2-Methoxyestradiol-3,17-O,O-bis-sulfamate (STX140) Inhibits Proliferation and Invasion via Senescence Pathway Induction in Human BRAFi-Resistant Melanoma Cells
Abstract
:1. Introduction
2. Results
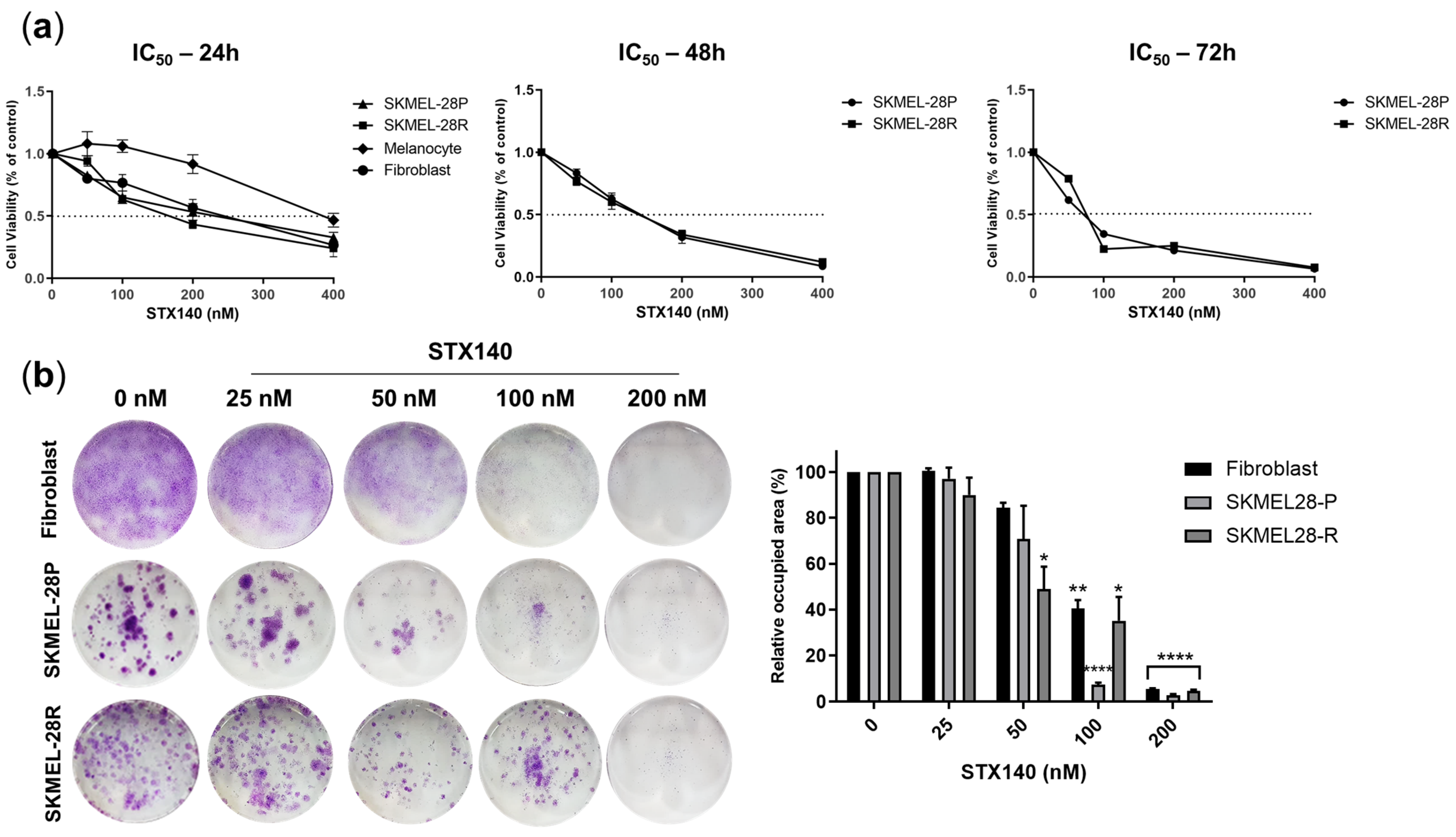
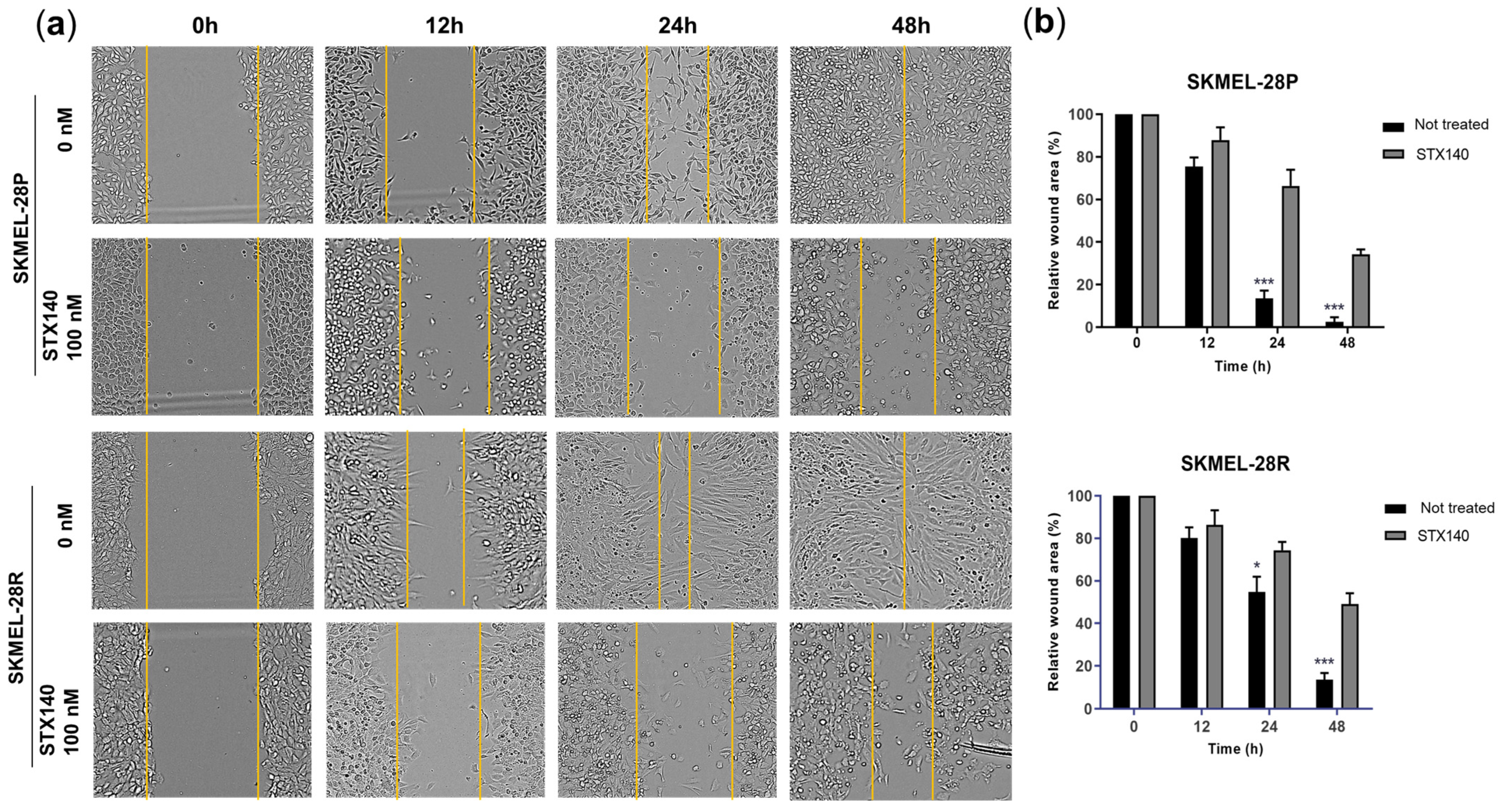
2.1. STX140 Showed Antiproliferative and Anti-Invasive Activity in Melanoma Cells
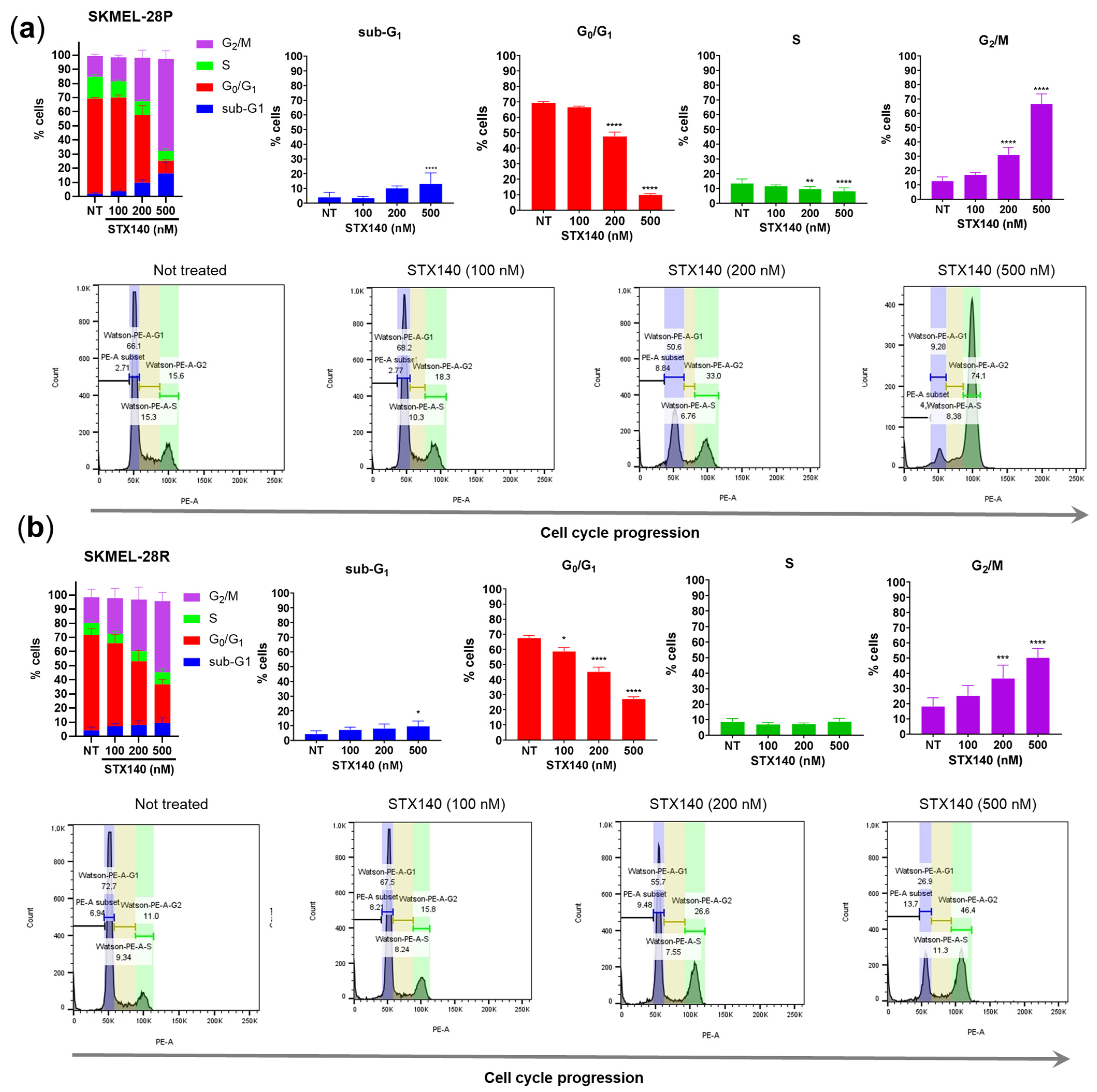
2.2. STX140 Treatment Led to Cell Cycle Arrest
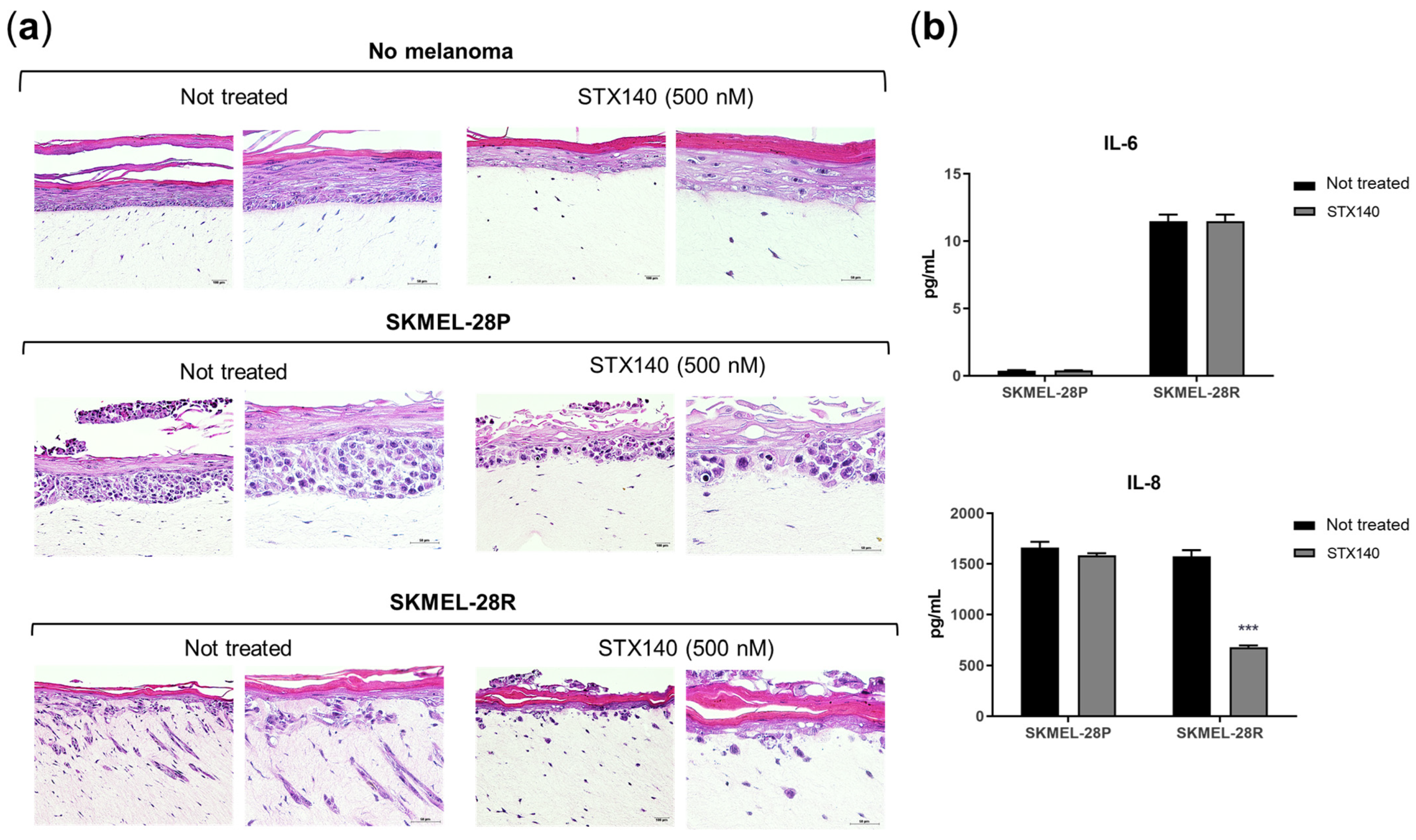
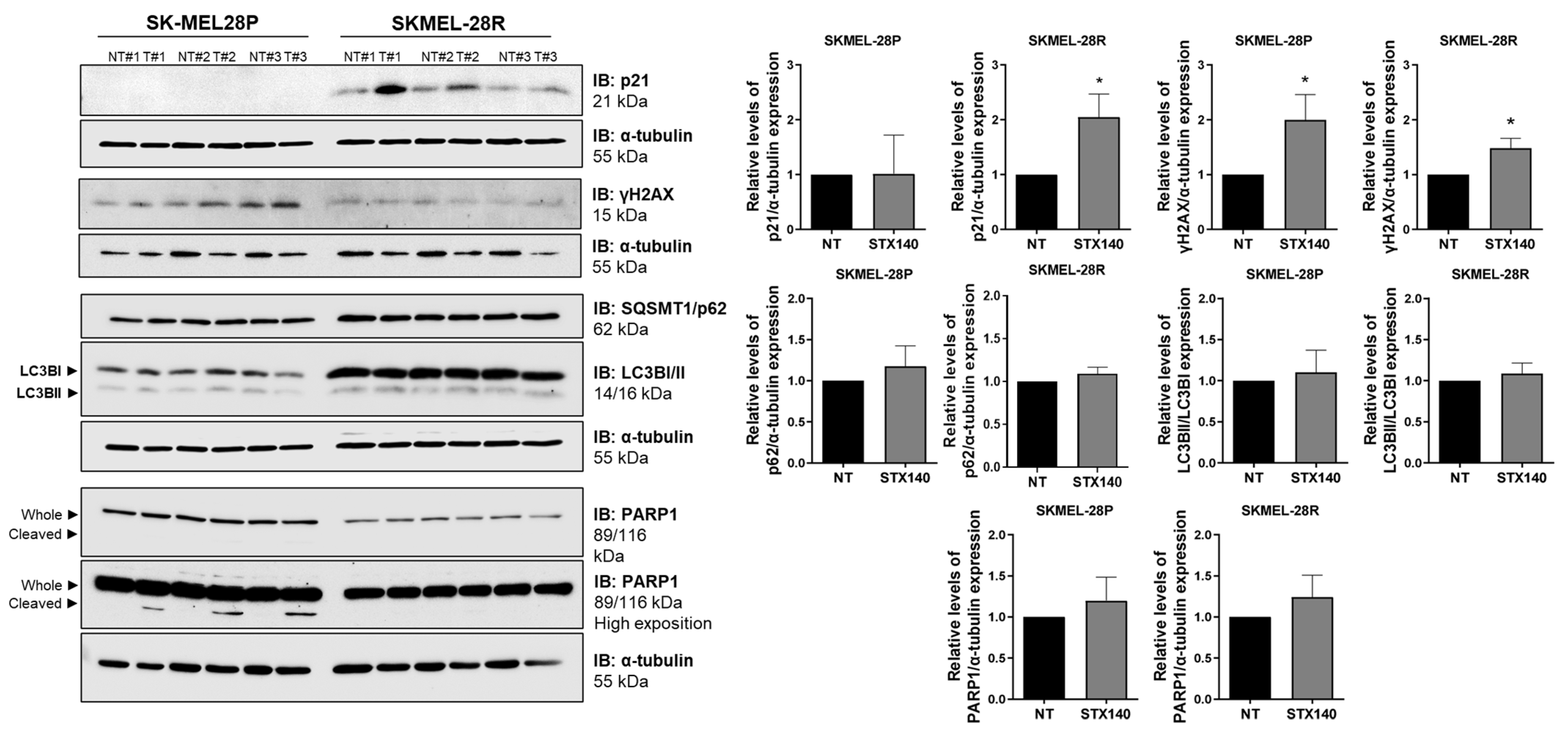
2.3. STX140 Triggered Senescence Phenotype
3. Discussion
4. Materials and Methods
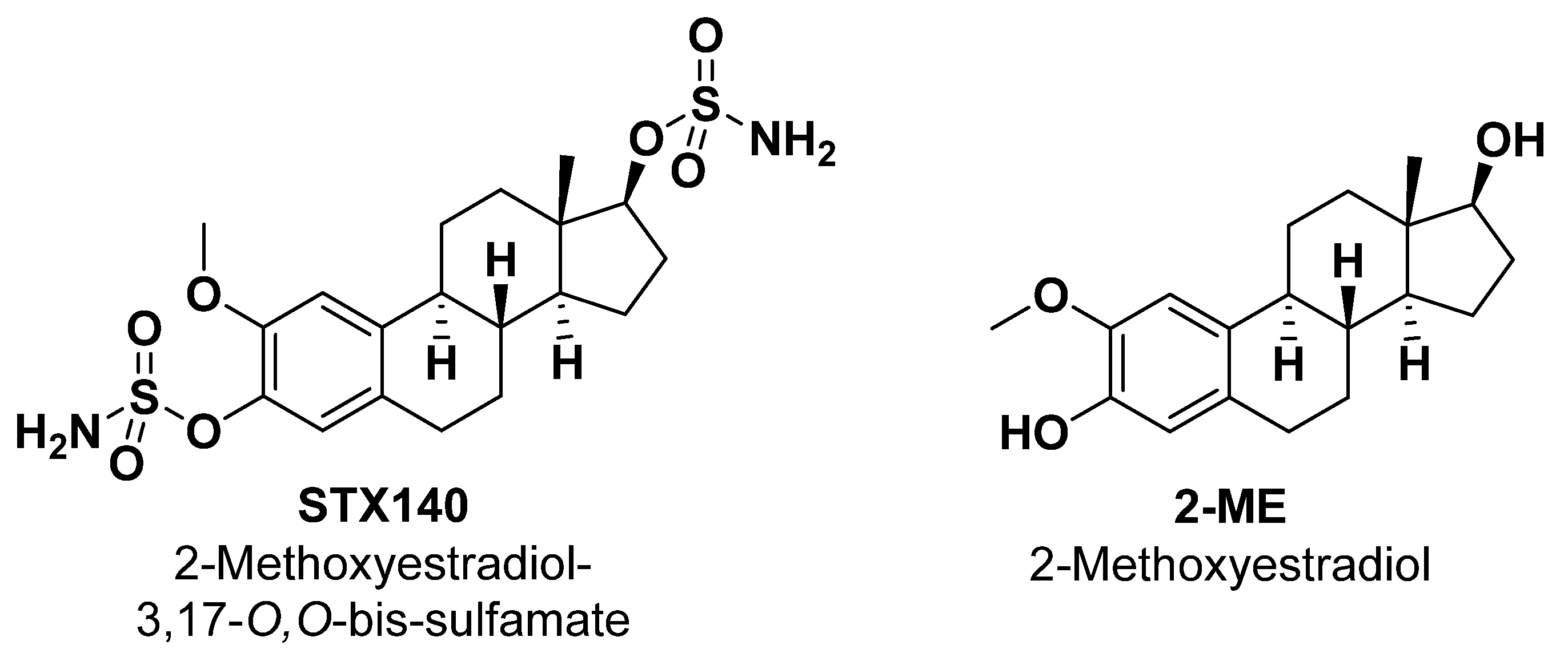
4.1. STX140 Synthesis, Cell Culture and Reagents
4.2. Cell Viability Evaluation
4.3. Clonogenic Assay
4.4. Senescence Evaluation with β-Galactosidase Staining
4.5. Wound-Healing Assay
4.6. IL-6 and IL-8 Quantification
4.7. Flow Cytometry—Apoptosis Evaluation
4.8. Flow Cytometry—Cell Cycle
4.9. Gene Expression Evaluation—RT-PCR
4.10. Reconstructed Human Skin (RHS)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global cancer statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Aplin, A.E.; Kaplan, F.M.; Shao, Y. Mechanisms of resistance to RAF inhibitors in melanoma. J. Investig. Dermatol. 2011, 131, 1817–1820. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Eldabaje, R.; Yang, L. Current status of biological therapies for the treatment of metastatic melanoma. Anticancer Res. 2016, 36, 3229–3241. [Google Scholar]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef]
- Platz, A.; Egyhazi, S.; Ringborg, U.; Hansson, J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol. Oncol. 2008, 1, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular markers and targets in melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Fedorenko, I.V.; Paraiso, K.H.T.; Smalley, K.S.M. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem. Pharm. 2011, 82, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zhang, G.; Li, G. Targeting MAPK pathway in melanoma therapy. Cancer Metastasis Rev. 2013, 32, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudchadkar, R.R.; Smalley, K.S.; Glass, L.F.; Trimble, J.S.; Sondak, V.K. Targeted therapy in melanoma. Clin. Dermatol. 2013, 31, 200–208. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Herlyn, M. Nongenetic mechanisms of drug resistance in melanoma. Annu. Rev. Cancer Biol. 2020, 4, 315–330. [Google Scholar] [CrossRef] [Green Version]
- Munhoz, R.R.; Postow, M.A. Combinatorial approaches to the treatment of advanced melanoma. Hematol. Oncol. Clin. 2021, 35, 145–158. [Google Scholar] [CrossRef]
- Massaro, R.R.; Faião-Flores, F.; Rebecca, V.W.; Sandri, S.; Alves-Fernandes, D.K.; Pennachi, P.C.; Smalley, K.S.M.; Maria-Engler, S.S. Inhibition of proliferation and invasion in 2D and 3D models by 2-methoxyestradiol in human melanoma cells. Pharm. Res. 2017, 119, 242–250. [Google Scholar] [CrossRef]
- Gorska-Ponikowska, M.; Kuban-Jankowska, A.; Daca, A.; Nussberger, S. 2-methoxyestradiol reverses the pro-carcinogenic effect of L-lactate in osteosarcoma 143B cells. Cancer Genom. Proteom. 2017, 14, 483–493. [Google Scholar]
- Kim, J.; Nam, J.; Kim, A.; Park, M.; Lee, H.; Park, J.; Kim, J.; Lee, Y. 2-methoxyestradiol inhibits radiation-induced skin injuries. Int. J. Mol. Sci. 2022, 23, 4171. [Google Scholar] [CrossRef]
- Hua, W.; Huang, X.; Li, J.; Feng, W.; Sun, Y.; Guo, C. 2-methoxyestradiol inhibits melanoma cell growth by activating adaptive immunity. Immunopharm. Immunotoxicol. 2022, 44, 541–547. [Google Scholar] [CrossRef]
- Ireson, C.R.; Chander, S.K.; Purohit, A.; Perera, S.; Newman, S.P.; Parish, D.; Leese, M.P.; Smith, A.C.; Potter, B.V.L.; Reed, M.J. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br. J. Cancer 2004, 90, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Dahut, W.L.; Lakhani, N.J.; Gulley, J.L.; Arlen, P.M.; Kohn, E.C.; Kotz, H.; McNally, D.; Parr, A.; Parr, A.; Nguyen, D.; et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol. Ther. 2006, 5, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, S.P.; Foster, P.A.; Stengel, C.; Day, J.M.; Ho, Y.T.; Judde, J.-G.; Lassalle, M.; Prevost, G.; Leese, M.P.; Potter, B.V.L.; et al. STX140 is efficacious in vitro and in vivo in taxane-resistant breast carcinoma cells. Clin. Cancer Res. 2008, 14, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, B.V.L. Steroid sulfatase inhibition by aryl sulfamates: Clinical progress, mechanism, and future prospects. J. Mol. Endocrinol. 2018, 61, T233–T252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.P.; Potter, B.V.L. Estrogen O-sulfamates and their analogues: Clinical steroid sulfatase inhibitors with broad potential. J. Steroid Biochem. Mol. Biol. 2015, 153, 160–169. [Google Scholar] [CrossRef]
- Thomas, M.P.; Potter, B.V.L. Discovery and development of the aryl O-sulfamate moiety for oncology and women’s health. J. Med. Chem. 2015, 58, 7634–7658. [Google Scholar] [CrossRef] [Green Version]
- Day, J.M.; Foster, P.A.; Tutill, H.J.; Newman, S.P.; Ho, Y.T.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A. BCRP expression does not result in resistance to STX140 in vivo, despite the increased expression of BCRP in A2780 cells in vitro after long-term STX140 exposure. Br. J. Cancer 2009, 100, 476–486. [Google Scholar] [CrossRef]
- Ho, Y.T.; Newman, S.P.; Purohit, A.; Leese, M.P.; Potter, B.V.L.; Reed, M.J. The effects of 2-methoxyoestrogens and their sulphamoylated derivatives in conjunction with TNF-α on endothelial and fibroblast cell growth, morphology, and apoptosis. J. Steroid Biochem. 2003, 86, 189–196. [Google Scholar] [CrossRef]
- Newman, S.P.; Leese, M.P.; Purohit, A.; James, D.R.C.; Rennie, C.E.; Potter, B.V.L.; Reed, M.J. Inhibition of in vitro angiogenesis by 2-methoxy- and 2-ethylestrogen sulfamates. Int. J. Cancer 2004, 109, 533–540. [Google Scholar] [CrossRef]
- Jourdan, F.; Leese, M.P.; Dohle, W.; Hamel, E.; Ferrandis, E.; Newman, S.P.; Purohit, A.; Reed, M.J.; Potter, B.V.L. Synthesis, antitubulin, and antiproliferative SAR of analogues of 2- methoxyestradiol-3,17-O,O-bis-sulfamate. J. Med. Chem. 2010, 53, 2942–2951. [Google Scholar] [CrossRef]
- Stengel, C.; Newman, S.P.; Leese, M.P.; Thomas, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A.; Foster, P.A. The in vitro and in vivo activity of the microtubule disruptor STX140 is mediated by Hif-1 alpha and CAIX expression. Anticancer Res. 2015, 35, 5249–5261. [Google Scholar] [PubMed]
- Meyer-Losic, F.; Newman, S.P.; Day, J.M.; Reed, M.J.; Kasprzyk, P.G.; Purohit, A.; Foster, A. STX140, but not paclitaxel, inhibits mammary tumour initiation and progression in C3(1)/SV40 T/t-antigen transgenic mice. PLoS ONE 2013, 8, e80305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosang, L.; Löhndorf, A.; Dohle, W.; Rosche, A.; Marry, S.; Diercks, B.P.; Müller-Kirschbaum, L.C.; Flügel, L.T.; Potter, B.V.L.; Odoardi, F.; et al. 2-Methoxyestradiol-3, 17-O, O-bis-sulfamate inhibits store-operated Ca2+ entry in T lymphocytes and prevents experimental autoimmune encephalomyelitis. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119485. [Google Scholar] [CrossRef]
- Foster, P.A.; Ho, Y.T.; Newman, S.P.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A. STX140 and STX641 cause apoptosis via the intrinsic mitochondrial pathway and down-regulate survivin and XIAP expression in ovarian and prostate cancer cells. Anticancer Res. 2009, 29, 3751–3758. [Google Scholar]
- Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress Bcl-2 is involved. Cancer Res. 1995, 55, 2284–2292. [Google Scholar] [PubMed]
- Sasaki, M.; Kumazaki, T.; Takano, H.; Nishiyama, M.; Mitsui, Y. Senescent cells are resistant to death despite low Bcl-2 level. Mech. Ageing Dev. 2001, 122, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of Bcl-w and Bcl-xL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, Z.; Liu, Y. GADD45 proteins: Roles in cellular senescence and tumor development. Exp. Biol. Med. 2014, 239, 773–778. [Google Scholar] [CrossRef]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Stivala, A.L.; Cazzalini, O.; Prosperi, E. The cyclin-dependent kinase inhibitor p21CDKN1A as a target of anti-cancer drugs. Curr. Cancer Drug Targets 2012, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Sasai, K.; Kawai, H.; Yuan, Z.; Bondaruk, J.; Suzuki, F.; Fujii, S.; Arlinghaus, R.B.; Czerniak, B.A.; Sen, S. Phosphorylation by aurora kinase a induces MDM2-mediated destabilization and inhibition of p53. Nat. Genet. 2004, 36, 55–62. [Google Scholar]
- Driscoll, D.L.; Chakravarty, A.; Bowman, D.; Shinde, V.; Lasky, K.; Shi, J.; Vos, T.; Stringer, B.; Amidon, B.; D’Amore, N.; et al. Plk1 inhibition causes post-mitotic DNA damage and senescence in a range of human tumor cell lines. PLoS ONE 2014, 9, e111060. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetov, M.K.; Gursoy-Yuzugullu, O.; Xu, C.; Xu, Y.; Price, B.D. DNA double-strand breaks promote methylation of histone h3 on lysine 9 and transient formation of repressive chromatin. Proc. Natl. Acad. Sci. USA 2014, 111, 9169–9174. [Google Scholar] [CrossRef] [PubMed]
- Chicas, A.; Wang, X.; Zhang, C.; McCurrach, M.; Zhao, Z.; Mert, O.; Dickins, R.A.; Narita, M.; Zhang, M.; Lowe, S.W. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 2010, 17, 376–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Takahashi, A.; Imai, Y.; Yamakoshi, K.; Kuninaka, S.; Ohtani, N.; Yoshimoto, S.; Hori, S.; Tachibana, M.; Anderton, E.; Takeuchi, T.; et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/CCDH1 in senescent cells. Mol. Cell 2012, 45, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Lázár-Molnár, E.; Hegyesi, H.; Tóth, S.; Falus, A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 2000, 12, 547–554. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobe, N.P.; Rosel, D.; Dvorankova, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brabek, J. Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem. Cell Biol. 2016, 146, 205–217. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Oñate, C.; Perez, G.; Alfaro, C.; Martín-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.G.; Citrin, D.E.; Hildesheim, J.; Ahmed, M.M.; Venkatachalam, S.; Riscuta, G.; Xi, D.; Zheng, G.; Deursen, J.V.; Goronzy, J.; et al. Therapy-induced senescence: Opportunities to improve anticancer therapy. J. Natl. Cancer Inst. 2021, 113, 1285–1298. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Leese, M.P.; Leblond, B.; Smith, A.; Newman, S.P.; Di Fiore, A.; De Simone, G.; Supuran, C.T.; Purohit, A.; Reed, M.J.; Potter, B.V.L. 2-Substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J. Med. Chem. 2006, 49, 7683–7696. [Google Scholar] [CrossRef] [PubMed]
- Sandri, S.; Faião-Flores, F.; Tiago, M.; Pennacchi, P.C.; Massaro, R.R.; Alves-Fernandes, D.K.; Berardinelli, G.N.; Evangelista, A.F.; de Lima Vazquez, V.; Reis, R.M.; et al. Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by mmp-2 upregulation. Pharm. Res. 2016, 111, 523–533. [Google Scholar] [CrossRef]
- Liu, W.; Meng, J.; Cui, J.; Luan, Y. Characterization and function of microRNA’s in plants. Front. Plant Sci. 2017, 8, 2200. [Google Scholar] [CrossRef] [Green Version]
- Brohem, C.A.; Cardeal, L.B.S.; Tiago, M.; Soengas, M.S.; Barros, S.B.M.; Maria-Engler, S.S. Artificial skin in perspective: Concepts and applications. Pigment Cell Melanoma Res. 2011, 24, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| SKMEL-28P | SKMEL-28R | Melanocyte | Fibroblasts | |
|---|---|---|---|---|
| 24 h | 95.3 | 112.3 | 222.3 | 133.5 |
| 48 h | 114.9 | 101.0 | ND | ND |
| 72 h | 61.9 | 68.2 | ND | ND |
| Gene | Sequence | Concentration |
|---|---|---|
| TP53 | FW: GGCGCACAGAGGAAGAGAAT RV: GGAGAGGAGCTGGTGTTGTTG | 300 nM |
| TP73 | FW: GCACCACGTTTGAGCACCTCT RV: GCAGATTGAACTGGGCCATGA | 300 nM |
| CDKN1A | FW: TGTCACTGTCTTGTACCCTTGT RV: GCCGGCGTTTGGAGTGGTAG | 300 nM |
| CDKN1B | FW: ACTCTGAGGACACGCATTTGGT RV: TCTGTTCTGTTGGCTCTTTTGTT | 300 nM |
| BBC3 | FW: GACCTCAACGCACAGTACGAG RV: AGGAGTCCCATGATGAGATTGT | 300 nM |
| PMAIP1 | FW: CGCGCAAGAACGCTCAACC RV: CACACTCGACTTCCAGCTCTGCT | 300 nM |
| BAX | FW: GAGCTGCAGAGGATGATTGC RV: CAGCTGCCACTCGGAAAA | 300 nM |
| CDKN2A | FW: CACCGAATAGTTACGGTCGGA RV: CACGGGTCGGGTGAGAGTG | 300 nM |
| GADD45A | FW: AAGGATGGATAAGGTGGGG RV: CTGGATCAGGGTGAAGTGG | 300 nM |
| SIVA1 | FW: TCTTCGAGAAGACCAAGCG RV: TGCCCAAGGCTCCTGATC | 300 nM |
| ACTB | FW: AGGCCAACCGCGAGAAG RV: ACAGCCTGGATAGCAACGTACA | 150 nM |
| HPRT1 | FW: GAACGTCTTGCTCGAGATGTGA RV: TCCAGCAGGTCAGCAAAGAAT | 150 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, Y.A.; de Moraes, M.O., Jr.; Carvalho, L.A.C.; Dohle, W.; da Silva, R.O.; Noma, I.H.Y.; Lima, K.; Potter, B.V.L.; Machado-Neto, J.A.; Maria-Engler, S.S. 2-Methoxyestradiol-3,17-O,O-bis-sulfamate (STX140) Inhibits Proliferation and Invasion via Senescence Pathway Induction in Human BRAFi-Resistant Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 11314. https://doi.org/10.3390/ijms241411314
Franco YA, de Moraes MO Jr., Carvalho LAC, Dohle W, da Silva RO, Noma IHY, Lima K, Potter BVL, Machado-Neto JA, Maria-Engler SS. 2-Methoxyestradiol-3,17-O,O-bis-sulfamate (STX140) Inhibits Proliferation and Invasion via Senescence Pathway Induction in Human BRAFi-Resistant Melanoma Cells. International Journal of Molecular Sciences. 2023; 24(14):11314. https://doi.org/10.3390/ijms241411314
Chicago/Turabian StyleFranco, Ylana Adami, Manoel Oliveira de Moraes, Jr., Larissa A. C. Carvalho, Wolfgang Dohle, Renaira Oliveira da Silva, Isabella Harumi Yonehara Noma, Keli Lima, Barry V. L. Potter, João A. Machado-Neto, and Silvya Stuchi Maria-Engler. 2023. "2-Methoxyestradiol-3,17-O,O-bis-sulfamate (STX140) Inhibits Proliferation and Invasion via Senescence Pathway Induction in Human BRAFi-Resistant Melanoma Cells" International Journal of Molecular Sciences 24, no. 14: 11314. https://doi.org/10.3390/ijms241411314





