Using a Dual CRISPR/Cas9 Approach to Gain Insight into the Role of LRP1B in Glioblastoma
Abstract
:1. Introduction
2. Results
2.1. Dual sgRNA CRISPR/Cas9-Mediated Strategy to Target LRP1B
2.2. Evaluation of LRP1B Alterations in Single-Cell-Derived Clones from CRISPR/Cas9-Transfected Cells
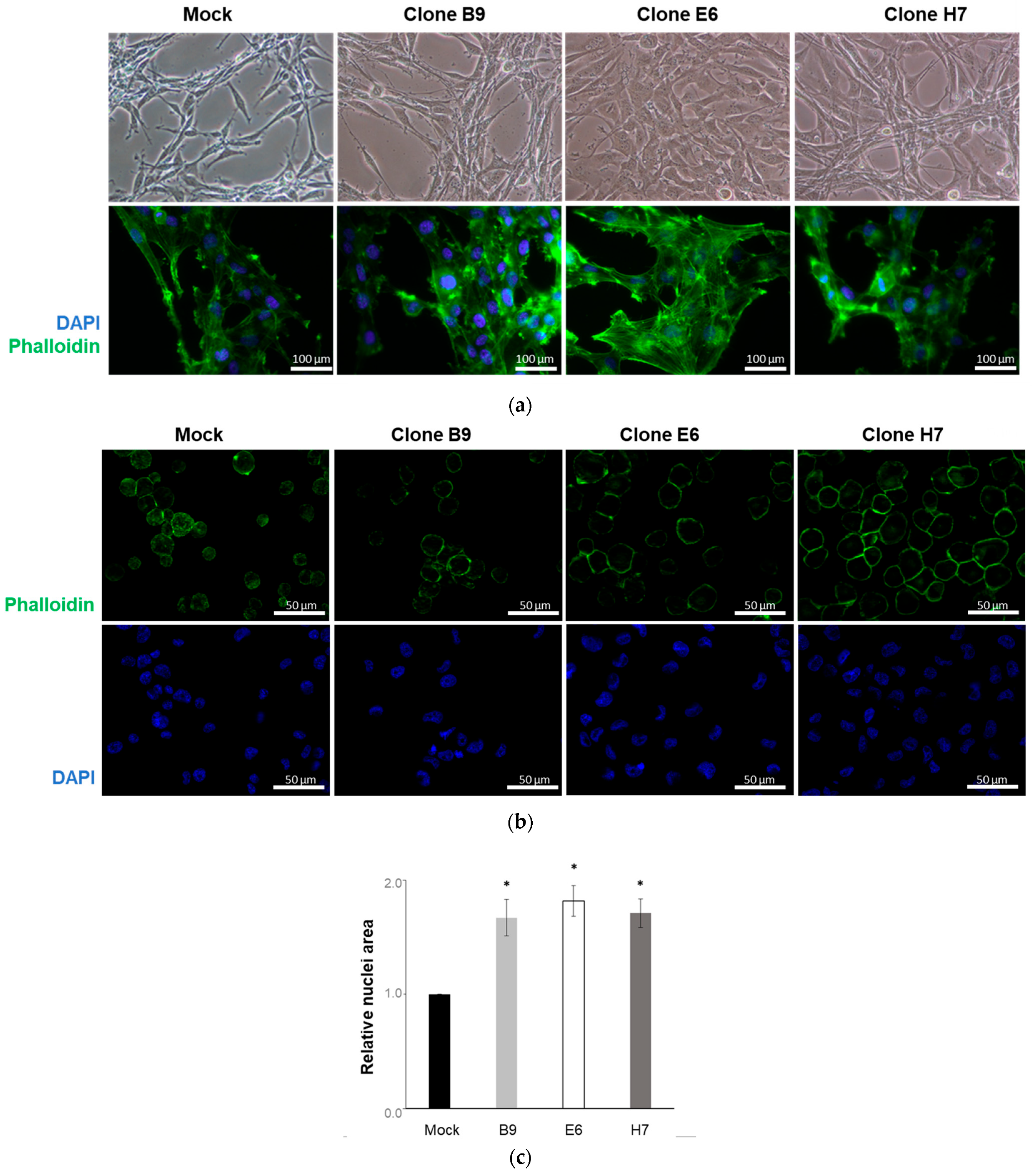
2.3. Evaluation of LRP1B Silencing in Cell Clones
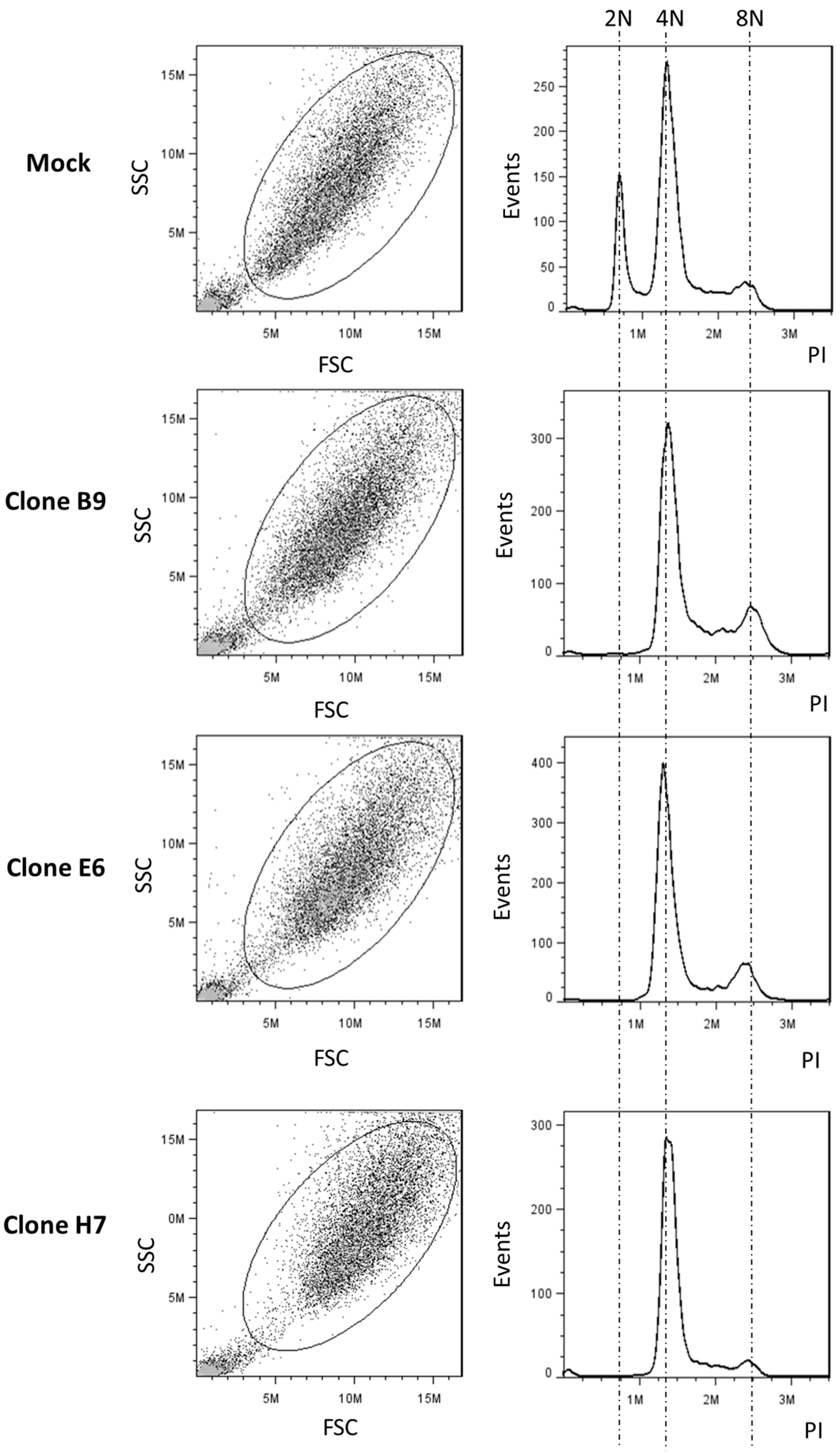
2.4. Effect of LRP1B Alterations on Tumor Growth In Vitro and In Vivo
2.5. Effect of LRP1B Alterations on Cell Secretome
3. Discussion
4. Materials and Methods
4.1. LRP1B Target Region
4.2. CRISPR/Cas9 Expression Vector
4.3. sgRNA Oligonucleotide Duplex Preparation and Cloning into PX459 Vector
4.4. Cell Culture
4.5. Cell Transfection
4.6. Genomic DNA Extraction, PCR, and Sanger Sequencing Analysis
4.7. RNA Extraction and LRP1B mRNA Expression Analysis
4.8. Cell Growth and Cell Cycle Profile Analysis
4.9. Immunofluorescence Analysis
4.10. CAM Assay
4.11. Collection of Secretome
4.12. Proteomic Evaluation Using Liquid Chromatography–Mass Spectrometry
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatoum, A.; Mohammed, R.; Zakieh, O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag. Res. 2019, 11, 1843–1855. [Google Scholar] [CrossRef]
- Alves, B.; Peixoto, J.; Macedo, S.; Pinheiro, J.; Carvalho, B.; Soares, P.; Lima, J.; Lima, R.T. High VEGFA Expression Is Associated with Improved Progression-Free Survival after Bevacizumab Treatment in Recurrent Glioblastoma. Cancers 2023, 15, 2196. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Kan, L.K.; Drummond, K.; Hunn, M.; Williams, D.; O’Brien, T.J.; Monif, M. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol. Open 2020, 2, e000069. [Google Scholar] [CrossRef]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- Principe, C.; Dionisio de Sousa, I.J.; Prazeres, H.; Soares, P.; Lima, R.T. LRP1B: A Giant Lost in Cancer Translation. Pharmaceuticals 2021, 14, 836. [Google Scholar] [CrossRef]
- Prazeres, H.; Salgado, C.; Duarte, C.; Soares, P. LRP1B (low density lipoprotein receptor-related protein 1B). Atlas Genet. Cytogenet. Oncol. Haematol. 2014, 18. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Z.; Liu, Y.; Guo, L.; Teng, L.; Guo, L.; Liang, L.; Wang, J.; Gao, J.; Li, R.; et al. Genomic characterization reveals distinct mutation landscapes and therapeutic implications in neuroendocrine carcinomas of the gastrointestinal tract. Cancer Commun. 2022, 42, 1367–1386. [Google Scholar] [CrossRef]
- Wang, L.; Yan, K.; He, X.; Zhu, H.; Song, J.; Chen, S.; Cai, S.; Zhao, Y.; Wang, L. LRP1B or TP53 mutations are associated with higher tumor mutational burden and worse survival in hepatocellular carcinoma. J. Cancer 2021, 12, 217–223. [Google Scholar] [CrossRef]
- Chen, H.; Chong, W.; Wu, Q.; Yao, Y.; Mao, M.; Wang, X. Association of LRP1B Mutation with Tumor Mutation Burden and Outcomes in Melanoma and Non-small Cell Lung Cancer Patients Treated with Immune Check-Point Blockades. Front. Immunol. 2019, 10, 1113. [Google Scholar] [CrossRef]
- Tabouret, E.; Labussiere, M.; Alentorn, A.; Schmitt, Y.; Marie, Y.; Sanson, M. LRP1B Deletion Is Associated With Poor Outcome for Glioblastoma Patients. J. Neurol. Sci. 2015, 358, 440–443. [Google Scholar] [CrossRef]
- Prazeres, H.; Torres, J.; Rodrigues, F.; Pinto, M.; Pastoriza, M.C.; Gomes, D.; Cameselle-Teijeiro, J.; Vidal, A.; Martins, T.C.; Sobrinho-Simoes, M.; et al. Chromosomal, epigenetic and microRNA-mediated inactivation of LRP1B, a modulator of the extracellular environment of thyroid cancer cells. Oncogene 2011, 30, 1302–1317. [Google Scholar] [CrossRef]
- Haas, J.; Beer, A.G.; Widschwendter, P.; Oberdanner, J.; Salzmann, K.; Sarg, B.; Lindner, H.; Herz, J.; Patsch, J.R.; Marschang, P. LRP1b shows restricted expression in human tissues and binds to several extracellular ligands, including fibrinogen and apoE—Carrying lipoproteins. Atherosclerosis 2011, 216, 342–347. [Google Scholar] [CrossRef]
- Beer, A.G.; Zenzmaier, C.; Schreinlechner, M.; Haas, J.; Dietrich, M.F.; Herz, J.; Marschang, P. Expression of a Recombinant Full-Length LRP1B Receptor In Human Non-Small Cell Lung Cancer Cells Confirms the Postulated Growth-Suppressing Function of This Large LDL Receptor Family Member. Oncotarget 2016, 7, 68721–68733. [Google Scholar] [CrossRef]
- Liu, C.X.; Ranganathan, S.; Robinson, S.; Strickland, D.K. Gamma-Secretase-Mediated Release of The Low Density Lipoprotein Receptor-Related Protein 1B Intracellular Domain Suppresses Anchorage-Independent Growth of Neuroglioma Cells. J. Biol. Chem. 2007, 282, 7504–7511. [Google Scholar] [CrossRef]
- Dionísio de Sousa, I.J.; Cunha, A.I.; Saraiva, I.A.; Portugal, R.V.; Gimba, E.R.P.; Guimarães, M.; Prazeres, H.; Lopes, J.M.; Soares, P.; Lima, R.T. LRP1B Expression as a Putative Predictor of Response to Pegylated Liposomal Doxorubicin Treatment in Ovarian Cancer. Pathobiology 2021, 88, 400–411. [Google Scholar] [CrossRef]
- Cowin, P.A.; George, J.; Fereday, S.; Loehrer, E.; Van Loo, P.; Cullinane, C.; Etemadmoghadam, D.; Ftouni, S.; Galletta, L.; Anglesio, M.S.; et al. LRP1B Deletion In High-Grade Serous Ovarian Cancers Is Associated with Acquired Chemotherapy Resistance To Liposomal Doxorubicin. Cancer Res. 2012, 72, 4060–4073. [Google Scholar] [CrossRef]
- Ni, S.; Hu, J.; Duan, Y.; Shi, S.; Li, R.; Wu, H.; Qu, Y.; Li, Y. Down expression of LRP1B promotes cell migration via R ho A/C dc42 pathway and actin cytoskeleton remodeling in renal cell cancer. Cancer Sci. 2013, 104, 817–825. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, P.; Gao, C.; Chen, J.; Li, J.; Chen, Z.; Xu, M.; Shao, J.; Zhang, Y.; Xie, J. Down-Regulation of LRP1B in Colon Cancer Promoted the Growth and Migration of Cancer Cells. Exp. Cell Res. 2017, 357, 1–8. [Google Scholar] [CrossRef]
- Zhen, Z.; Shen, Z.; Sun, P. Downregulation of Low-density lipoprotein receptor-related protein 1B (LRP1B) inhibits the progression of hepatocellular carcinoma cells by activating the endoplasmic reticulum stress signaling pathway. Bioengineered 2022, 13, 9467–9481. [Google Scholar] [CrossRef]
- Liu, F.; Hou, W.; Liang, J.; Zhu, L.; Luo, C. LRP1B Mutation: A Novel Independent Prognostic Factor and a Predictive Tumor Mutation Burden In Hepatocellular Carcinoma. J. Cancer 2021, 12, 4039–4048. [Google Scholar] [CrossRef]
- Yu, G.; Mu, H.; Fang, F.; Zhou, H.; Li, H.; Wu, Q.; Xiong, Q.; Cui, Y. LRP1B mutation associates with increased tumor mutation burden and inferior prognosis in liver hepatocellular carcinoma. Medicine 2022, 101, e29763. [Google Scholar] [CrossRef]
- Lan, S.W.; Li, H.; Liu, Y.; Ma, L.X.; Liu, X.H.; Liu, Y.; Yan, S.; Cheng, Y. Somatic Mutation of LRP1B Is Associated with Tumor Mutational Burden In Patients with Lung Cancer. Lung Cancer 2019, 132, 154–156. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, X.; Qian, F.; Jin, C.; Hou, K. Correlation Between LRP1B Mutations and Tumor Mutation Burden In Gastric Cancer. Comput. Math. Methods Med. 2021, 2021, 1522250. [Google Scholar] [CrossRef]
- Brown, L.C.; Tucker, M.D.; Sedhom, R.; Schwartz, E.B.; Zhu, J.; Kao, C.; Labriola, M.K.; Gupta, R.T.; Marin, D.; Wu, Y.; et al. LRP1B Mutations Are Associated with Favorable Outcomes to Immune Checkpoint Inhibitors across Multiple Cancer Types. J. Immunother. Cancer 2021, 9, e001792. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, R.; Li, X.; Wang, B.; Cheng, Y.; Xiao, S.; Sun, P.; Yu, W.; Li, C.; Lin, X.; et al. LRP1B Is a Potential Biomarker for Tumor Immunogenicity and Prognosis of HCC Patients Receiving ICI Treatment. J. Hepatocell. Carcinoma 2022, 9, 203–220. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Aparicio-Prat, E.; Arnan, C.; Sala, I.; Bosch, N.; Guigó, R.; Johnson, R. DECKO: Single-Oligo, Dual-Crispr Deletion of Genomic Elements Including Long Non-Coding RNAs. BMC Genom. 2015, 16, 846. [Google Scholar] [CrossRef]
- Arnan, C.; Ullrich, S.; Pulido-Quetglas, C.; Nurtdinov, R.; Esteban, A.; Blanco-Fernandez, J.; Aparicio-Prat, E.; Johnson, R.; Pérez-Lluch, S.; Guigó, R. Paired guide RNA CRISPR-Cas9 screening for protein-coding genes and lncRNAs involved in transdifferentiation of human B-cells to macrophages. BMC Genom. 2022, 23, 402. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Jin, R.; Cheng, H.; Chen, H.; Li, L.; Guo, K. Effects of LRP1B regulated by HSF1 on lipid metabolism in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2020, 7, 361–376. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information—The Genome Decoration Page. Available online: https://www.ncbi.nlm.nih.gov/genome/tools/gdp (accessed on 2 April 2020).
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Ferreira, A.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE Reference Annotation for The Human and Mouse Genomes. Nucleic Acids Res. 2018, 47, D766–D773. [Google Scholar] [CrossRef]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; et al. Ensembl 2020. Nucleic Acids Res. 2019, 48, D682–D688. [Google Scholar] [CrossRef]
- Ensembl Archive Release 100. Available online: http://apr2020.archive.ensembl.org/index.html (accessed on 2 April 2020).
- Campenhout, C.V.; Cabochette, P.; Veillard, A.C.; Laczik, M.; Zelisko-Schmidt, A.; Sabatel, C.; Dhainaut, M.; Vanhollebeke, B.; Gueydan, C.; Kruys, V. Guidelines for Optimized Gene Knockout Using CRISPR/Cas9. Biotechniques 2019, 66, 295–302. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA Targeting Specificity of RNA-Guided Cas9 Nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using The CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Guschin, D.Y.; Waite, A.J.; Katibah, G.E.; Miller, J.C.; Holmes, M.C.; Rebar, E.J. A Rapid and General Assay For Monitoring Endogenous Gene Modification. Methods Mol. Biol. 2010, 649, 247–256. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of The Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Kozak, M. The Scanning Model for Translation: An Update. J. Cell Biol. 1989, 108, 229–241. [Google Scholar] [CrossRef]
- Liu, H.; Wong, L. Data Mining Tools for Biological Sequences. J. Bioinform. Comput. Biol. 2003, 1, 139–167. [Google Scholar] [CrossRef]
- Pedersen, A.G.; Nielsen, H. Neural Network Prediction of Translation Initiation Sites In Eukaryotes: Perspectives for EST and Genome Analysis. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1997, 5, 226–233. [Google Scholar] [PubMed]
- Tuladhar, R.; Yeu, Y.; Tyler Piazza, J.; Tan, Z.; Rene Clemenceau, J.; Wu, X.; Barrett, Q.; Herbert, J.; Mathews, D.H.; Kim, J.; et al. CRISPR-Cas9-based mutagenesis frequently provokes on-target mRNA misregulation. Nat. Commun. 2019, 10, 4056. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.K. When a ribosome encounters a premature termination codon. BMB Rep. 2013, 46, 9–16. [Google Scholar] [CrossRef]
- Motaln, H.; Koren, A.; Gruden, K.; Ramšak, Ž.; Schichor, C.; Lah, T.T. Heterogeneous glioblastoma cell cross-talk promotes phenotype alterations and enhanced drug resistance. Oncotarget 2015, 6, 40998–41017. [Google Scholar] [CrossRef]
- Mukherjee, R.N.; Chen, P.; Levy, D.L. Recent advances in understanding nuclear size and shape. Nucleus 2016, 7, 167–186. [Google Scholar] [CrossRef]
- Danielsen, H.; Lindmo, T.; Reith, A. A method for determining ploidy distributions in liver tissue by stereological analysis of nuclear size calibrated by flow cytometric DNA analysis. Cytom. J. Int. Soc. Anal. Cytol. 1986, 7, 475–480. [Google Scholar] [CrossRef]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef]
- Roversi, G.; Pfundt, R.; Moroni, R.F.; Magnani, I.; van Reijmersdal, S.; Pollo, B.; Straatman, H.; Larizza, L.; Schoenmakers, E.F. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene 2006, 25, 1571–1583. [Google Scholar] [CrossRef]
- Chen, X.; Xu, F.; Zhu, C.; Ji, J.; Zhou, X.; Feng, X.; Guang, S. Dual sgRNA-Directed Gene Knockout Using CRISPR/Cas9 Technology In Caenorhabditis elegans. Sci. Rep. 2014, 4, 7581. [Google Scholar] [CrossRef]
- Treuren, T.V.; Vishwanatha, J.K. CRISPR Deletion of MIEN1 in Breast Cancer Cells. PLoS ONE 2018, 13, e0204976. [Google Scholar] [CrossRef]
- Grotz, A.K.; Abaitua, F.; Navarro-Guerrero, E.; Hastoy, B.; Ebner, D.; Gloyn, A.L. A CRISPR/Cas9 Genome Editing Pipeline In The EndoC-betaH1 Cell Line to Study Genes Implicated in Beta Cell Function. Wellcome Open Res. 2019, 4, 150. [Google Scholar] [CrossRef]
- Ho, T.; Zhou, N.; Huang, J.; Koirala, P.; Xu, M.; Fung, R.; Wu, F.; Mo, Y. Targeting Non-Coding RNAs with The CRISPR/Cas9 System In Human Cell Lines. Nucleic Acids Res. 2015, 43, e17. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Cai, A.; Calloway, C.L.; Xu, Y.F.; Zhang, R.; Fung, K.M.; Ding, W.Q. miR-23b and miR-27b Are Oncogenic microRNAs In Breast Cancer: Evidence from a CRISPR/Cas9 Deletion Study. BMC Cancer 2019, 19, 642. [Google Scholar] [CrossRef]
- Li, Y.; Rivera, C.M.; Ishii, H.; Jin, F.; Selvaraj, S.; Lee, A.Y.; Dixon, J.R.; Ren, B. CRISPR Reveals a Distal Super-Enhancer Required for Sox2 Expression In Mouse Embryonic Stem Cells. PLoS ONE 2014, 9, e114485. [Google Scholar] [CrossRef]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA Design To Maximize Activity and Minimize off-Target Effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef]
- Javaid, N.; Choi, S. CRISPR/Cas system and factors affecting its precision and efficiency. Front. Cell Dev. Biol. 2021, 9, 761709. [Google Scholar] [CrossRef]
- Zhao, Z.; Shang, P.; Mohanraju, P.; Geijsen, N. Prime editing: Advances and therapeutic applications. Trends Biotechnol. 2023. [Google Scholar] [CrossRef]
- Baralle, D.; Baralle, M. Splicing In Action: Assessing Disease Causing Sequence Changes. J. Med. Genet. 2005, 42, 737–748. [Google Scholar] [CrossRef]
- Guo, T.; Feng, Y.; Xiao, J.; Liu, Q.; Sun, X.; Xiang, J.; Kong, N.; Liu, S.; Chen, G.; Wang, Y.; et al. Harnessing Accurate Non-Homologous End Joining for Efficient Precise Deletion In CRISPR/Cas9-Mediated Genome Editing. Genome Biol. 2018, 19, 170. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease In Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, J. Cas9-Catalyzed DNA Cleavage Generates Staggered Ends: Evidence From Molecular Dynamics Simulations. Sci. Rep. 2016, 6, 37584. [Google Scholar] [CrossRef]
- Lemos, B.R.; Kaplan, A.C.; Bae, J.E.; Ferrazzoli, A.E.; Kuo, J.; Anand, R.P.; Waterman, D.P.; Haber, J.E. CRISPR/Cas9 Cleavages In Budding Yeast Reveal Templated Insertions and Strand-Specific Insertion/Deletion Profiles. Proc. Natl. Acad. Sci. USA 2018, 115, E2040–E2047. [Google Scholar] [CrossRef]
- Taheri-Ghahfarokhi, A.; Taylor, B.J.M.; Nitsch, R.; Lundin, A.; Cavallo, A.; Madeyski-Bengtson, K.; Karlsson, F.; Clausen, M.; Hicks, R.; Mayr, L.M.; et al. Decoding Non-Random Mutational Signatures At Cas9 Targeted Sites. Nucleic Acids Res. 2018, 46, 8417–8434. [Google Scholar] [CrossRef]
- Gisler, S.; Gonçalves, J.P.; Akhtar, W.; de Jong, J.; Pindyurin, A.V.; Wessels, L.F.A.; van Lohuizen, M. Multiplexed Cas9 Targeting Reveals Genomic Location Effects and gRNA-Based Staggered Breaks Influencing Mutation Efficiency. Nat. Commun. 2019, 10, 1598. [Google Scholar] [CrossRef]
- Ge, Y.; Porse, B.T. The Functional Consequences of Intron Retention: Alternative Splicing Coupled To NMD as a Regulator of Gene Expression. Bioessays 2014, 36, 236–243. [Google Scholar] [CrossRef]
- Jacob, A.G.; Smith, C.W.J. Intron Retention as a Component of Regulated Gene Expression Programs. Hum. Genet. 2017, 136, 1043–1057. [Google Scholar] [CrossRef]
- Law, M.E.; Templeton, K.L.; Kitange, G.; Smith, J.; Misra, A.; Feuerstein, B.G.; Jenkins, R.B. Molecular Cytogenetic Analysis of Chromosomes 1 and 19 In Glioma Cell Lines. Cancer Genet. Cytogenet. 2005, 160, 1–14. [Google Scholar] [CrossRef]
- Turtoi, A.; Blomme, A.; Bianchi, E.; Maris, P.; Vannozzi, R.; Naccarato, A.G.; Delvenne, P.; De Pauw, E.; Bevilacqua, G.; Castronovo, V. Accessibilome of Human Glioblastoma: Collagen-VI-alpha-1 Is a New Target and a Marker of Poor Outcome. J. Proteome Res. 2014, 13, 5660–5669. [Google Scholar] [CrossRef]
- Pérès, E.A.; Gérault, A.N.; Valable, S.; Roussel, S.; Toutain, J.; Divoux, D.; Guillamo, J.-S.; Sanson, M.; Bernaudin, M.; Petit, E. Silencing erythropoietin receptor on glioma cells reinforces efficacy of temozolomide and X-rays through senescence and mitotic catastrophe. Oncotarget 2014, 6, 2101–2119. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wakefield, L.; Peters, A.; Peto, M.; Spellman, P.; Grompe, M. Proliferative polyploid cells give rise to tumors via ploidy reduction. Nat. Commun. 2021, 12, 646. [Google Scholar] [CrossRef]
- Tovar, C.; Higgins, B.; Deo, D.; Kolinsky, K.; Liu, J.-J.; Heimbrook, D.C.; Vassilev, L.T. Small-molecule inducer of cancer cell polyploidy promotes apoptosis or senescence: Implications for therapy. Cell Cycle 2010, 9, 3384–3395. [Google Scholar] [CrossRef]
- Singh, T.D.; Park, S.-Y.; Bae, J.-s.; Yun, Y.; Bae, Y.-C.; Park, R.-W.; Kim, I.-S. MEGF10 functions as a receptor for the uptake of amyloid-β. FEBS Lett. 2010, 584, 3936–3942. [Google Scholar] [CrossRef]
- Potere, N.; Del Buono, M.G.; Mauro, A.G.; Abbate, A.; Toldo, S. Low Density Lipoprotein Receptor-Related Protein-1 in Cardiac Inflammation and Infarct Healing. Front. Cardiovasc. Med. 2019, 6, 51. [Google Scholar] [CrossRef]
- Hsu, H.W.; Rodriguez-Ortiz, C.J.; Zumkehr, J.; Kitazawa, M. Inflammatory Cytokine IL-1β Downregulates Endothelial LRP1 via MicroRNA-mediated Gene Silencing. Neuroscience 2021, 453, 69–80. [Google Scholar] [CrossRef]
- Niu, N.; Yao, J.; Bast, R.C.; Sood, A.K.; Liu, J. IL-6 promotes drug resistance through formation of polyploid giant cancer cells and stromal fibroblast reprogramming. Oncogenesis 2021, 10, 65. [Google Scholar] [CrossRef]
- Schneider, V.A.; Graves-Lindsay, T.; Howe, K.; Bouk, N.; Chen, H.C.; Kitts, P.A.; Murphy, T.D.; Pruitt, K.D.; Thibaud-Nissen, F.; Albracht, D.; et al. Evaluation of GRCh38 and De Novo Haploid Genome Assemblies Demonstrates The Enduring Quality of The Reference Assembly. Genome Res. 2017, 27, 849–864. [Google Scholar] [CrossRef]
- Luria, S.E.; Adams, J.N.; Ting, R.C. Transduction of Lactose-Utilizing Ability amongs Strains of E.coli and S.dysenteriae and the Properties of the Transducing Phage Particles. Virology 1960, 12, 348–390. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool To Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Lima, R.T.; Sousa, D.; Paiva, A.M.; Palmeira, A.; Barbosa, J.; Pedro, M.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. Modulation of Autophagy by a Thioxanthone Decreases the Viability of Melanoma Cells. Molecules 2016, 21, 1343. [Google Scholar] [CrossRef]
- Filippi-Chiela, E.C.; Oliveira, M.M.; Jurkovski, B.; Callegari-Jacques, S.M.; da Silva, V.D.; Lenz, G. Nuclear morphometric analysis (NMA): Screening of senescence, apoptosis and nuclear irregularities. PLoS ONE 2012, 7, e42522. [Google Scholar] [CrossRef]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polonia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Leite, M.; Marques, M.S.; Melo, J.; Pinto, M.T.; Cavadas, B.; Aroso, M.; Gomez-Lazaro, M.; Seruca, R.; Figueiredo, C. Helicobacter Pylori Targets the EPHA2 Receptor Tyrosine Kinase in Gastric Cells Modulating Key Cellular Functions. Cells 2020, 9, 513. [Google Scholar] [CrossRef]
- Pinto, F.; Santos-Ferreira, L.; Pinto, M.T.; Gomes, C.; Reis, C.A. The Extracellular Small Leucine-Rich Proteoglycan Biglycan Is a Key Player in Gastric Cancer Aggressiveness. Cancers 2021, 13, 1330. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Osório, H.; Silva, C.; Ferreira, M.; Gullo, I.; Máximo, V.; Barros, R.; Mendonça, F.; Oliveira, C.; Carneiro, F. Proteomics Analysis of Gastric Cancer Patients with Diabetes Mellitus. J. Clin. Med. 2021, 10, 407. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021, 50, D543–D552. [Google Scholar] [CrossRef]


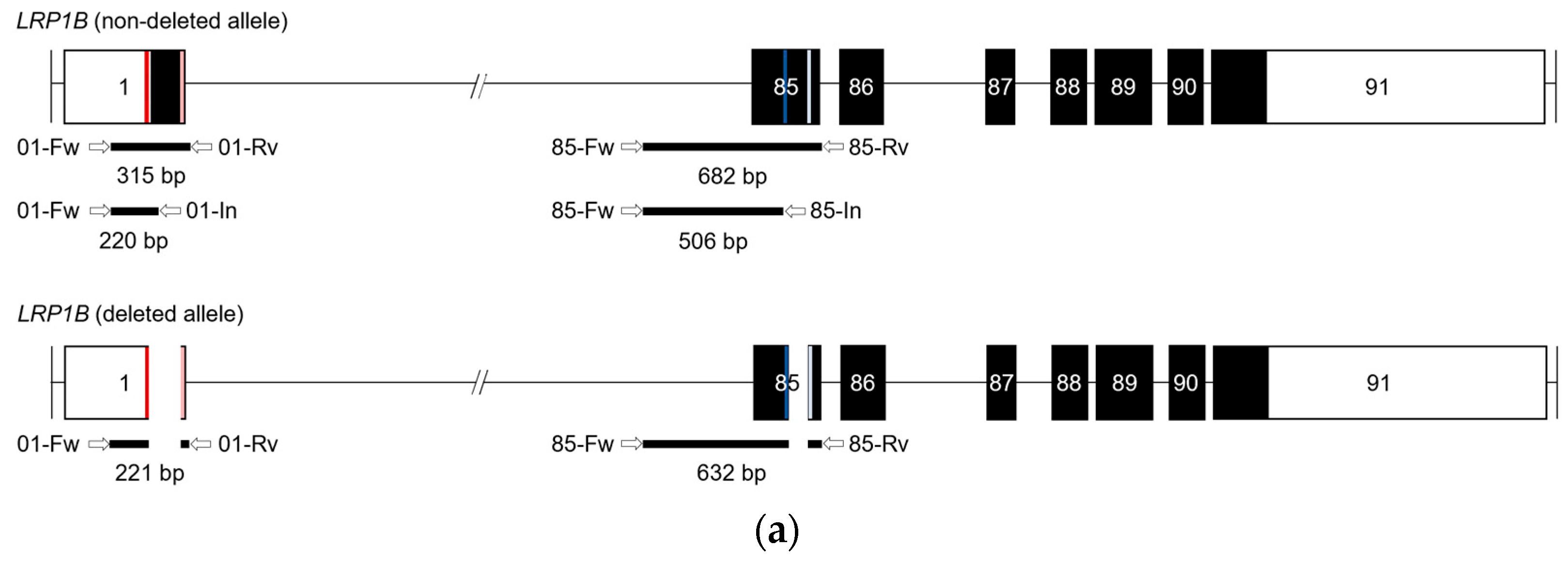
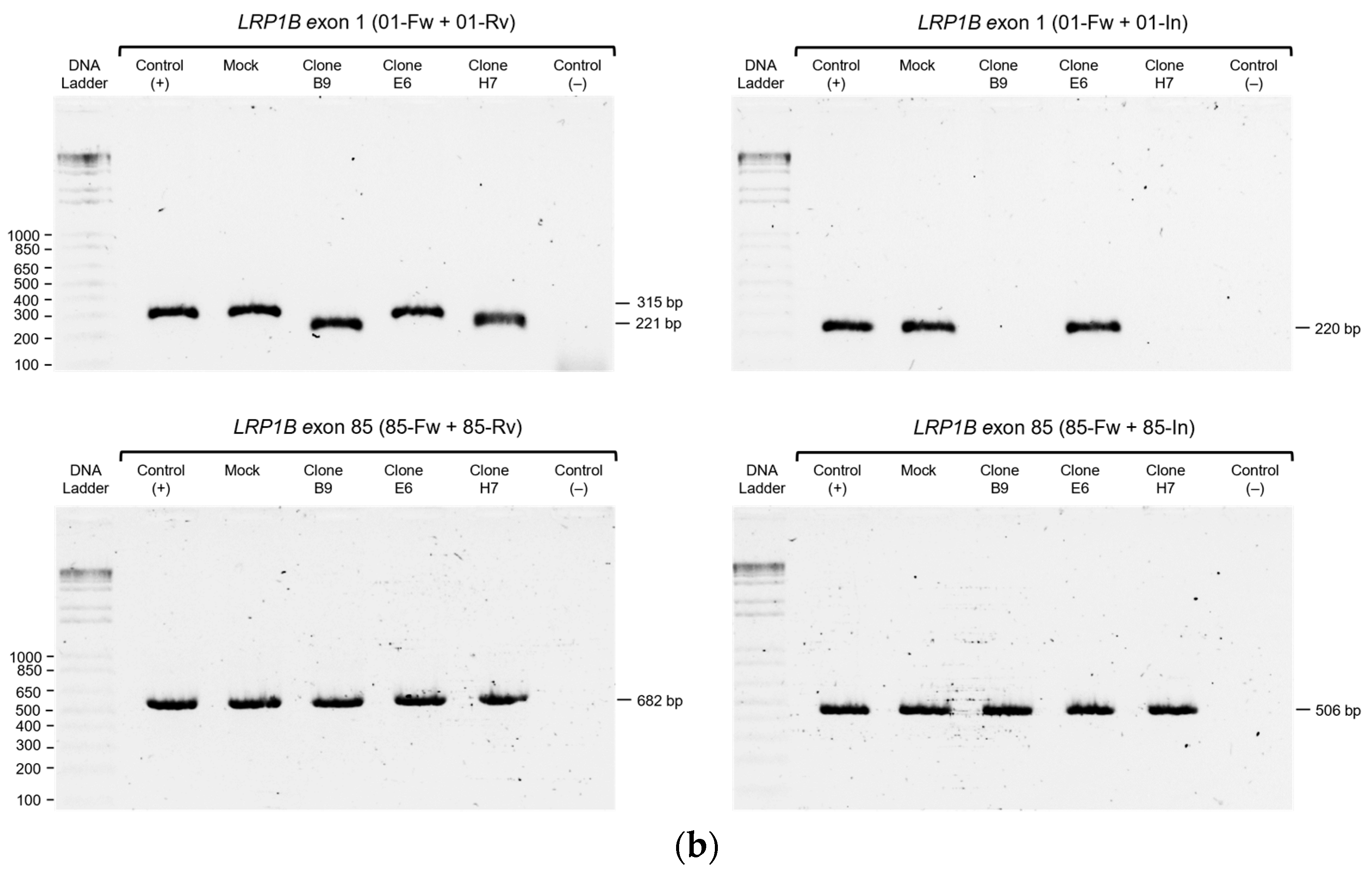
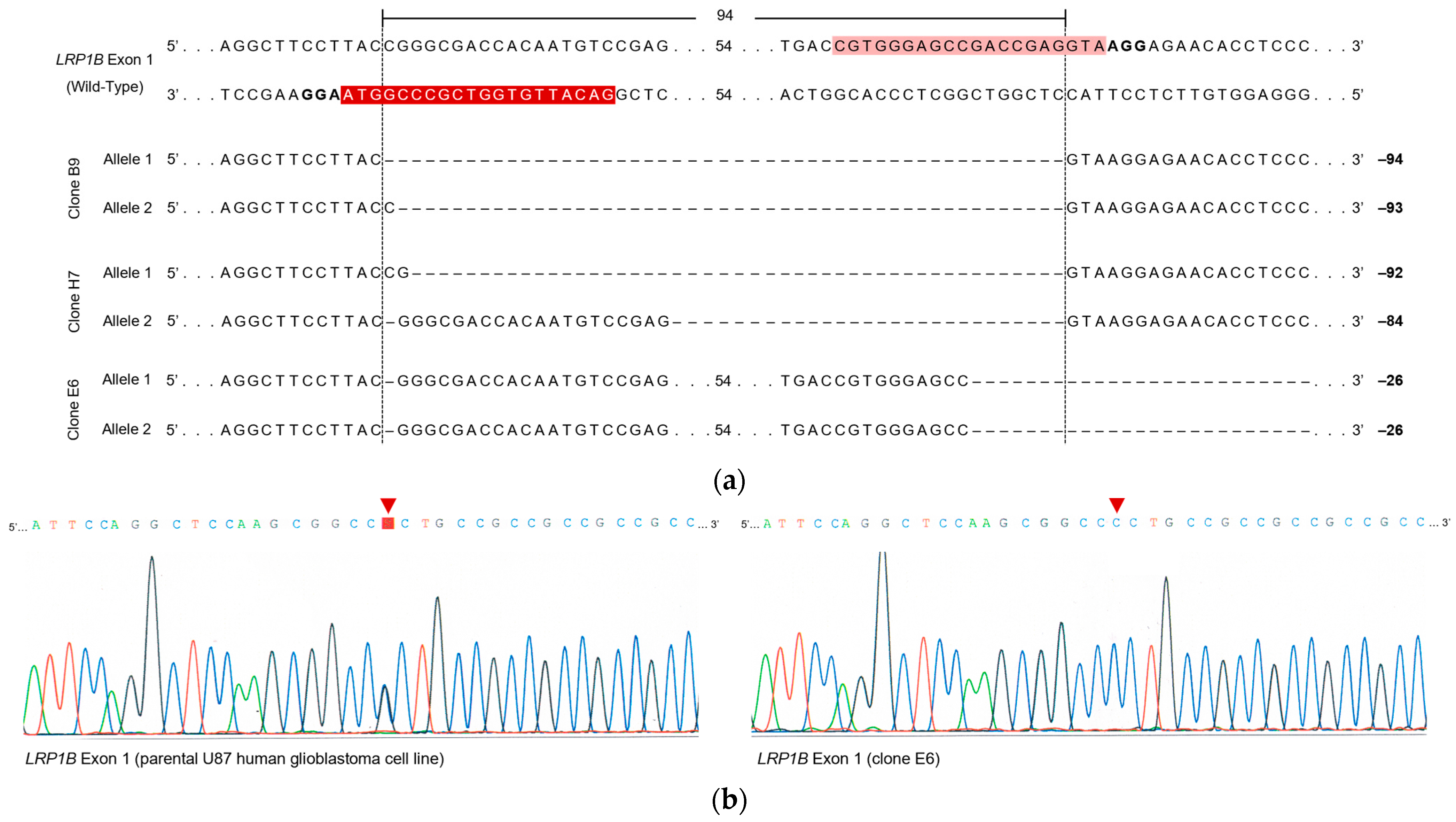

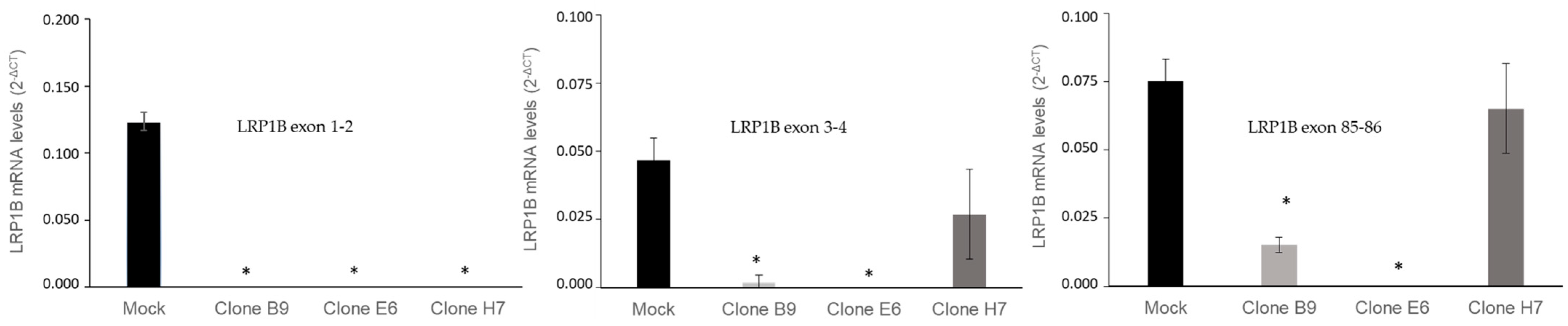



| Target Exon | Clone | Deletion Length (bp) | Predicted Effect on LRP1B mRNA |
|---|---|---|---|
| 1 | B9 | 94 (precise deletion) | Loss of the putative Kozac consensus sequence a and the canonical AUG start codon |
| 93 (1 bp insertion) | Loss of the putative Kozac consensus sequence a and the canonical AUG start codon | ||
| E6 | 26 (1 bp deletion in sgRNA1-binding region) and 25 bp deletion in the sgRNA2-binding region) | Loss of the short consensus sequence at exon 1–intron 1 junction (5′ splice site) | |
| H7 | 92 (2 bp insertion) | Loss of the putative Kozac consensus sequence a and the canonical AUG start codon | |
| 84 (20 bp insertion) | Preservation of the putative Kozac consensus sequence a and the canonical AUG start codon | ||
| 85 | B9 | No deletion | NA |
| E6 | 2 bp deletion in the sgRNA4-binding region | Introduction of an ORF-interrupting PTC | |
| H7 | 1 bp deletion in the sgRNA4-binding region2 bp deletion in the sgRNA4-binding region | Introduction of an ORF-interrupting PTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, J.; Príncipe, C.; Pestana, A.; Osório, H.; Pinto, M.T.; Prazeres, H.; Soares, P.; Lima, R.T. Using a Dual CRISPR/Cas9 Approach to Gain Insight into the Role of LRP1B in Glioblastoma. Int. J. Mol. Sci. 2023, 24, 11285. https://doi.org/10.3390/ijms241411285
Peixoto J, Príncipe C, Pestana A, Osório H, Pinto MT, Prazeres H, Soares P, Lima RT. Using a Dual CRISPR/Cas9 Approach to Gain Insight into the Role of LRP1B in Glioblastoma. International Journal of Molecular Sciences. 2023; 24(14):11285. https://doi.org/10.3390/ijms241411285
Chicago/Turabian StylePeixoto, Joana, Catarina Príncipe, Ana Pestana, Hugo Osório, Marta Teixeira Pinto, Hugo Prazeres, Paula Soares, and Raquel T. Lima. 2023. "Using a Dual CRISPR/Cas9 Approach to Gain Insight into the Role of LRP1B in Glioblastoma" International Journal of Molecular Sciences 24, no. 14: 11285. https://doi.org/10.3390/ijms241411285






