Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro
Abstract
:1. Introduction
2. Results
2.1. Sertindole Is Cytotoxic to Various Human Urinary Bladder TCC Cell Lines While Showing Less Cytotoxicity to Normal Human Urothelial Cells
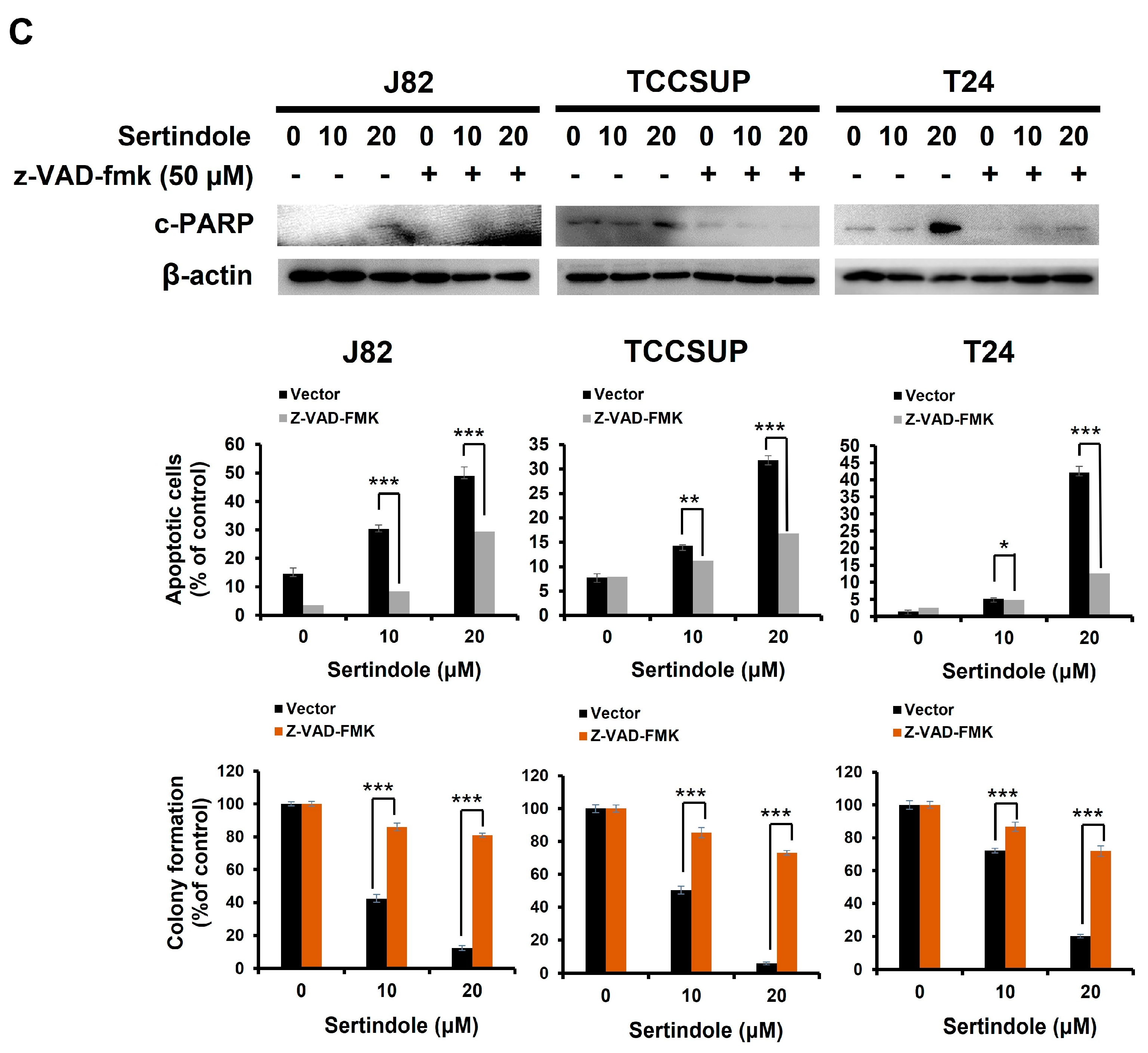
2.2. Sertindole-Induced Bladder Cancer Cytotoxicity Depends on Apoptosis Induction
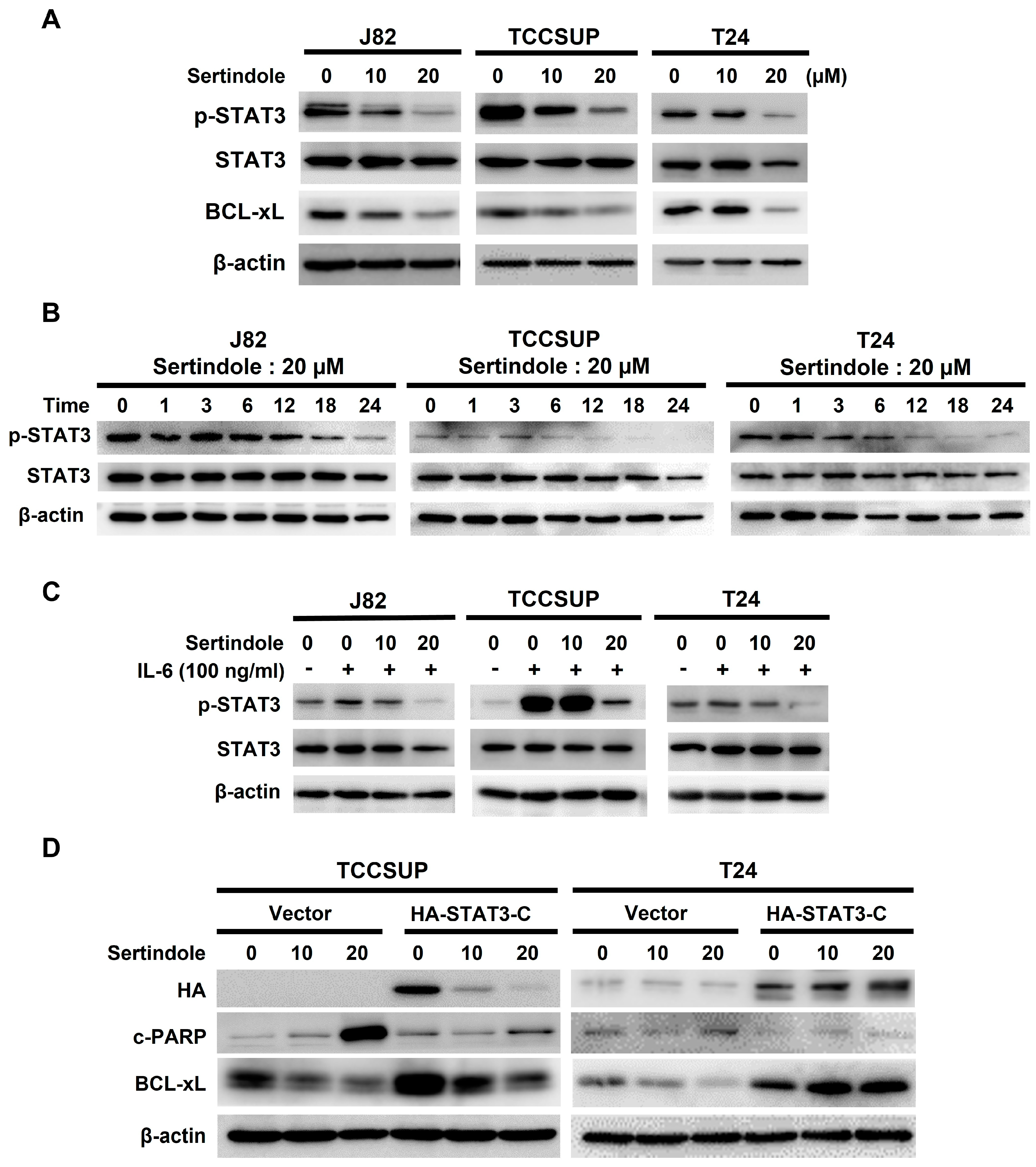
2.3. Suppression of STAT3 Activation Is Pivotal to Sertindole-Induced Human Bladder Cancer Apoptosis
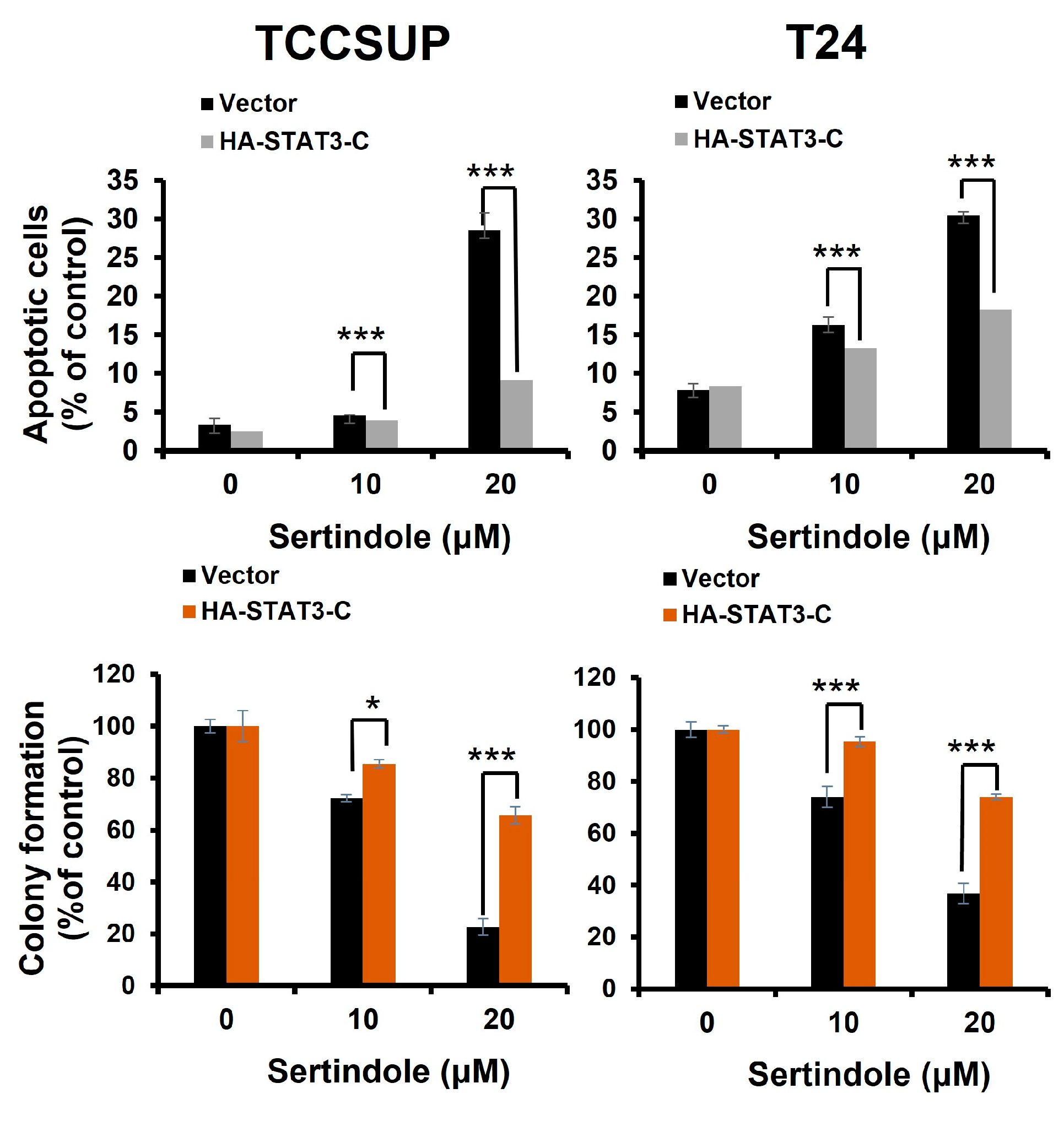
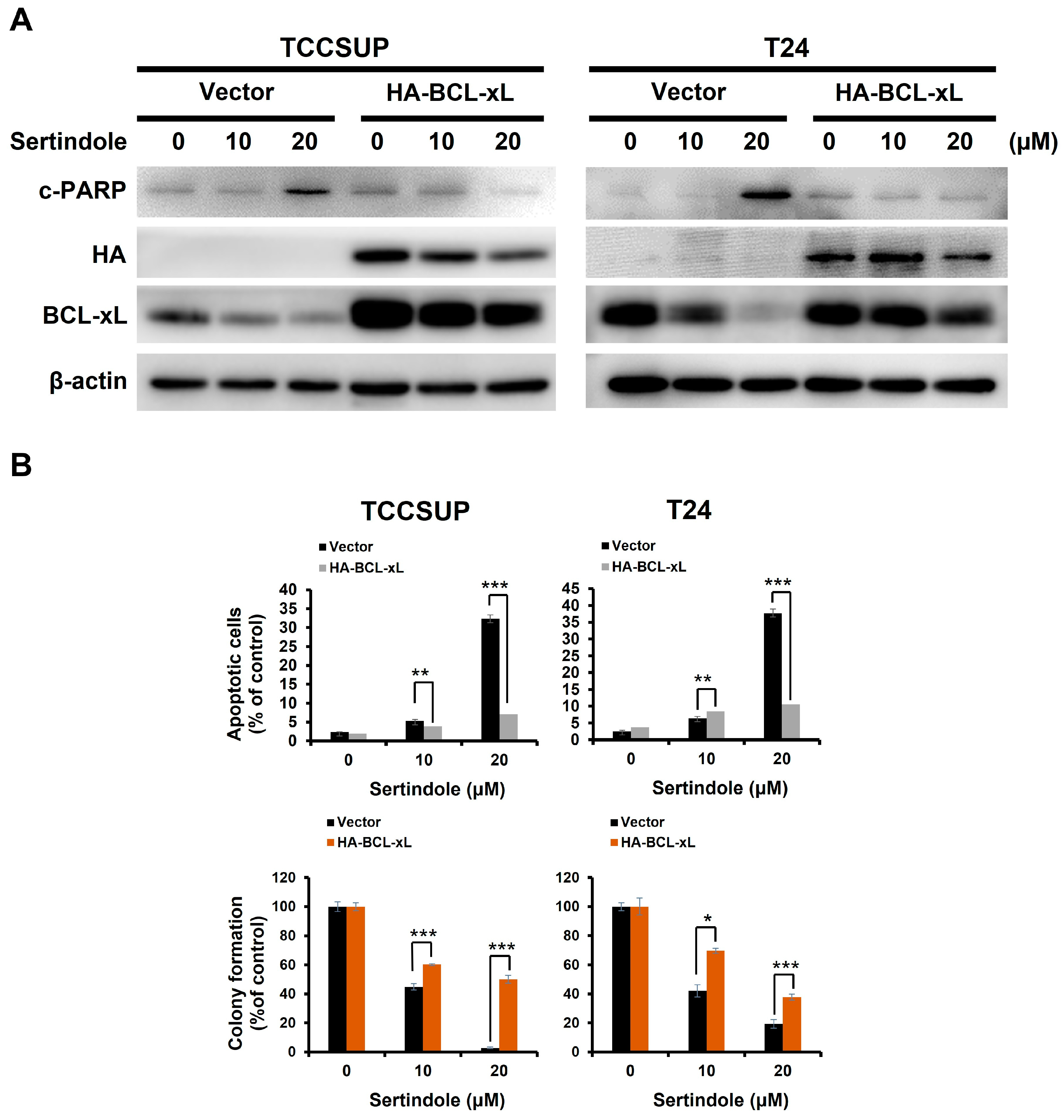
2.4. BCL-xL Downregulation Is Responsible for Human Bladder Cell Apoptosis Resulting from Sertindole-Induced STAT3 Inhibition
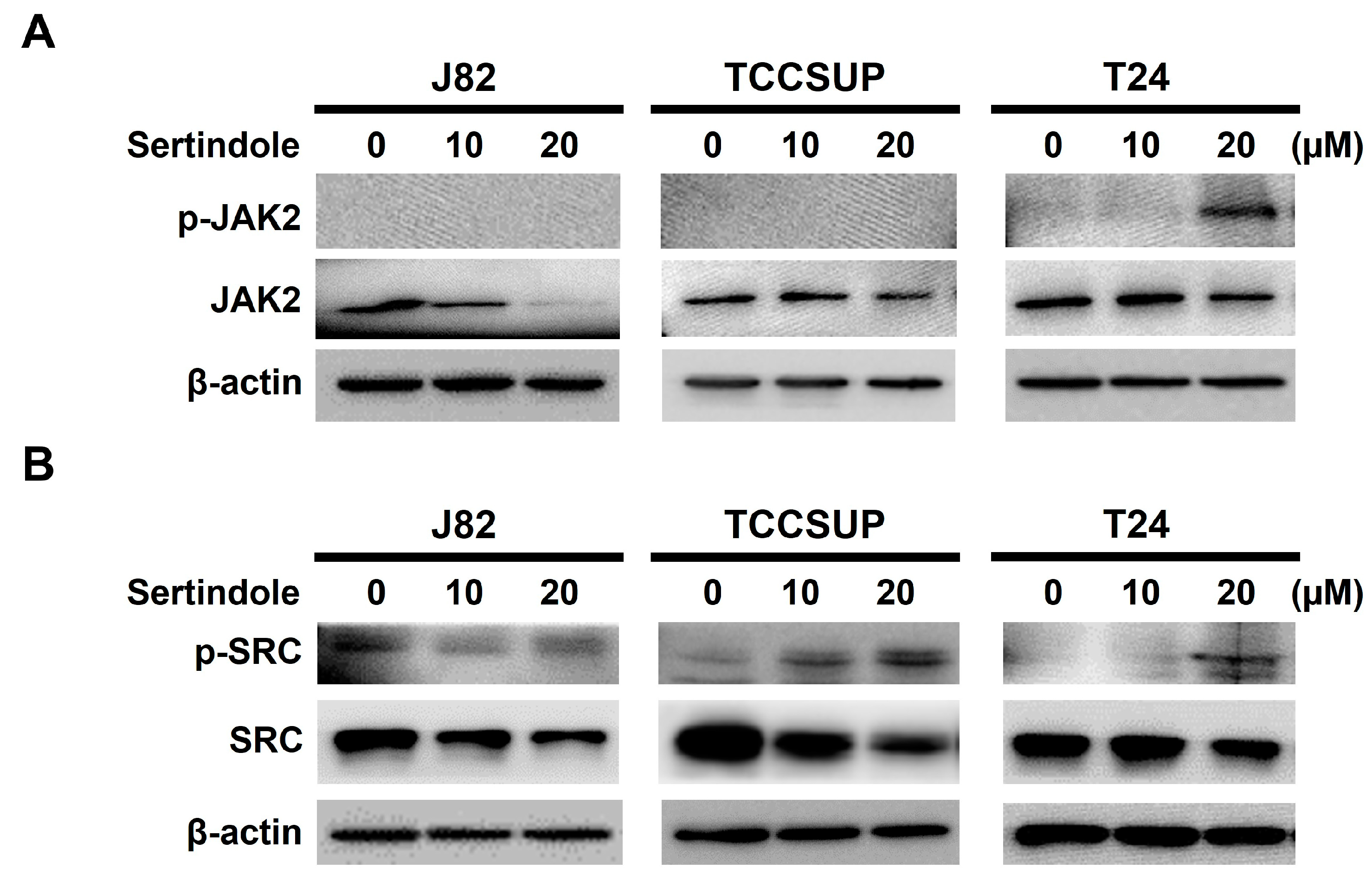
2.5. Neither JAK2 nor SRC Appears to Involve in Sertindole-Inhibited STAT3 Activation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plasmids
4.3. Cell Culture
4.4. Cytotoxicity Assay
4.5. Immunoblotting
4.6. Apoptosis Assay
4.7. Establishment of HA-STAT3-C and HA-BCL-xL Stable Clones in TCCSUP and T24 Cells
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobruch, J.; Oszczudłowski, M. Bladder cancer: Current challenges and future directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef]
- Crocetto, F.; Buonerba, C.; Caputo, V.; Ferro, M.; Persico, F.; Trama, F.; Iliano, E.; Rapisarda, S.; Bada, M.; Facchini, G.; et al. Urologic malignancies: Advances in the analysis and interpretation of clinical findings. Future Sci. OA 2021, 7, FSO674. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Fung, F.D.H.; Leung, C.; Cheung, W.W.L.; Goggins, W.B.; Ng, C.F. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018, 8, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The role of tobacco smoke in bladder and kidney carcinogenesis: A comparison of exposures and meta-analysis of incidence and mortality risks. Eur. Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Jee, S.H.; Shin, H.R.; Park, E.H.; Shin, A.; Jung, K.W.; Hwang, S.S.; Cha, E.S.; Yun, Y.H.; Park, S.K.; et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer 2014, 14, 406. [Google Scholar] [CrossRef] [Green Version]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Aveta, A.; Cacciapuoti, C.; Barone, B.; Di Zazzo, E.; Del Giudice, F.; Maggi, M.; Ferro, M.; Terracciano, D.; Busetto, G.M.; Lucarelli, G.; et al. The impact of meat intake on bladder cancer incidence: Is it really a relevant risk? Cancers 2022, 14, 4775. [Google Scholar] [CrossRef]
- Ferro, M.; Chiujdea, S.; Musi, G.; Lucarelli, G.; Del Giudice, F.; Hurle, R.; Damiano, R.; Cantiello, F.; Mari, A.; Minervini, A.; et al. Impact of age on outcomes of patients with pure carcinoma in situ of the bladder: Multi-institutional cohort analysis. Clin. Genitourin. Cancer 2022, 20, e166–e172. [Google Scholar] [CrossRef]
- Piyathilake, C. Dietary factors associated with bladder cancer. Investig. Clin. Urol. 2016, 57 (Suppl. 1), S14–S25. [Google Scholar] [CrossRef] [Green Version]
- Czene, K.; Lichtenstein, P.; Hemminki, K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int. J. Cancer 2002, 99, 260–266. [Google Scholar] [CrossRef]
- Charlton, M.E.; Adamo, M.P.; Sun, L.; Deorah, S. Bladder cancer collaborative stage variables and their data quality, usage, and clinical implications: A review of SEER data, 2004–2010. Cancer 2014, 120 (Suppl. 23), 3815–3825. [Google Scholar] [CrossRef] [Green Version]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou-Redorta, J.; Rouprêt, M.; European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011, 59, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. MedComm 2022, 3, e124. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Guan, X.; Qin, J.J. Inhibiting STAT3 signaling pathway by natural products for cancer prevention and therapy: In vitro and in vivo activity and mechanisms of action. Pharmacol. Res. 2022, 182, 106357. [Google Scholar] [CrossRef]
- Turkson, J.; Ryan, D.; Kim, J.S.; Zhang, Y.; Chen, Z.; Haura, E.; Laudano, A.; Sebti, S.; Hamilton, A.D.; Jove, R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J. Biol. Chem. 2001, 276, 45443–45455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Bromberg, J.; Darnell, J.E., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 2000, 19, 2468–2473. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Yi, E.H.; Ye, S.K. Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Arch. Pharm. Res. 2016, 39, 1085–1099. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Mahabady, M.K.; Nabavi, N.; Zabolian, A.; Banihashemi, S.M.; Haddadi, A.; Entezari, M.; Hushmandi, K.; Makvandi, P.; et al. Pre-clinical investigation of STAT3 pathway in bladder cancer: Paving the way for clinical translation. Biomed. Pharmacother. 2021, 133, 111077. [Google Scholar] [CrossRef]
- Murray, P.J. The JAK-STAT signaling pathway: Input and output integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korac-Prlic, J.; Degoricija, M.; Vilović, K.; Haupt, B.; Ivanišević, T.; Franković, L.; Grivennikov, S.; Terzić, J. Targeting Stat3 signaling impairs the progression of bladder cancer in a mouse model. Cancer Lett. 2020, 490, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Grinshpoon, A.; Barchana, M.; Ponizovsky, A.; Lipshitz, I.; Nahon, D.; Tal, O.; Weizman, A.; Levav, I. Cancer in schizophrenia: Is the risk higher or lower? Schizophr. Res. 2005, 73, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tabarés-Seisdedos, R.; Dumont, N.; Baudot, A.; Valderas, J.M.; Climent, J.; Valencia, A.; Crespo-Facorro, B.; Vieta, E.; Gómez-Beneyto, M.; Martínez, S.; et al. No paradox, no progress: Inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011, 12, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Tabarés-Seisdedos, R.; Rubenstein, J.L. Inverse cancer comorbidity: A serendipitous opportunity to gain insight into CNS disorders. Nat. Rev. Neurosci. 2013, 14, 293–304. [Google Scholar] [CrossRef]
- Moore, N.; Hall, G.; Sturkenboom, M.; Mann, R.; Lagnaoui, R.; Begaud, B. Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: The example of sertindole. Pharmacoepidemiol. Drug Saf. 2003, 12, 271–281. [Google Scholar] [CrossRef]
- Spina, E.; Zoccali, R. Sertindole: Pharmacological and clinical profile and role in the treatment of schizophrenia. Expert Opin. Drug Metab. Toxicol. 2008, 4, 629–638. [Google Scholar] [CrossRef]
- Kasper, S.; Möller, H.J.; Hale, A. The European post-marketing observational sertindole study: An investigation of the safety of antipsychotic drug treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 59–68. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, S.J.; Kim, E.S.; Jo, Y.K.; Hong, J.; Cho, D.H. Sertindole, a potent antagonist at dopamine D₂ receptors, induces autophagy by increasing reactive oxygen species in SH-SY5Y neuroblastoma cells. Biol. Pharm. Bull. 2012, 35, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, C.; Liu, F.; Mao, Y.; Xu, W.; Fan, T.; Sun, Q.; He, S.; Chen, Y.; Guo, W.; et al. Antiproliferative activities of the second-generation antipsychotic drug sertindole against breast cancers with a potential application for treatment of breast-to-brain metastases. Sci. Rep. 2018, 8, 15753. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Liu, P.; Wang, X.; Yin, Y.; Jin, W.; Shen, L.; Chen, Y.; Chen, Z.; Wang, Y. The antipsychotic agent sertindole exhibited antiproliferative activities by inhibiting the stat3 signaling pathway in human gastric cancer cells. J. Cancer 2020, 11, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar] [PubMed]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Catlett-Falcone, R.; Landowski, T.H.; Oshiro, M.M.; Turkson, J.; Levitzki, A.; Savino, R.; Ciliberto, G.; Moscinski, L.; Fernández-Luna, J.L.; Nuñez, G.; et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999, 10, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Cheng, X.D.; Zhang, W.D.; Qin, J.J. Recent update on development of small-molecule STAT3 inhibitors for cancer therapy: From phosphorylation inhibition to protein degradation. J. Med. Chem. 2021, 64, 8884–8915. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Vlachos, N.; Lampros, M.; Voulgaris, S.; Alexiou, G.A. Repurposing antipsychotics for cancer treatment. Biomedicines 2021, 9, 1785. [Google Scholar] [CrossRef]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Buccarelli, M.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Anticancer properties of the antipsychotic drug chlorpromazine and its synergism with temozolomide in restraining human glioblastoma proliferation in vitro. Front. Oncol. 2021, 11, 635472. [Google Scholar] [CrossRef]
- Dong, Y.; Furuta, T.; Sabit, H.; Kitabayashi, T.; Jiapaer, S.; Kobayashi, M.; Ino, Y.; Todo, T.; Teng, L.; Hirao, A.; et al. Identification of antipsychotic drug fluspirilene as a potential anti-glioma stem cell drug. Oncotarget 2017, 8, 111728–111741. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol induced cell cycle arrest and apoptosis in glioblastoma cells. Biomedicines 2020, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Srivastava, S.K. Penfluridol suppresses glioblastoma tumor growth by Akt-mediated inhibition of GLI1. Oncotarget 2017, 8, 32960–32976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan, A.; Kaushik, I.; Srivastava, S.K. Pimozide suppresses the growth of brain tumors by targeting STAT3-mediated autophagy. Cells 2020, 9, 2141. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.; Saki, M.; Vlashi, E.; Cheng, F.; Duhachek-Muggy, S.; Alli, C.; Yu, G.; Medina, P.; He, L.; Damoiseaux, R.; et al. The dopamine receptor antagonist trifluoperazine prevents phenotype conversion and improves survival in mouse models of glioblastoma. Proc. Natl. Acad. Sci. USA 2020, 117, 11085–11096. [Google Scholar] [CrossRef]
- Nielsen, J.; Matz, J.; Mittoux, A.; Polcwiartek, C.; Struijk, J.J.; Toft, E.; Kanters, J.K.; Graff, C. Cardiac effects of sertindole and quetiapine: Analysis of ECGs from a randomized double-blind study in patients with schizophrenia. Eur. Neuropsychopharmacol. 2015, 25, 303–311. [Google Scholar] [CrossRef]
- Dou, Z.; Zhao, D.; Chen, X.; Xu, C.; Jin, X.; Zhang, X.; Wang, Y.; Xie, X.; Li, Q.; Di, C.; et al. Aberrant Bcl-x splicing in cancer: From molecular mechanism to therapeutic modulation. J. Exp. Clin. Cancer Res. 2021, 40, 194. [Google Scholar] [CrossRef]
- Yoshimine, S.; Kikuchi, E.; Kosaka, T.; Mikami, S.; Miyajima, A.; Okada, Y.; Oya, M. Prognostic significance of Bcl-xL expression and efficacy of Bcl-xL targeting therapy in urothelial carcinoma. Br. J. Cancer 2013, 108, 2312–2320. [Google Scholar] [CrossRef] [Green Version]
- Kunze, D.; Wuttig, D.; Fuessel, S.; Kraemer, K.; Kotzsch, M.; Meye, A.; Grimm, M.O.; Hakenberg, O.W.; Wirth, M.P. Multitarget siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-X(L)) in bladder cancer cells. Anticancer Res. 2008, 28, 2259–2263. [Google Scholar]
- Rieger, C.; Huebner, D.; Temme, A.; Wirth, M.P.; Fuessel, S. Antisense- and siRNA-mediated inhibition of the anti-apoptotic gene Bcl-xL for chemosensitization of bladder cancer cells. Int. J. Oncol. 2015, 47, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Morales, L.D.; Jang, I.S.; Cho, Y.Y.; Kim, D.J. Protein tyrosine phosphatases as potential regulators of STAT3 signaling. Int. J. Mol. Sci. 2018, 19, 2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.C.; Teng, H.W.; Shiau, C.W.; Tai, W.T.; Hung, M.H.; Yang, S.H.; Jiang, J.K.; Chen, K.F. Pharmacological targeting SHP-1-STAT3 signaling is a promising therapeutic approach for the treatment of colorectal cancer. Neoplasia 2015, 17, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Song, H.; Yoon, Y.J.; Park, S.J.; Kim, S.Y.; Han, D.C.; Kwon, B.M. Ethacrynic acid inhibits STAT3 activity through the modulation of SHP2 and PTP1B tyrosine phosphatases in DU145 prostate carcinoma cells. Biochem. Pharmacol. 2020, 175, 113920. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.Y.; Yang, W.T.; Ho, Y.J.; Chang, G.M.; Chang, H.H.; Hsu, C.Y.; Chang, C.C.; Chen, Y.H. Hispolon methyl ether, a hispolon analog, suppresses the SRC/STAT3/Survivin signaling axis to induce cytotoxicity in human urinary bladder transitional carcinoma cell lines. Int. J. Mol. Sci. 2023, 24, 138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.P.; Li, S.; Chuang, W.L.; Li, C.H.; Chen, G.J.; Chang, C.C.; Or, C.R.; Lin, P.Y.; Chang, C.C. Blockade of STAT3 signaling contributes to anticancer effect of 5-acetyloxy-6,7,8,4′-tetra-methoxyflavone, a tangeretin derivative, on human glioblastoma multiforme cells. Int. J. Mol. Sci. 2019, 20, 3366. [Google Scholar] [CrossRef] [Green Version]
- Or, C.R.; Huang, C.W.; Chang, C.C.; Lai, Y.C.; Chen, Y.J.; Chang, C.C. Obatoclax, a pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/β-catenin signaling. Int. J. Mol. Sci. 2020, 21, 1773. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Yang, W.-T.; Lin, J.-H.; Lu, C.-H.; Hu, K.-C.; Lan, T.-H.; Chang, C.-C. Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro. Int. J. Mol. Sci. 2023, 24, 11852. https://doi.org/10.3390/ijms241411852
Hsu C-Y, Yang W-T, Lin J-H, Lu C-H, Hu K-C, Lan T-H, Chang C-C. Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro. International Journal of Molecular Sciences. 2023; 24(14):11852. https://doi.org/10.3390/ijms241411852
Chicago/Turabian StyleHsu, Chao-Yu, Wei-Ting Yang, Ju-Hwa Lin, Chien-Hsing Lu, Kai-Cheng Hu, Tsuo-Hung Lan, and Chia-Che Chang. 2023. "Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro" International Journal of Molecular Sciences 24, no. 14: 11852. https://doi.org/10.3390/ijms241411852






