The Combined Use of Copper Sulfate and Trichlorfon Exerts Stronger Toxicity on the Liver of Zebrafish
Abstract
:1. Introduction
2. Results
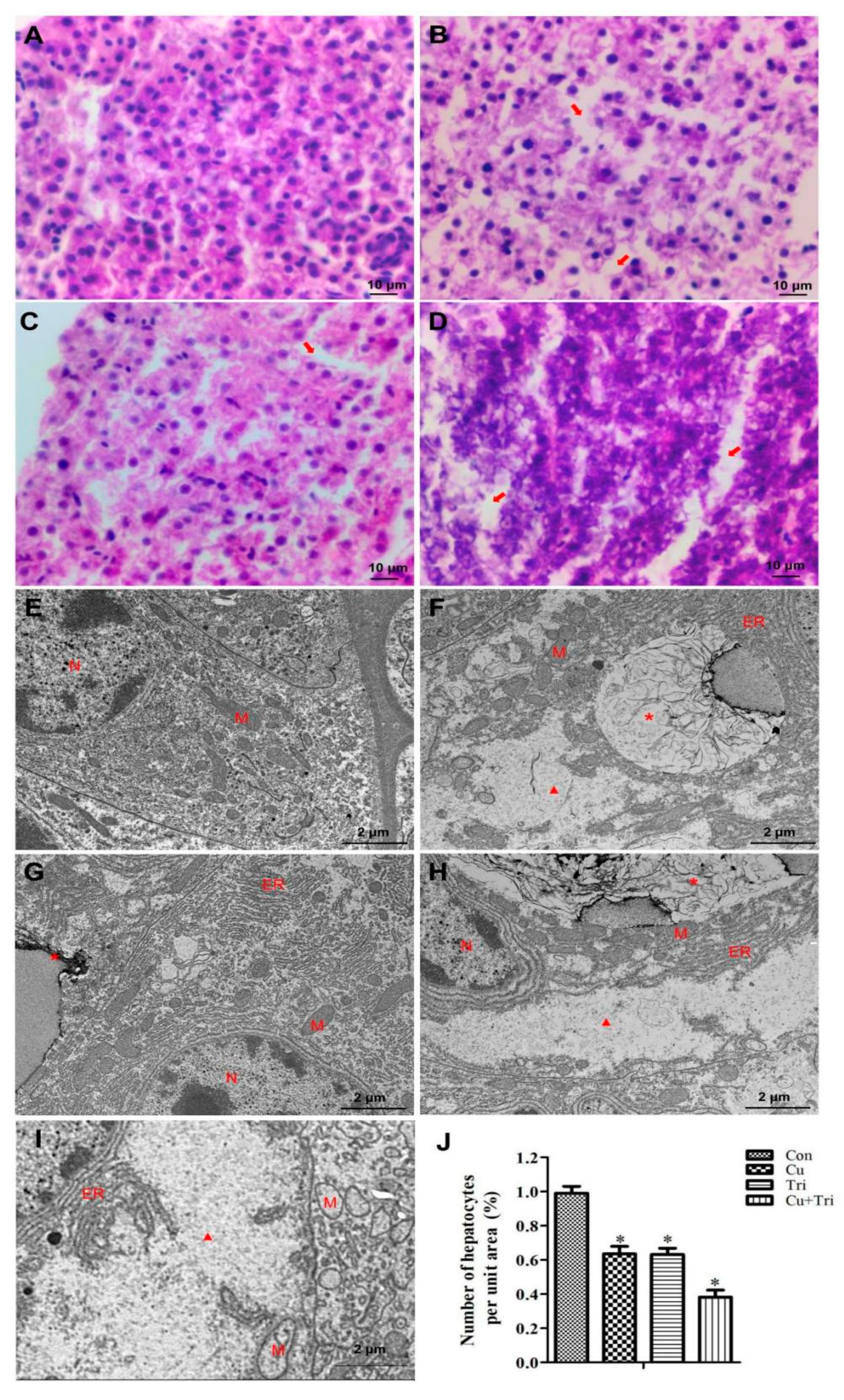
2.1. Morphology Observation
2.2. Oxidative Stress and Immune Enzyme Activity
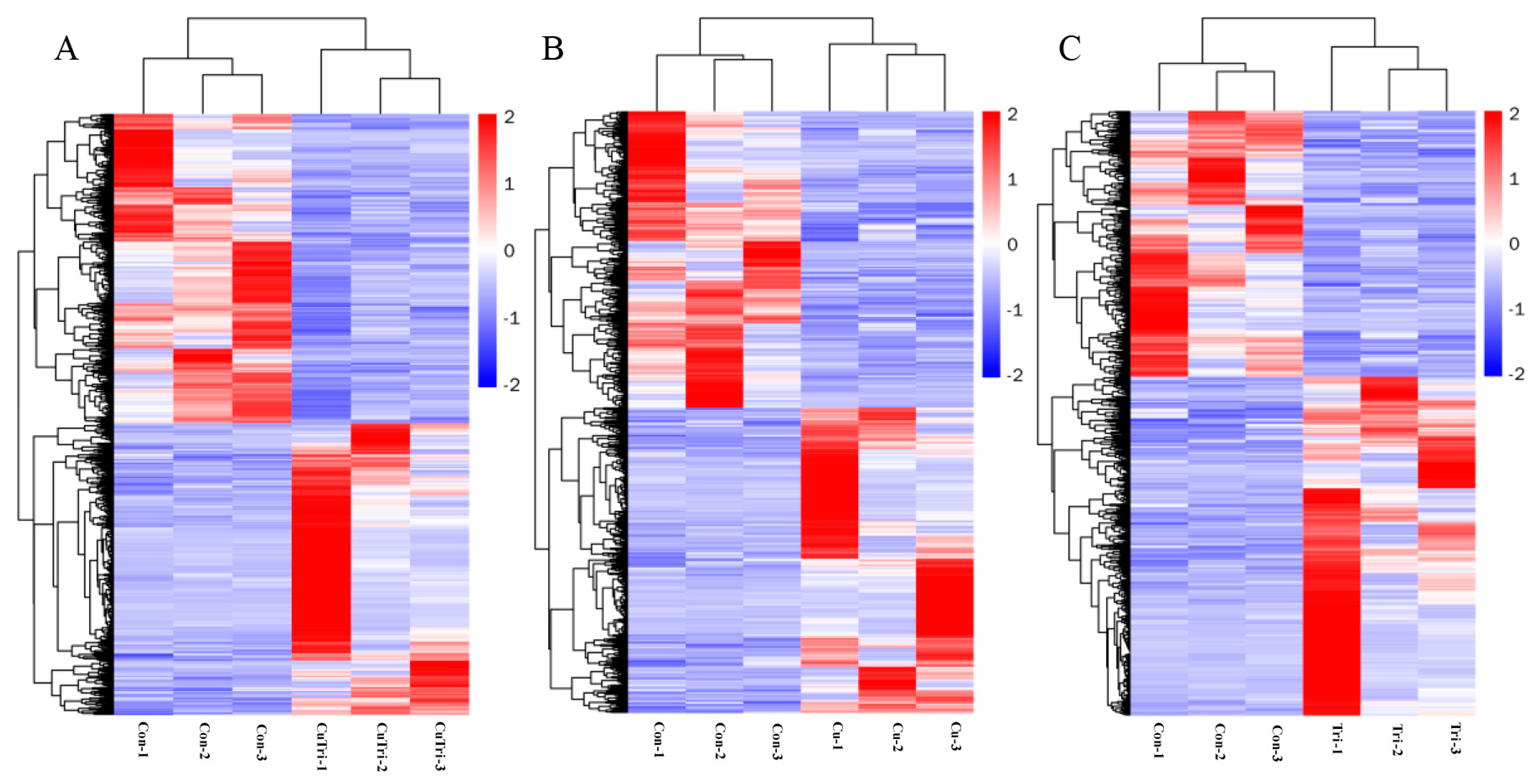
2.3. Transcriptome Analysis
2.4. GO Enrichment Analysis
2.5. KEGG Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Drug Exposure
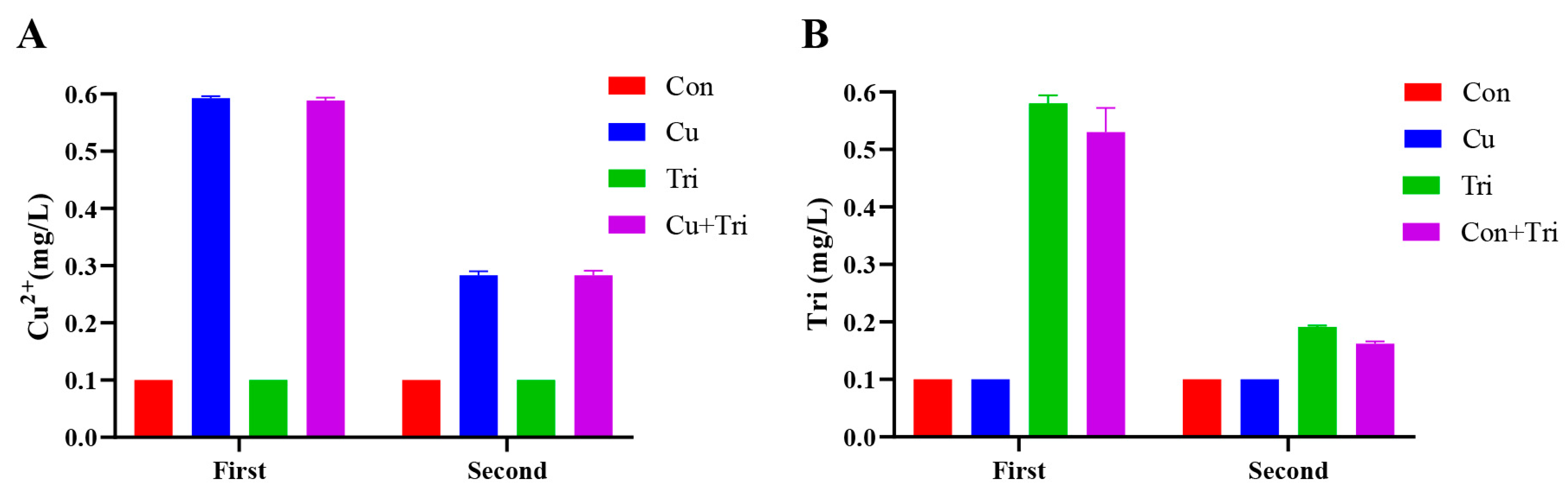
4.2. Analysis of Actual Cu2+ and Trichlorfon Concentration in Water Samples
4.3. Sampling and Morphometry
4.4. Hepatic Histological Examination
4.5. Enzyme Activity Assay
4.6. Transcriptomic Analyses
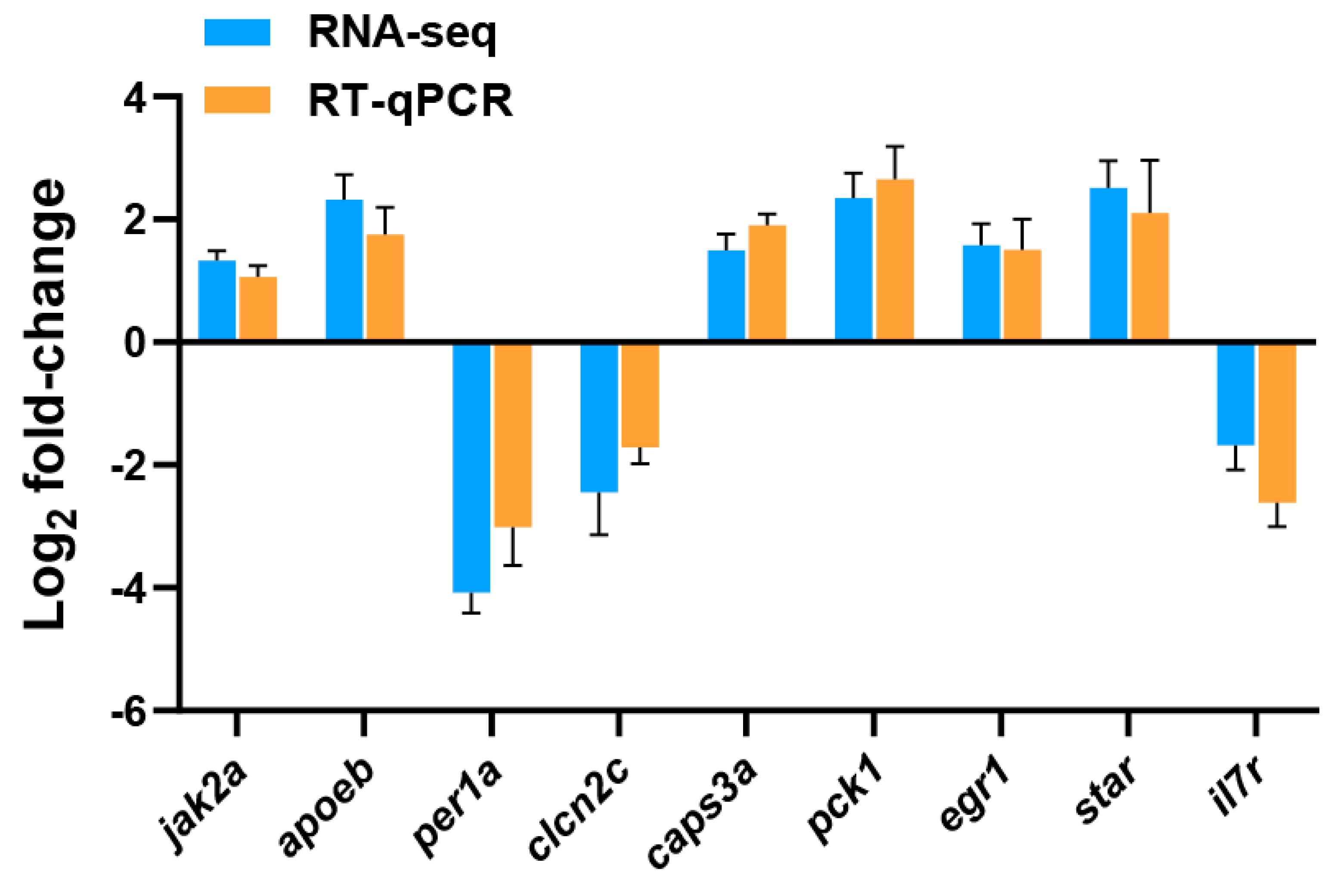
4.7. qPCR Verification of DEGs
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stern, B.R. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. J. Toxicol. Environ. Health A 2010, 73, 114–127. [Google Scholar] [CrossRef]
- Rowland, S.J.; Mifsud, C.; Nixon, M.; Read, P.; Landos, M. Use of formalin and copper to control ichthyophthiriosis in the Australian freshwater fish silver perch (Bidyanus bidyanus Mitchell). Aquac. Res. 2008, 40, 44–54. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Liu, J.; Shao, Y.; Li, J.; Luo, L.; Xing, M. Copper and arsenic-induced oxidative stress and immune imbalance are associated with activation of heat shock proteins in chicken intestines. Int. Immunopharmacol. 2018, 60, 64–75. [Google Scholar]
- Tom-Petersen, A.; Brandt, K.K.; Nybroe, O.; Jørgensen, N.O.G. Copper bioavailability and impact on bacterial growth in flow-through rainbow trout aquaculture systems. Aquaculture 2011, 322–323, 259–262. [Google Scholar] [CrossRef]
- Cao, H.; Yang, Y.; Lu, L.; Yang, X.; Ai, X. Effect of copper sulfate on Bdellovibrio growth and bacteriolytic activity towards gibel carp-pathogenic Aeromonas hydrophila. Can. J. Microbiol. 2018, 64, 1054–1058. [Google Scholar]
- Farmer, B.D.; Straus, D.L.; Beck, B.H.; Mitchell, A.J.; Freeman, D.; Meinelt, T. Effectiveness of copper sulphate, potassium permanganate and peracetic acid to reduce mortality and infestation ofIchthyobodo necatorin channel catfish Ictalurus punctatus (Rafinesque 1818). Aquac. Res. 2013, 44, 1103–1109. [Google Scholar]
- Sun, Q.; Hu, K.; Yang, X.L. The efficacy of copper sulfate in controlling infection of Saprolegnia parasitica. J. World Aquac. Soc. 2014, 45, 220–225. [Google Scholar] [CrossRef]
- Xu, D.H.; Zhang, Q.Z.; Zhang, D. Two in vitro methods for screening potential parasiticides against Ichthyophthirius multifiliis using Tetrahymena thermophila. J. Fish Dis. 2016, 39, 285–294. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.R.; Uapipatanakul, B.; Huang, J.C.; Chen, K.H.; Hsiao, C.D. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Oda, S.S.; El-Manakhly, E.M.; Abou-Srag, M.A.; Tohamy, H.G. Assessment of reproductive toxicity of carbofuran and copper sulfate in male Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. Int. 2022, 29, 15896–15904. [Google Scholar] [CrossRef]
- Trivedi, S.P.; Kumar, V.; Singh, S.; Trivedi, A.; Kumar, M. Ethanolic root extract of Rauwolfia serpentina alleviates copper induced genotoxicity and hepatic impairments in spotted snakehead fish, Channa punctatus (Bloch, 1793). J. Environ. Biol. 2021, 42, 1433–1441. [Google Scholar] [CrossRef]
- Rożyński, M.; Sikora, A.; Demska-Zakęś, K.; Zakęś, Z. Influence of brief immersion in an aqueous solution of sodium chloride and/or copper sulphate on pikeperch (Sander lucioperca (L.)) blood parameters. Ann. Anim. Sci. 2022, 22, 643–651. [Google Scholar]
- da Cruz, M.G.; Jerônimo, G.T.; dos Santos Torres, G.; de Matos, L.V.; Monteiro dos Santos, D.K.; Serra, B.N.V.; Santana, T.M.; Gonçalves, L.U. Acute toxicity of trichlorfon and histological changes in the gills of Arapaima gigas, a neotropical fish from Amazon. Aquac. Rep. 2022, 25, 101229. [Google Scholar]
- Mişe Yonar, S.; Yonar, M.E.; Pala, A.; Sağlam, N.; Sakin, F. Effect of trichlorfon on some haematological and biochemical changes in Cyprinus carpio: The ameliorative effect of lycopene. Aquac. Rep. 2020, 16, 100246. [Google Scholar]
- Woo, S.J.; Chung, J.K. Effects of trichlorfon on oxidative stress, neurotoxicity, and cortisol levels in common carp, Cyprinus carpio L., at different temperatures. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 229, 108698. [Google Scholar]
- Chandrasekara, H.U.; Pathiratne, A. Influence of low concentrations of Trichlorfon on haematological parameters and brain acetylcholinesterase activity in common carp, Cyprinus carpio L. Aquac. Res. 2005, 36, 144–149. [Google Scholar]
- Li, H.; Wu, M.; Jiang, J.; Sun, X.; Chen, L.; Feng, M.; Yuan, D.; Wen, Z.; Qin, C. The extracts of Angelica sinensis restore the digestive and absorptive capacity through improving antioxidant status in digestive organs of fish treated with trichlorfon. Aquac. Res. 2018, 50, 490–504. [Google Scholar]
- Xu, W.; Liu, W.; Lu, K.; Jiang, Y.; Li, G. Effect of trichlorfon on oxidative stress and hepatocyte apoptosis of Carassius auratus gibelio in vivo. Fish. Physiol. Biochem. 2012, 38, 769–775. [Google Scholar] [CrossRef]
- Vaz, C.; Afonso, F.; Barata, M.; Ribeiro, L.; Pousao-Ferreira, P.; Soares, F. Effect of copper exposure and recovery period in reared Diplodus sargus. Ecotoxicology 2019, 28, 1075–1084. [Google Scholar]
- Viriyatum, R.; Boyd, C.E. Slow-release coated copper sulfate as an algicide for aquaculture. J. World Aquac. Soc. 2016, 47, 667–675. [Google Scholar] [CrossRef]
- Woo, S.J.; Kim, N.Y.; Kim, S.H.; Ahn, S.J.; Seo, J.S.; Jung, S.H.; Cho, M.Y.; Chung, J.K. Toxicological effects of trichlorfon on hematological and biochemical parameters in Cyprinus carpio L. following thermal stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 209, 18–27. [Google Scholar] [CrossRef]
- Cao, Q.; Jiang, Y.; Yang, H.; Zhang, Y.; Wei, W. Comprehensive toxic effects of povidone iodine on microalgae Chlorella pyrenoidosa under different concentrations. Aquac. Res. 2021, 53, 1833–1841. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, H.; Mi, K.; Xue, W.; Wei, W.; Zhang, Y. Toxicity comparison of nano-sized and micron-sized microplastics to Goldfish Carassius auratus Larvae. J. Hazard. Mater. 2020, 388, 122058. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Chen, B.; Zhao, H.; Huang, W. Toxic effects of aflatoxin B(1) in Chinese sea bass (Lateolabrax maculatus). Toxins 2021, 13, 844. [Google Scholar] [CrossRef]
- Liu, X.L.; Xi, Q.Y.; Yang, L.; Li, H.Y.; Jiang, Q.Y.; Shu, G.; Zhang, Y.L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yi, M.; Xiao, P.; Meng, L.; Li, X.; Sun, G.; Liu, Y. The impact of Aeromonas salmonicida infection on innate immune parameters of Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 2015, 44, 307–315. [Google Scholar] [CrossRef]
- Meng, X.; Tian, X.; Nie, G.; Wang, J.; Liu, M.; Jiang, K.; Wang, B.; Guo, Q.; Huang, J.; Wang, L. The transcriptomic response to copper exposure in the digestive gland of Japanese scallops (Mizuhopecten yessoensis). Fish Shellfish Immunol. 2015, 46, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Thompson, E.L.; Nair, S.V.; Raftos, D.A. Differential effects of metal contamination on the transcript expression of immune- and stress-response genes in the Sydney Rock oyster, Saccostrea glomerata. Environ. Pollut. 2013, 178, 65–71. [Google Scholar] [CrossRef]
- Abubakar, M.; Ali, H.; Shad, S.A.; Anees, M.; Binyameen, M. Trichlorfon resistance: Its stability and impacts on biological parameters of Bactrocera zonata (Diptera: Tephritidae). Appl. Entomol. Zool. 2021, 56, 473–482. [Google Scholar] [CrossRef]
- Pena, S.C.; Pocsidio, G.N. Influence of copper on the feeding rate, growth and reproduction of the golden apple snail, Pomacea canaliculata Lamarck. Bull. Environ. Contam. Toxicol. 2007, 79, 606–608. [Google Scholar] [CrossRef]
- Chen, M.; Luo, Y.; Xu, J.; Chang, M.X.; Liu, J.X. Copper regulates the susceptibility of zebrafish larvae to inflammatory stimuli by controlling neutrophil/macrophage survival. Front. Immunol. 2019, 10, 2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhao, H.; Wang, Y.; Shao, Y.; Zhang, L.; Xing, M. Impacts of simultaneous exposure to arsenic (III) and copper (II) on inflammatory response, immune homeostasis, and heat shock response in chicken thymus. Int. Immunopharmacol. 2018, 64, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Li, T.; Zhao, H.; Wang, X.; Kang, Y.; Kang, Y. Methylome and transcriptome profiling revealed epigenetic silencing of LPCAT1 and PCYT1A associated with lipidome alterations in polycystic ovary syndrome. J. Cell. Physiol. 2021, 236, 6362–6375. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Wu, L.; Yu, J.; Song, Z.; Liu, F.; Li, J.; Song, X.; Li, X. Acute low-dose phosphate disrupts glycerophospholipid metabolism and induces stress in juvenile turbot (Scophthalmus maximus). Sci. Total Environ. 2023, 861, 160430. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Pillai, B.R.; Adhikari, S. Bioaccumulation of copper in post-larvae and juveniles of freshwater prawn Macrobrachium rosenbergii (de Man) exposed to sub-lethal levels of copper sulfate. Aquaculture 2006, 252, 356–360. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Cao, Q.; Liu, S.; Wei, W.; Yang, H.; Zhang, Y. Long-term BPA exposure leads to bone malformation and abnormal expression of MAPK/Wnt/FoxO signaling pathway genes in zebrafish offspring. Ecotoxicol. Environ. Saf. 2022, 245, 114082. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| GO_ID | Term | Test | Ref. | p-Value | ||
|---|---|---|---|---|---|---|
| Cu | Up | GO:0051321 | Meiotic cell cycle | 12 | 101 | 1.71 × 10−7 |
| GO:0006030 | Chitin metabolic process | 5 | 10 | 3.45 × 10−7 | ||
| GO:0007049 | Cell cycle | 39 | 1030 | 3.72 × 10−6 | ||
| GO:0015793 | Glycerol transport | 4 | 9 | 1.02 × 10−5 | ||
| GO:0034508 | Centromere complex assembly | 5 | 19 | 1.40 × 10−5 | ||
| Down | GO:0006955 | Immune response | 37 | 573 | 3.24 × 10−12 | |
| GO:0060326 | Cell chemotaxis | 15 | 152 | 4.73 × 10−8 | ||
| GO:0019882 | Antigen processing and presentation | 8 | 34 | 7.51 × 10−8 | ||
| GO:0050900 | Leukocyte migration | 13 | 135 | 4.97 × 10−7 | ||
| GO:0071345 | Cellular response to cytokine stimulus | 16 | 219 | 1.06 × 10−6 | ||
| Tri | Up | GO:0035082 | Axoneme assembly | 7 | 38 | 5.14 × 10−6 |
| GO:0007049 | Cell cycle | 40 | 1030 | 7.18 × 10−6 | ||
| GO:0019755 | One-carbon compound transport | 5 | 16 | 7.50 × 10−6 | ||
| GO:0042981 | Regulation of apoptotic process | 27 | 579 | 1.04 × 10−5 | ||
| GO:0001578 | Microtubule bundle formation | 7 | 47 | 2.22 × 10−5 | ||
| Down | GO:0006629 | Lipid metabolic process | 22 | 710 | 5.38 × 10−6 | |
| GO:0016126 | Sterol biosynthetic process | 5 | 30 | 1.33 × 10−5 | ||
| GO:0008610 | Lipid biosynthetic process | 13 | 294 | 1.34 × 10−5 | ||
| GO:0008202 | Steroid metabolic process | 7 | 85 | 2.94 × 10−5 | ||
| GO:0044281 | Small molecule metabolic process | 27 | 1144 | 6.11 × 10−5 | ||
| CuTri | Up | GO:0055114 | Oxidation–reduction process | 40 | 880 | 7.50 × 10−8 |
| GO:0009410 | Response to xenobiotic stimulus | 10 | 101 | 1.37 × 10−5 | ||
| GO:1901615 | Organic hydroxy compound metabolic process | 14 | 203 | 1.91 × 10−5 | ||
| GO:0071466 | Cellular response to xenobiotic stimulus | 9 | 86 | 2.36 × 10−5 | ||
| GO:0006066 | Alcohol metabolic process | 12 | 157 | 2.73 × 10−5 | ||
| Down | GO:0022414 | Reproductive process | 21 | 252 | 9.57 × 10−9 | |
| GO:0006260 | DNA replication | 14 | 119 | 4.05 × 10−8 | ||
| GO:0060046 | Regulation of acrosome reaction | 6 | 14 | 9.75 × 10−8 | ||
| GO:2000344 | Positive regulation of acrosome reaction | 6 | 14 | 9.75 × 10−8 | ||
| GO:0009988 | Cell–cell recognition | 6 | 15 | 1.60 × 10−7 |
| ID | Description | Test | Ref. | p-Value | ||
|---|---|---|---|---|---|---|
| Cu | Up | dre00520 | Amino sugar and nucleotide sugar metabolism | 7 | 63 | 5.35 × 10−5 |
| dre00140 | Steroid hormone biosynthesis | 6 | 67 | 0.000613967 | ||
| dre00983 | Drug metabolism—other enzymes | 7 | 87 | 0.000414833 | ||
| dre00980 | Metabolism of xenobiotics by cytochrome P450 | 5 | 61 | 0.002632369 | ||
| dre00040 | Pentose and glucuronate interconversions | 4 | 42 | 0.004145541 | ||
| Down | dre05168 | Herpes simplex virus 1 infection | 11 | 185 | 0.000308054 | |
| dre04514 | Cell adhesion molecules | 8 | 152 | 0.004449689 | ||
| dre04672 | Intestinal immune network for IgA production | 4 | 37 | 0.00351935 | ||
| dre03010 | Ribosome | 6 | 130 | 0.023987246 | ||
| dre04060 | Cytokine–cytokine receptor interaction | 8 | 204 | 0.023658627 | ||
| Tri | Up | dre00030 | Pentose phosphate pathway | 3 | 33 | 0.018950865 |
| dre00051 | Fructose and mannose metabolism | 3 | 45 | 0.04248255 | ||
| dre00760 | Nicotinate and nicotinamide metabolism | 3 | 45 | 0.04248255 | ||
| dre01240 | Biosynthesis of cofactors | 7 | 188 | 0.044177541 | ||
| dre04210 | Apoptosis | 8 | 192 | 0.017937474 | ||
| Down | dre00100 | Steroid biosynthesis | 6 | 22 | 8.02 × 10−8 | |
| dre01100 | Metabolic pathways | 36 | 1719 | 6.16 × 10−6 | ||
| dre00510 | N-glycan biosynthesis | 4 | 59 | 0.003553768 | ||
| dre00591 | Linoleic acid metabolism | 3 | 29 | 0.003544349 | ||
| dre04672 | Intestinal immune network for IgA production | 3 | 37 | 0.007089709 | ||
| CuTri | Up | dre00830 | Retinol metabolism | 14 | 80 | 6.54 × 10−9 |
| dre01100 | Metabolic pathways | 75 | 1719 | 1.90 × 10−8 | ||
| dre00982 | Drug metabolism—cytochrome P450 | 9 | 59 | 1.19 × 10−5 | ||
| dre00591 | Linoleic acid metabolism | 6 | 29 | 6.12 × 10−5 | ||
| dre00350 | Tyrosine metabolism | 6 | 38 | 0.000295976 | ||
| Down | dre00564 | Glycerophospholipid metabolism | 7 | 107 | 0.001203711 | |
| dre00561 | Glycerolipid metabolism | 5 | 69 | 0.003967923 | ||
| dre04110 | Cell cycle | 7 | 150 | 0.007960662 | ||
| dre04114 | Oocyte meiosis | 7 | 146 | 0.006895526 | ||
| dre00062 | Fatty acid elongation | 3 | 42 | 0.026082223 |
| Genes | Name | Primer Sequences |
|---|---|---|
| jak2a | F R | TAATGATGAGAGAACA GATCATCCACAGTTCAG |
| apoeb | F R | CTCTTGTGGTATTCTTTGCTCTGGCAGTTT TTGCACCATGCCGTCAGTTTGTGTGTTGAG |
| Per1a | F R | GTACATCTCATCCCAGGCGG AAAGGTGCTGACGTCCTGAG |
| clcn2c | F R | GAGACCTGAGGTGTGTGAGC TAACACACATTGGTCGCGGA |
| caps3a | F R | GATCGCAGGACAGGCATGAA AATCTGCGCAACTGTCTGGT |
| pck1 | F R | CGCGTACTGGAGTGGATGTT GTGTGTTGCGTGTCTTCAGC |
| egr1 | F R | AAGTGAGATCAGCCTGGTGC GGTTGCAAAGGCCTTGATGG |
| star | F R | ACCTGTTTTCTGGCTGGGATG GGGTCCATTCTCAGCCCTTAC |
| il7r | F R | TGCAAAGAGCAAAGGAGGGC CAACATAATCCACGCGGTGT |
| actb | F R | GTCCGTGACATCAAAGAG ACCGCAAGATTCCATAC |
| ef1α | F R | AGGAAGATTGAACGCAAGAGTG AGGAAGATTGAACGCAAGAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhu, M.; Wang, Q.; Yang, H. The Combined Use of Copper Sulfate and Trichlorfon Exerts Stronger Toxicity on the Liver of Zebrafish. Int. J. Mol. Sci. 2023, 24, 11203. https://doi.org/10.3390/ijms241311203
Zhang J, Zhu M, Wang Q, Yang H. The Combined Use of Copper Sulfate and Trichlorfon Exerts Stronger Toxicity on the Liver of Zebrafish. International Journal of Molecular Sciences. 2023; 24(13):11203. https://doi.org/10.3390/ijms241311203
Chicago/Turabian StyleZhang, Jianlu, Mingzhen Zhu, Qijun Wang, and Hui Yang. 2023. "The Combined Use of Copper Sulfate and Trichlorfon Exerts Stronger Toxicity on the Liver of Zebrafish" International Journal of Molecular Sciences 24, no. 13: 11203. https://doi.org/10.3390/ijms241311203




