]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2016, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Snyder-Talkington, B.N.; Dymacek, J.; Porter, D.W.; Wolfarth, M.G.; Mercer, R.R.; Pacurari, M.; Denvir, J.; Castranova, V.; Qian, Y.; Guo, N.L. System-based identification of toxicity pathways associated with multi-walled carbon nanotube-induced pathological responses. Toxicol. Appl. Pharmacol. 2013, 272, 476–489. [Google Scholar] [CrossRef] [Green Version]
- Seidel, C.; Zhernovkov, V.; Cassidy, H.; Kholodenko, B.; Matallanas, D.; Cosnier, F.; Gate, L. Inhaled multi-walled carbon nanotubes differently modulate global gene and protein expression in rat lungs. Nanotoxicology 2021, 15, 238–256. [Google Scholar] [CrossRef]
- Hamaneh, M.; Yu, Y.K. An 8-Gene Signature for Classifying Major Subtypes of Non-Small-Cell Lung Cancer. Cancer Inform. 2022, 21, 11769351221100718. [Google Scholar] [CrossRef] [PubMed]
- Dazon, C.; Witschger, O.; Bau, S.; Fierro, V.; Llewellyn, P.L. Toward an operational methodology to identify industrial-scaled nanomaterial powders with the volume specific surface area criterion. Nanoscale Adv. 2019, 1, 3232–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosens, I.; Cassee, F.R.; Zanella, M.; Manodori, L.; Brunelli, A.; Costa, A.L.; Bokkers, B.G.; de Jong, W.H.; Brown, D.; Hristozov, D.; et al. Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology 2016, 10, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Marwah, V.S.; Scala, G.; Kinaret, P.A.S.; Serra, A.; Alenius, H.; Fortino, V.; Greco, D. eUTOPIA: solUTion for Omics data PreprocessIng and Analysis. Source Code Biol. Med. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentino, S.A.; Chezeau, L.; Seidel, C.; Sebillaud, S.; Lorcin, M.; Chalansonnet, M.; Cosnier, F.; Gate, L. Exposure to TiO2 Nanostructured Aerosol Induces Specific Gene Expression Profile Modifications in the Lungs of Young and Elderly Rats. Nanomaterials 2021, 11, 1466. [Google Scholar] [CrossRef]
- Scala, G.; Serra, A.; Marwah, V.S.; Saarimaki, L.A.; Greco, D. FunMappOne: A tool to hierarchically organize and visually navigate functional gene annotations in multiple experiments. BMC Bioinform. 2019, 20, 79. [Google Scholar] [CrossRef]
- Guichard, Y.; Maire, M.A.; Sébillaud, S.; Fontana, C.; Langlais, C.; Micillino, J.C.; Darne, C.; Roszak, J.; Stępnik, M.; Fessard, V.; et al. Genotoxicity of Synthetic Amorphous Silica Nanoparticles in Rats Following Short-Term Exposure, Part 2: Intratracheal Instillation and Intravenous Injection. Environ. Mol. Mutagen. 2015, 56, 228–244. [Google Scholar] [CrossRef]


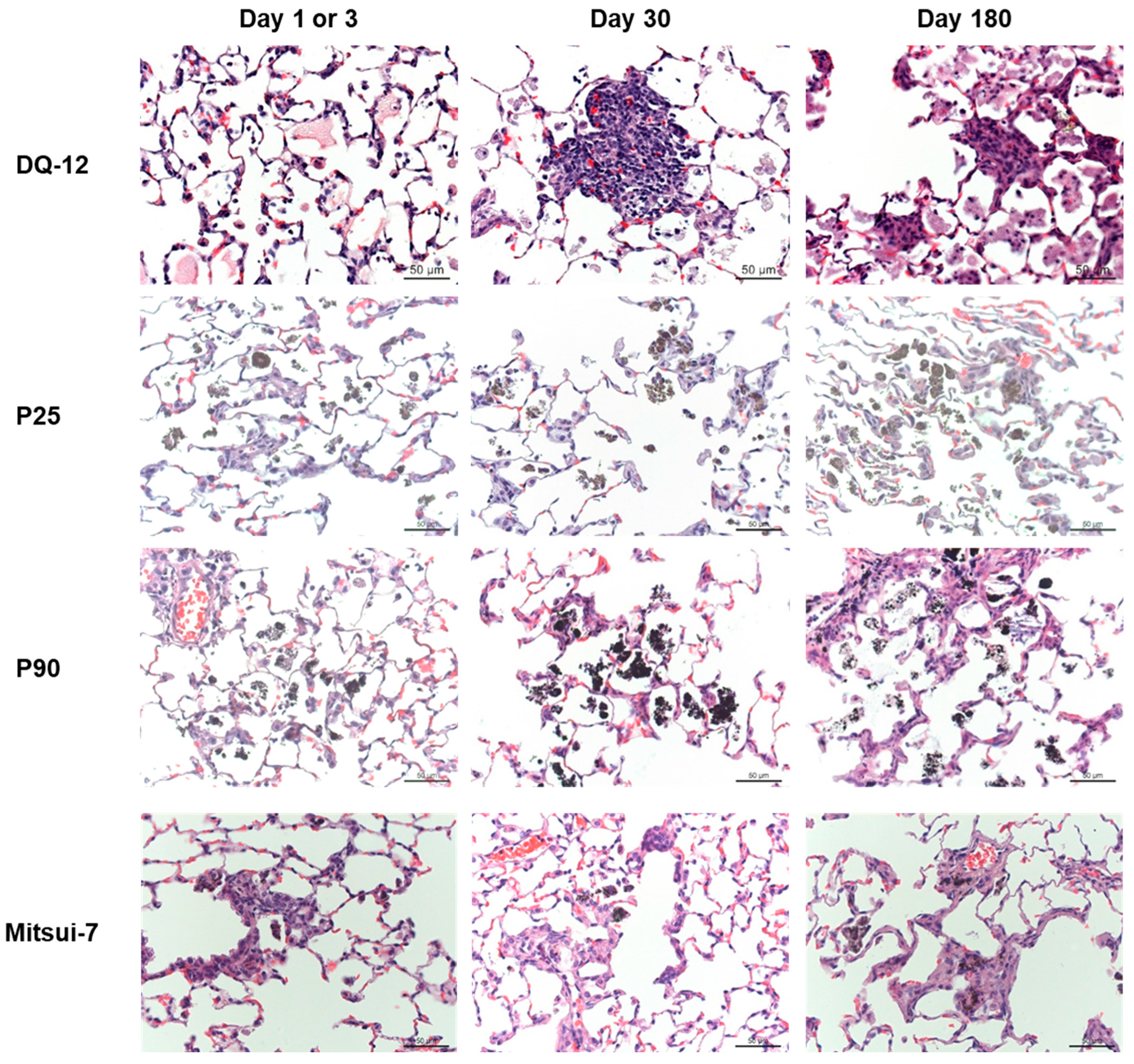

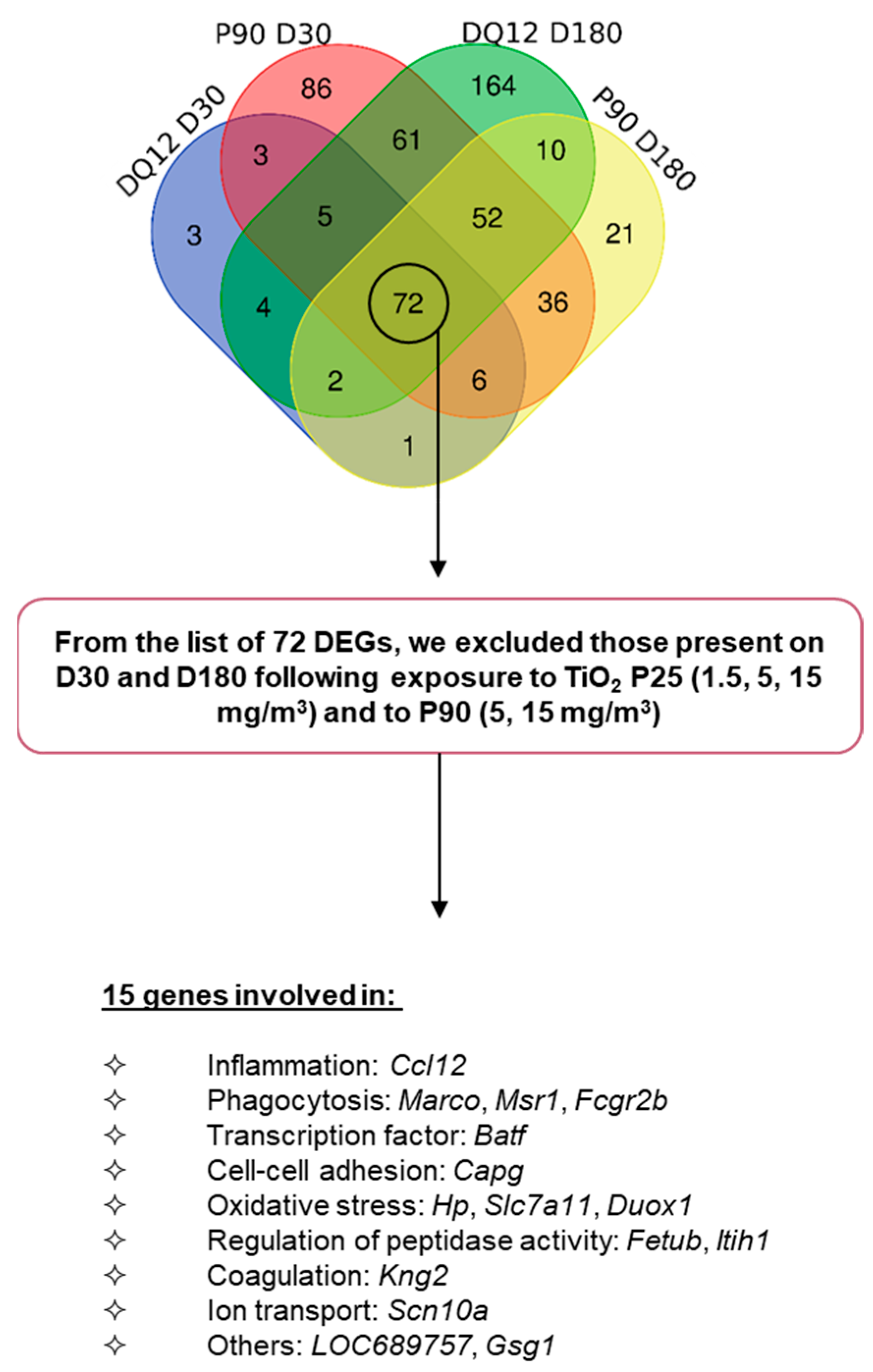
| Day 1 or 3 | Day 30 | Day 180 | ||
|---|---|---|---|---|
| DQ-12 | 10 mg/rat | 278 | 103 | 419 |
| P25 | 1.5 mg/m3 | 317 | 39 | 61 |
| 5 mg/m3 | 329 | 49 | 332 | |
| 15 mg/m3 | 290 | 186 | 365 | |
| P90 | 5 mg/m3 | 32 | 7 | 2 |
| 15 mg/m3 | 214 | 101 | 26 | |
| 50 mg/m3 | 465 | 350 | 217 | |
| Mitsui-7 | 0.15 mg/m3 | 13 | 7 | 4 |
| 0.5 mg/m3 | 141 | 63 | 24 | |
| 1.5 mg/m3 | 236 | 108 | 106 |
| DQ-12 | P90 | |||
|---|---|---|---|---|
| D30 | D180 | D30 | D180 | |
| Ccl12 | 3.3 | 6.5 | 3.5 | 2.1 |
| Marco | 2.1 | 2.4 | 2.3 | 2.4 |
| Msr1 | 2.2 | 2.5 | 3.4 | 2.2 |
| Fcgr2b | 2.3 | 4.1 | 4.3 | 2.7 |
| Batf | 2.0 | 2.0 | 3.0 | 2.4 |
| Capg | 2.1 | 3.0 | 2.9 | 2.3 |
| Hp | 2.0 | 2.7 | 2.4 | 2.1 |
| Slc7a11 | 2.5 | 4.6 | 2.6 | 3.0 |
| Duox1 | 2.1 | 2.4 | 2.1 | 2.3 |
| Fetub | 2.1 | 2.3 | 2.8 | 2.3 |
| Itih1 | 2.9 | 3.2 | 4.1 | 2.6 |
| Kng2 | 2.4 | 3.1 | 2.5 | 2.4 |
| Scn10a | 2.8 | 3.1 | 3.5 | 2.9 |
| LOC689757 | 2.0 | 7.0 | 4.1 | 2.9 |
| Gsg1 | 2.9 | 7.5 | 3.4 | 2.4 |
| DEGs from the Literature | |||||
|---|---|---|---|---|---|
| Gene List | Sellamuthu et al., 2013 [36] | Umbright et al., 2017 [37] | Cai et al., 2021 [35] | Bo et al., 2022 [34] | Zhang et al., 2023 |
| Ccl12 | x | x | |||
| Marco | x | ||||
| Msr1 | x | x | |||
| Fcgr2b | x | x | x | x | |
| Baft | x | ||||
| Capg | x | x | x | x | |
| Hp | x | x | x | x | |
| Slc7a11 | x | x | |||
| Duox1 | x | x | |||
| Fetub | x | x | x | x | x |
| Itih1 | x | x | |||
| Kng2 | x | x | |||
| Scn10a | x | ||||
| LOC689757 | x | ||||
| Gsg1 | |||||
| Total | 7/15 | 2/15 | 10/15 | 9/15 | 5/15 |
| Diameter (nm) | SBET d (m2/g) | Purity (%) | Crystallinity Rate (%) | ||
|---|---|---|---|---|---|
| DQ-12 a After grinding | Crystalline silica | 100–1000 b 220 c | 10.3 ± 2.6 e | Si: 99.4% Co: 808 ± 48 µg/g W: 4829 ± 87 µg/g e | 66 e |
| P25 | Titanium dioxide | Anatase: 21 ± 1.5 Rutile: 40 ± 1.5 | 55 | 99.9 | 87% anatase and 13% rutile crystallites |
| P90 | Carbon black | 14 | 316 | ~99 | |
| Mitsui-7 | Carbon nanotube | Diameter: 88 ± 5 nm Length: 5.0 ± 4.5 µm | 15 | 99 | |
| Target Concentration (mg/m3) | Actual Mass Concentration (mg/m3) a | Number Concentration (Particles/cm3) b | MMAD (µm) c | CMAD (µm) d | GSDd | Aerosol Effective Density (g/cm3) e | |
|---|---|---|---|---|---|---|---|
| P25 | 15 | 15.3 ± 4.7 | 5.1 × 104 ± 1.7 × 104 | 1.56 | 0.31 | 1.72 | 1.70 |
| 5 | 5.02 ± 0.39 | ||||||
| 1.5 | 1.59 ± 0.45 | ||||||
| P90 | 50 | 50.1 ± 3.9 | 3.5 × 105 ± 1.4 × 105 | 0.94 | 0.03 and 0.20 f | 1.97 and 2.11 f | 0.35 |
| 15 | 15.0 ± 1.24 | ||||||
| 5 | 4.89 ± 0.39 | ||||||
| Mitsui-7 | 1.5 | 1.69 ± 0.49 | 1.4 × 103 ± 0.5 × 103 | 1.78 | 0.40 | 1.69 | 0.45 |
| 0.5 | 0.47 ± 0.15 | ||||||
| 0.15 | 0.13 ± 0.02 |
| Materials | Mode of Administration | Dose/Concentration | Post-Exposure Time Considered | Number of Animals |
|---|---|---|---|---|
| DQ-12 | Instillation | Control, 10 mg/rat | 1, 30, and 180 days after exposure | 6 per exposure group and post-exposure time |
| P25 | Inhalation (6 h/day, 5 days/week for 4 weeks) | Control, 1.5 mg/m3 a, 5 mg/m3 a, 15 mg/m3 | 3, 30, and 180 days after exposure | 6 per exposure group and post-exposure time |
| P90 | Inhalation (6 h/day, 5 days/week for 4 weeks) | Control, 5 mg/m3 a, 15 mg/m3 a, 50 mg/m3 | 3, 30, and 180 days after exposure | 6 per exposure group and post-exposure time |
| Mitsui-7 | Inhalation (6 h/day, 5 days/week for 4 weeks) | Control, 0.15 mg/m3 a, 0.5 mg/m3 a, 1.5 mg/m3 | 1, 30, and 180 days after exposure | 6 per exposure group and post-exposure time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentino, S.A.; Seidel, C.; Lorcin, M.; Sébillaud, S.; Wolff, H.; Grossmann, S.; Viton, S.; Nunge, H.; Saarimäki, L.A.; Greco, D.; et al. Identification of a Gene Signature Predicting (Nano)Particle-Induced Adverse Lung Outcome in Rats. Int. J. Mol. Sci. 2023, 24, 10890. https://doi.org/10.3390/ijms241310890
Valentino SA, Seidel C, Lorcin M, Sébillaud S, Wolff H, Grossmann S, Viton S, Nunge H, Saarimäki LA, Greco D, et al. Identification of a Gene Signature Predicting (Nano)Particle-Induced Adverse Lung Outcome in Rats. International Journal of Molecular Sciences. 2023; 24(13):10890. https://doi.org/10.3390/ijms241310890
Chicago/Turabian StyleValentino, Sarah Amandine, Carole Seidel, Mylène Lorcin, Sylvie Sébillaud, Henrik Wolff, Stéphane Grossmann, Stéphane Viton, Hervé Nunge, Laura Aliisa Saarimäki, Dario Greco, and et al. 2023. "Identification of a Gene Signature Predicting (Nano)Particle-Induced Adverse Lung Outcome in Rats" International Journal of Molecular Sciences 24, no. 13: 10890. https://doi.org/10.3390/ijms241310890






