Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction
Abstract
:1. Introduction
2. Transcriptional Activity and Chromatin Rearrangement in Mammalian Late Diplotene Oocytes
2.1. Chromosome Aggregation and Karyosphere Formation
2.2. Transcriptional Activity of GV Oocytes during Karyosphere Formation
3. Morphodynamics of Heterochromatin in Mouse and Human GV Oocytes
3.1. NSN and SN Chromatin Configurations in the Mouse GV
3.2. Chromatin Configurations in the Human GV
3.3. Is It Possible to Develop a Unified Classification of Chromatin Configurations for Humans and Mice?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wennberg, A.L.; Opdahl, S.; Bergh, C.; Aaris Henningsen, A.-K.; Gissler, M.; Romundstad, L.B.; Pinborg, A.; Tiitinen, A.; Skjærven, R.; Wennerholm, U.-B. Effect of maternal age on maternal and neonatal outcomes after assisted reproductive technology. Fertil. Steril. 2016, 106, 1142–1149.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienzi, L.; Balaban, B.; Ebner, T.; Mandelbaum, J. The oocyte. Hum. Reprod. 2012, 27 (Suppl. S1), i2–i21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, J.G.; Wu, Z.; Murphy, C.; Gao, H. Structure in the amphibian germinal vesicle. Exp. Cell Res. 2004, 296, 28–34. [Google Scholar] [CrossRef]
- Escrich, L.; Galiana, Y.; Grau, N.; Insua, F.; Soler, N.; Pellicer, A.; Escribá, M. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod. Biomed. Online 2018, 37, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L. Present state and future outlook for the application of in vitro oocyte maturation in human infertility treatment. Biol. Reprod. 2022, 106, 235–242. [Google Scholar] [CrossRef]
- Ebner, T.; Moser, M.; Sommergruber, M.; Tews, G. Selection based on morphological assessment of oocytes and embryos at different stages of pre-implantation development. Hum. Reprod. Update 2003, 9, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mai, Q.; Chen, X.; Wang, L.; Gao, L.; Zhou, C.; Zhou, Q. Assessment of the developmental competence of human somatic cell nuclear transfer embryos by oocyte morphology classification. Hum. Reprod. 2009, 24, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, D.; Grøndahl, M.L.; Hreinsson, J.; Andersen, C.Y. Human oocyte morphology and outcomes of infertility treatment: A systematic review. Reprod. Sci. 2022, 29, 2768–2785. [Google Scholar] [CrossRef]
- Zuccotti, M.; Giorgi Rossi, P.; Martinez, A.; Garagna, S.; Forabosco, A.; Redi, C.A. Meiotic and developmental competence of mouse antral oocytes. Biol. Reprod. 1998, 58, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Zuccotti, M.; Ponce, R.H.; Boiani, M.; Guizzardi, S.; Govoni, P.; Scandroglio, R.; Garagna, S.; Redi, C.A. The analysis of chromatin organisation allows selection of mouse antral oocytes competent for development to blastocyst. Zygote 2002, 10, 73–78. [Google Scholar] [CrossRef]
- Monti, M.; Zanonim, M.; Calligaro, A.; Ko, M.S.H.; Mauri, P.; Redi, C.A. Developmental arrest and mouse antral not-surrounded nucleolus oocytes. Biol. Reprod. 2013, 88, 2. [Google Scholar] [CrossRef]
- Escrich, L.; Grau, N.; Meseguer, M.; Pellicer, A.; Escribá, M.J. Morphologic indicators predict the stage of chromatin condensation of human germinal vesicle oocytes recovered from stimulated cycles. Fertil. Steril. 2010, 93, 2557–2564. [Google Scholar] [CrossRef]
- Bogolyubova, I.; Bogolyubov, D. Heterochromatin morphodynamics in late oogenesis and early embryogenesis of mammals. Cells 2020, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Zybina, E.V.; Zybina, T.G. Changes in the arrangement of chromosomes and nucleoli related to functional peculiarities of developing mammalian oocytes during meiotic prophase I. Tsitologiya 1992, 34, 3–23. [Google Scholar]
- Tan, J.-H.; Wang, H.-L.; Sun, X.-S.; Liu, Y.; Suim, H.-S.; Zhang, J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol. Hum. Reprod. 2009, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogolyubova, I.O.; Bogolyubov, D.S. Oocyte nuclear structure during mammalian oogenesis. In Recent Advances in Germ Cells Research; Nova Biomedical: Waltham, MA, USA, 2013; pp. 105–132. [Google Scholar]
- Luciano, A.M.; Franciosi, F.; Dieci, C.; Lodde, V. Changes in large-scale chromatin structure and function during oogenesis: A journey in company with follicular cells. Anim. Reprod. Sci. 2014, 149, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.E. Meiosis overview. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 3, pp. 5–12. [Google Scholar] [CrossRef]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of oocyte maturation and related epigenetic regulation. Front. Cell Dev. Biol. 2021, 9, 654028. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zheng, H.; Kawamura, Y.K.; Zhang, K.; Gassler, J.; Powell, S.; Xu, Q.; Lin, Z.; Xu, K.; Zhou, Q.; et al. Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol. Cell 2020, 77, 825–839.e7. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, T.; Maslova, A.; Starshova, P.; Rodriguez Ramos, J.S.; Krasikova, A. Comparison of the somatic TADs and lampbrush chromomere-loop complexes in transcriptionally active prophase I oocytes. Chromosoma 2022, 131, 207–223. [Google Scholar] [CrossRef]
- Zatsepina, O.V.; Bouniol-Baly, C.; Amirand, C.; Debey, P. Functional and molecular reorganization of the nucleolar apparatus in maturing mouse oocytes. Dev. Biol. 2000, 223, 354–370. [Google Scholar] [CrossRef] [Green Version]
- Fair, T.; Hyttel, P.; Lonergan, P.; Boland, M.P. Immunolocalization of nucleolar proteins during bovine oocyte growth, meiotic maturation, and fertilization. Biol. Reprod. 2001, 64, 1516–1525. [Google Scholar] [CrossRef] [Green Version]
- Shishova, K.V.; Lavrentyeva, E.A.; Dobrucki, J.W.; Zatsepina, O.V. Nucleolus-like bodies of fully-grown mouse oocytes contain key nucleolar proteins but are impoverished for rRNA. Dev. Biol. 2015, 397, 267–281. [Google Scholar] [CrossRef]
- Parfenov, V.; Potchukalina, G.; Dudina, L.; Kostyuchek, D.; Gruzova, M. Human antral follicles: Oocyte nucleus and the karyosphere formation (electron microscopic and autoradiographic data). Gamete Res. 1989, 22, 219–231. [Google Scholar] [CrossRef]
- Miyara, F.; Migne, C.; Dumont-Hassan, M.; Le Meur, A.; Cohen-Bacrie, P.; Aubriot, F.-X.; Glissant, A.; Nathan, C.; Douard, S.; Stanovici, A.; et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol. Reprod. Dev. 2003, 64, 458–470. [Google Scholar] [CrossRef]
- Szöllösi, M.S.; Debey, P.; Szöllösi, D.; Rime, H.; Vautier, D. Chromatin behaviour under influence of puromycin and 6-DMAP at different stages of mouse oocyte maturation. Chromosoma 1991, 100, 339–354. [Google Scholar] [CrossRef]
- Fulka, J.; Benc, M.; Loi, P.; Langerova, A.; Fulka, H. Function of atypical mammalian oocyte/zygote nucleoli and its implications for reproductive biology and medicine. Int. J. Dev. Biol. 2019, 63, 105–112. [Google Scholar] [CrossRef]
- Kyogoku, H.; Kitajima, T.S.; Miyano, T. Nucleolus precursor body (NPB): A distinct structure in mammalian oocytes and zygotes. Nucleus 2014, 5, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Fulka, H.; Langerova, A. Nucleoli in embryos: A central structural platform for embryonic chromatin remodeling? Chromosome Res. 2019, 27, 129–140. [Google Scholar] [CrossRef]
- Sui, H.-S.; Liu, Y.; Miao, D.-Q.; Yuan, J.-H.; Qiao, T.-W.; Luo, M.-J.; Tan, J.-H. Configurations of germinal vesicle (GV) chromatin in the goat differ from those of other species. Mol. Reprod. Dev. 2005, 71, 227–236. [Google Scholar] [CrossRef]
- Kopecny, V.; Biggiogera, M.; Laurincik, J.; Pivko, J.; Grafenau, P.; Martin, T.E.; Fu, X.-D.; Fakan, S. Fine structural cytochemical and immunocytochemical analysis of nucleic acids and ribonucleoprotein distribution in nuclei of pig oocytes and early preimplantation embryos. Chromosoma 1996, 104, 561–574. [Google Scholar] [CrossRef]
- Pan, L.-Z.; Zhu, S.; Zhang, M.; Sun, M.-J.; Lin, J.; Chen, F.; Tan, J.-H. A new classification of the germinal vesicle chromatin configurations in pig oocytes. Biol. Reprod. 2018, 99, 1149–1158. [Google Scholar] [CrossRef]
- Benc, M.; Fulka, J.J.; Strejček, F.; Morovič, M.; Murín, M.; Martínková, S.; Jettmarová, D.; Laurinčík, J. Enucleolation and nucleolus transfer in mammalian oocytes and zygotes. Int. J. Dev. Biol. 2019, 63, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Kopečný, V.; Landa, V.; Malatesta, M.; Martin, T.E.; Fakan, S. Immunoelectron microscope analyses of rat germinal vesicle-stage oocyte nucleolus-like bodies. Reprod. Nutr. Dev. 1996, 36, 667–679. [Google Scholar]
- Pochukalina, G.N.; Parfenov, V.N. Nucleolus transformation in mouse antral follicles: Distribution of coilin and components of RNA polymerase I complex. Cell Tissue Biol. 2008, 2, 522–530. [Google Scholar] [CrossRef]
- Pochukalina, G.N.; Ilicheva, N.V.; Podgornaya, O.I.; Voronin, A.P. Nucleolus-like body of mouse oocytes contains lamin A and B and TRF2 but not actin and topo II. Mol. Cytogenet. 2016, 9, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulka, H.; Martinkova, S.; Kyogoku, H.; Langerova, A.; Fulka, J. Production of giant mouse oocyte nucleoli and assessment of their protein content. J. Reprod. Dev. 2012, 58, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spector, D.L.; Lamond, A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011, 3, a000646. [Google Scholar] [CrossRef] [Green Version]
- Galganski, L.; Urbanek, M.O.; Krzyzosiak, W.J. Nuclear speckles: Molecular organization, biological function and role in disease. Nucleic Acids Res. 2017, 45, 10350–10368. [Google Scholar] [CrossRef] [Green Version]
- Faber, G.P.; Nadav-Eliyahu, S.; Shav-Tal, Y. Nuclear speckles—A driving force in gene expression. J. Cell Sci. 2022, 135, jcs259594. [Google Scholar] [CrossRef]
- Ilık, İ.A.; Aktaş, T. Nuclear speckles: Dynamic hubs of gene expression regulation. FEBS J. 2022, 289, 7234–7245. [Google Scholar] [CrossRef]
- Parfenov, V.N.; Davis, D.S.; Pochukalina, G.N.; Kostyuchek, D.; Murti, K.G. Nuclear distribution of RNA polymerase II in human oocytes from antral follicles: Dynamics relative to the transcriptional state and association with splicing factors. J. Cell. Biochem. 2000, 77, 654–665. [Google Scholar] [CrossRef]
- Otsuki, J.; Nagai, Y. A phase of chromosome aggregation during meiosis in human oocytes. Reprod. Biomed. Online 2007, 15, 191–197. [Google Scholar] [CrossRef]
- Gruzova, M.N.; Parfenov, V.N. Karyosphere in oogenesis and intranuclear morphogenesis. Int. Rev. Cytol. 1993, 144, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Bogolyubov, D.S. Karyosphere (karyosome): A peculiar structure of the oocyte nucleus. Int. Rev. Cell Mol. Biol. 2018, 337, 1–48. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, R.; Viveiros, M.M.; Burns, K.H.; Adashi, E.Y.; Matzuk, M.M.; Eppig, J.J. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev. Biol. 2004, 275, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.M.; Franciosi, F.; Dey, P.; De Guevara, M.L.; Monferini, N.; Bonumallu, S.K.N.; Musmeci, G.; Fagali Franchi, F.; Garcia Barros, R.; Colombo, M.; et al. Progress toward species-tailored prematuration approaches in carnivores. Theriogenology 2023, 196, 202–213. [Google Scholar] [CrossRef]
- Liu, H.; Aoki, F. Transcriptional activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote 2002, 10, 327–332. [Google Scholar] [CrossRef]
- Bouniol-Baly, C.; Hamraoui, L.; Guibert, J.; Beaujean, N.; Szöllösi, M.S.; Debey, P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol. Reprod. 1999, 60, 580–587. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, R.; Eppig, J.J. Transcriptional activity of the mouse oocyte genome: Companion granulosa cells modulate transcription and chromatin remodeling. Dev. Biol. 2001, 229, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, D.; Li, W.; Gao, Q.; Tao, J.; Liu, H. Comprehensive analysis of nonsurrounded nucleolus and surrounded nucleolus oocytes on chromatin accessibility using ATAC-seq. Mol. Reprod. Dev. 2023, 90, 87–97. [Google Scholar] [CrossRef]
- Motlík, J.; Kopečný, V.; Trávnik, P.; Pivko, J. RNA synthesis in pig follicular oocytes. Autoradiographic and cytochemical study. Biol. Cell 1984, 50, 229–235. [Google Scholar] [CrossRef]
- Sun, X.-S.; Liu, Y.; Yue, K.-Z.; Ma, S.-F.; Tan, J.-H. Changes in germinal vesicle (GV) chromatin configurations during growth and maturation of porcine oocytes. Mol. Reprod. Dev. 2004, 69, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Oqani, R.K.; Lee, M.G.; Diao, Y.F.; Han, R.X.; Jin, D.I. Halogenated nucleotide labeling of nascent RNAs reveals dynamic transcription in growing pig oocytes. Dev. Dyn. 2013, 242, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Murin, M.; Nemcova, L.; Bartkova, A.; Gad, A.; Lucas-Hahn, A.; Strejcek, F.; Prochazka, R.; Laurincik, J. Porcine oocytes matured in a chemically defined medium are transcriptionally active. Theriogenology 2023, 203, 89–98. [Google Scholar] [CrossRef]
- Fair, T.; Hyttel, P.; Greve, T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol. Reprod. Dev. 1995, 42, 437–442. [Google Scholar] [CrossRef]
- Fair, T.; Hyttel, P.; Greve, T.; Boland, M. Nucleolus structure and transcriptional activity in relation to oocyte diameter in cattle. Mol. Reprod. Dev. 1996, 43, 503–512. [Google Scholar] [CrossRef]
- Lodde, V.; Modina, S.; Galbusera, C.; Franciosi, F.; Luciano, A.M. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol. Reprod. Dev. 2007, 74, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Tesařík, J.; Kopečný, V.; Kurilo, L.F. Preovulatory RNA synthesis in human oocytes of large antral follicles. Histochem. J. 1984, 16, 438–440. [Google Scholar] [CrossRef]
- Xia, M.; He, H.; Wang, Y.; Liu, M.; Zhou, T.; Lin, M.; Zhou, Z.; Huo, R.; Zhou, Q.; Sha, J. PCBP1 is required for maintenance of the transcriptionally silent state in fully grown mouse oocytes. Cell Cycle 2012, 11, 2833–2842. [Google Scholar] [CrossRef] [Green Version]
- Lowther, K.M.; Mehlmann, L.M. Embryonic poly(A)-binding protein is required during early stages of mouse oocyte development for chromatin organization, transcriptional silencing, and meiotic competence. Biol. Reprod. 2015, 93, 43. [Google Scholar] [CrossRef]
- De Smedt, V.; Crozet, N.; Gall, L. Morphological and functional changes accompanying the acquisition of meiotic competence of ovarian goat oocytes. J. Exp. Zool. 1994, 269, 128–139. [Google Scholar] [CrossRef]
- Wang, H.-L.; Sui, H.-S.; Liu, Y.; Miao, D.-Q.; Lu, J.-H.; Liang, B.; Tan, J.-H. Dynamic changes of germinal vesicle chromatin configuration and transcriptional activity during maturation of rabbit follicles. Fertil. Steril. 2009, 91, 1589–1594. [Google Scholar] [CrossRef]
- Frehlick, L.J.; Eirín-López, J.M.; Ausió, J. New insights into the nucleophosmin/nucleoplasmin family of nuclear chaperones. Bioessays 2007, 29, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.; Viveiros, M.M.; Ren, Y.; Wang, P.; DeMayo, F.J.; Frail, D.E.; Eppig, J.J.; Matzuk, M.M. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 2003, 300, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Debey, P.; Szöllösi, M.S.; Szöllösi, D.; Vautier, D.; Girousse, A.; Besombes, D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol. Reprod. Dev. 1993, 36, 59–74. [Google Scholar] [CrossRef]
- Zuccotti, M.; Piccinelli, A.; Giorgi Rossi, P.; Garagna, S.; Redi, C.A. Chromatin organization during mouse oocyte growth. Mol. Reprod. Dev. 1995, 41, 479–485. [Google Scholar] [CrossRef]
- Ostromyshenskii, D.I.; Chernyaeva, E.N.; Kuznetsova, I.S.; Podgornaya, O.I. Mouse chromocenters DNA content: Sequencing and in silico analysis. BMC Genom. 2018, 19, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garagna, S.; Merico, V.; Sebastiano, V.; Monti, M.; Orlandini, G.; Gatti, R.; Scandroglio, R.; Redi, C.A.; Zuccotti, M. Three-dimensional localization and dynamics of centromeres in mouse oocytes during folliculogenesis. J. Mol. Histol. 2004, 35, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bonnet-Garnier, A.; Feuerstein, P.; Chebrout, M.; Fleurot, R.; Jan, H.-U.; Debey, P.; Beaujean, N. Genome organization and epigenetic marks in mouse germinal vesicle oocytes. Int. J. Dev. Biol. 2012, 56, 877–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogolyubova, I.; Bogolyubov, D. DAXX is a crucial factor for proper development of mammalian oocytes and early embryos. Int. J. Mol. Sci. 2021, 22, 1313. [Google Scholar] [CrossRef]
- De La Fuente, R.; Viveiros, M.M.; Wigglesworth, K.; Eppig, J.J. ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev. Biol. 2004, 272, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, R.; Baumann, C.; Viveiros, M.M. Chromatin structure and ATRX function in mouse oocytes. Results Probl. Cell Differ. 2012, 55, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Viveiros, M.M.; De La Fuente, R. Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo. PLoS Genet. 2010, 6, e1001137. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Nakajima, R.; Nagata, M.; Aoki, F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Hum. Reprod. 2008, 23, 1377–1384. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-Y.; Li, M.; Luo, Y.-B.; Song, S.; Tian, D.; Yang, J.; Zhang, B.; Hou, Y.; Schatten, H.; Liu, Z.; et al. Maternal factors required for oocyte developmental competence in mice: Transcriptome analysis of non-surrounded nucleolus (NSN) and surrounded nucleolus (SN) oocytes. Cell Cycle 2013, 12, 1928–1938. [Google Scholar] [CrossRef] [Green Version]
- Combelles, C.M.H.; Cekleniak, N.A.; Racowsky, C.; Albertini, D.F. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum. Reprod. 2002, 17, 1006–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuki, J.; Nagai, Y.; Sankai, T. Aggregated chromosomes transfer in human oocytes. Reprod. Biomed. Online 2014, 28, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, B.A.; Albertini, D.F. Oogenesis: Chromatin and microtubule dynamics during meiotic prophase. Mol. Reprod. Dev. 1990, 25, 374–383. [Google Scholar] [CrossRef]
- Schramm, R.D.; Tennier, M.T.; Boatman, D.E.; Bavister, B.D. Chromatin configurations and meiotic competence of oocytes are related to follicular diameter in nonstimulated rhesus monkeys. Biol. Reprod. 1993, 48, 349–356. [Google Scholar] [CrossRef] [Green Version]


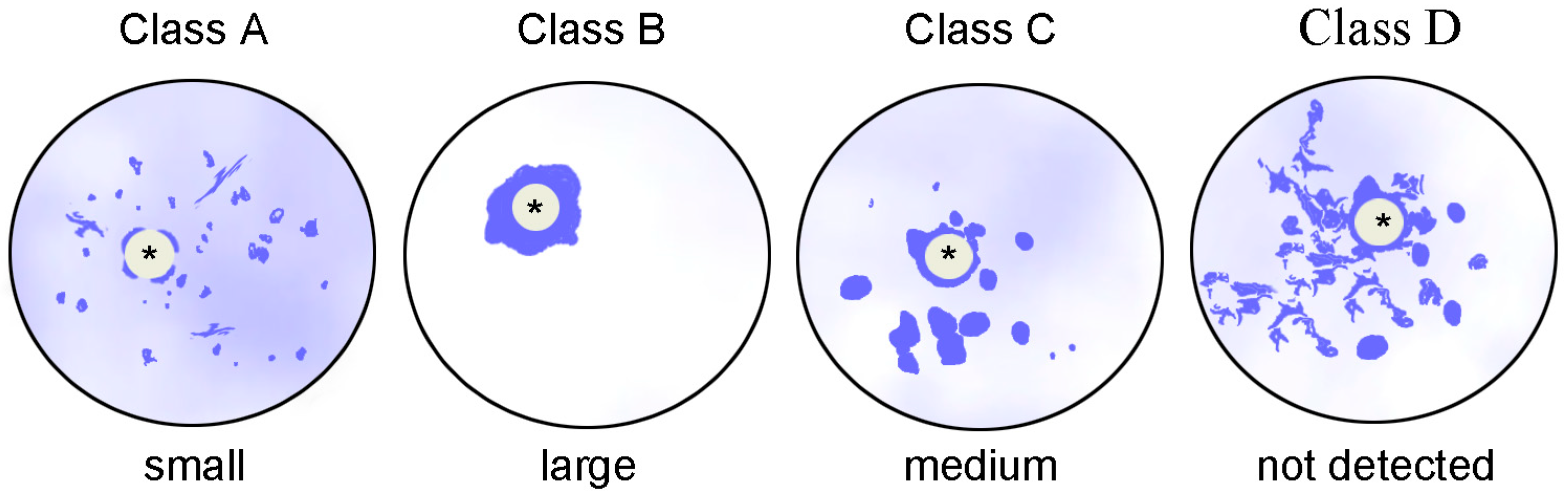
| Class | General Characteristics of the GV |
|---|---|
| NSN | Chromatin is weakly condensed, not associated with atypical nucleoli, and distributed throughout the GV |
| pNSN | Chromatin is distributed throughout the GV, and only single blocks of condensed chromatin are found in close association with the atypical nucleolus |
| pSN | Chromatin is distributed throughout the GV, while an incomplete heterochromatin ring or its separate fragments is formed around the atypical nucleolus |
| SN | The atypical nucleolus is completely surrounded by a heterochromatic ring |
| Class | Characteristics of the GV |
|---|---|
| According to Combelles et al. [78]: | |
| A | The atypical nucleolus is only partially surrounded by chromatin; a significant part of the chromatin (“fibrillar chromatin” in the cited paper) is dispersed throughout the GV |
| B | All chromatin is centered around the atypical nucleolus 2 |
| C | The atypical nucleolus is completely surrounded by chromatin, but a significant portion of condensed chromatin (the authors’ “masses of condensed chromatin”) is observed in the rest of the GV |
| D | The atypical nucleolus is completely surrounded by chromatin, the strands of which (“threads of dispersed chromatin… without any evidence of fibrillar chromatin patterning”) are present in the rest of the GV |
| According to Miyara et al. [26]: | |
| Small oocytes | Chromatin is distributed throughout the GV |
| Oocytes of intermediate size | Condensed chromatin is distributed throughout the GV in the form of large blocks (“aggregates” in the cited paper) and partially surrounds the atypical nucleolus 3 |
| Large oocytes | All chromatin is centered around the atypical nucleolus 4 |
| Class | Characteristics of the GV | Mouse Traditional Nomenclature [50,67] | Human Nomenclature According to Combelles et al. [78] | Human Nomenclature According to Miyara et al. [26] 1 | ||
|---|---|---|---|---|---|---|
| General | Chromatin State 1 | Karyosphere State | ||||
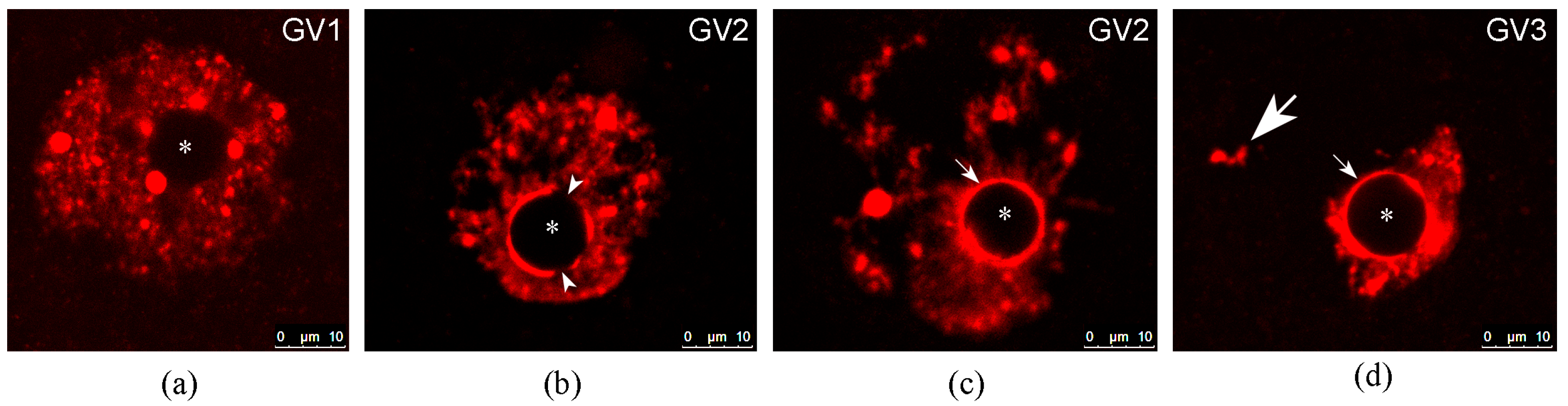
| GV1 | Chromatin is weakly condensed and distributed throughout the GV. Only single blocks of heterochromatin are associated with the atypical nucleolus, or there is no association between chromatin and the atypical nucleolus at all | Disperse chromatin configuration | Absent | NSN + pNSN | A | Small oocytes |
| GV2 | Chromatin is moderately condensed and distributed throughout the GV. An incomplete or complete heterochromatin ring is detected around the atypical nucleolus | Intermediate chromatin configuration | Partially formed | pSN + a portion of SN | C + D | Oocytes of intermediate size |
| GV3 | Heterochromatin is concentrated (completely in humans) in a limited area of the GV around the atypical nucleolus | Condensed/compact chromatin configuration | Fully formed | SN | B | Large oocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogolyubova, I.; Salimov, D.; Bogolyubov, D. Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction. Int. J. Mol. Sci. 2023, 24, 11517. https://doi.org/10.3390/ijms241411517
Bogolyubova I, Salimov D, Bogolyubov D. Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction. International Journal of Molecular Sciences. 2023; 24(14):11517. https://doi.org/10.3390/ijms241411517
Chicago/Turabian StyleBogolyubova, Irina, Daniil Salimov, and Dmitry Bogolyubov. 2023. "Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction" International Journal of Molecular Sciences 24, no. 14: 11517. https://doi.org/10.3390/ijms241411517






