Tricellulin, α-Catenin and Microfibrillar-Associated Protein 5 Exhibit Concomitantly Altered Immunosignals along with Vascular, Extracellular and Cytoskeletal Elements after Experimental Focal Cerebral Ischemia
Abstract
:1. Introduction
2. Results
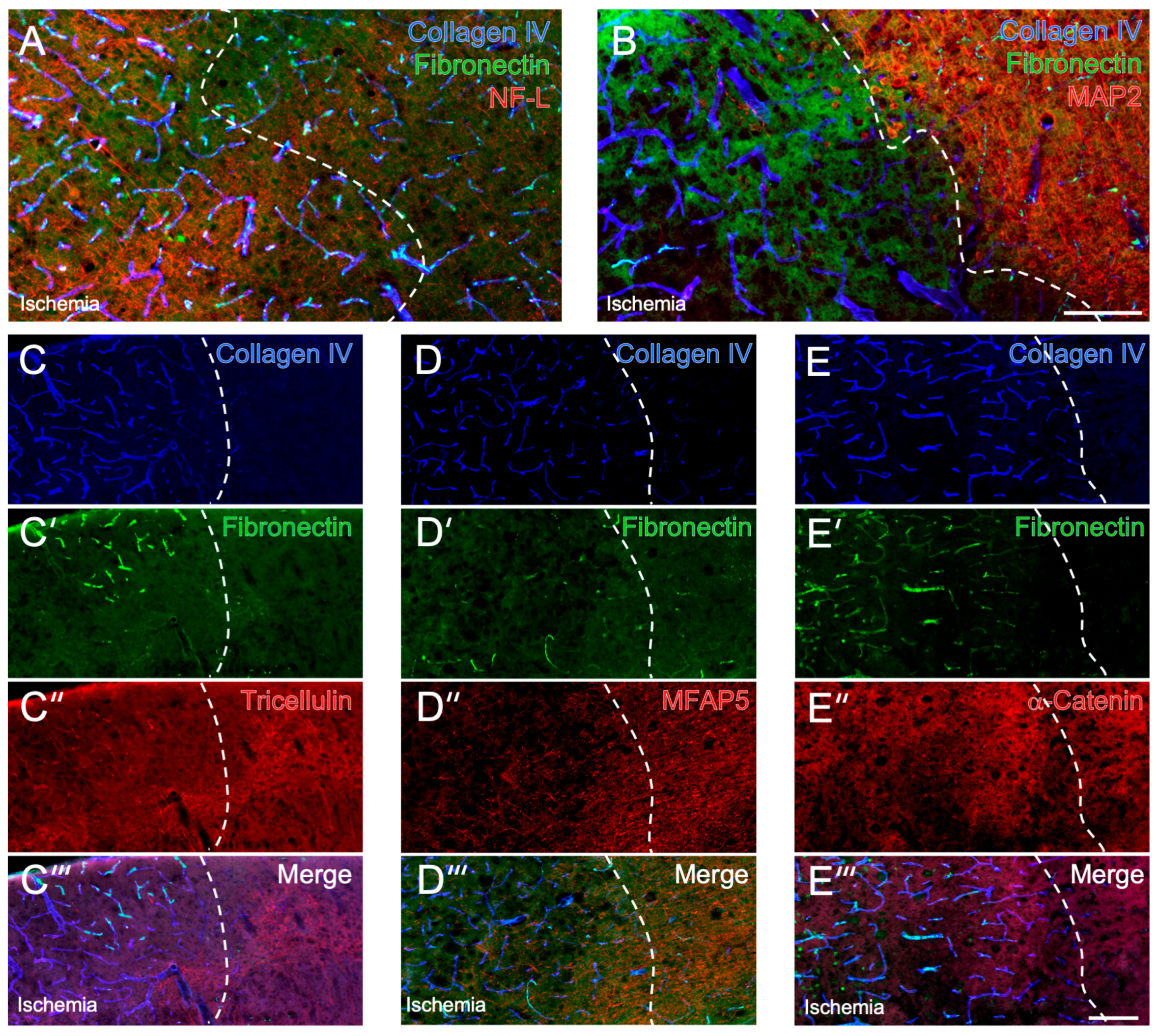
2.1. Cellular and Extracellular Consequences of Focal Cerebral Ischemia
2.2. Immunosignals of Tricellulin, MFAP5 and α-Catenin in Naive and Ischemic Brain Regions
2.3. Histochemical Patterns of Tricellulin, MFAP5 and α-Catenin Compared with MAP2 and NF-L in the Ischemia-Affected Neocortex and Subcortex
2.4. Detection of Cell-Stabilizing Elements in Brains Subjected to Short-Term Focal Ischemia
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Experimental Focal Cerebral Ischemia
4.3. Tissue Preparation and Immunofluorescence Labeling
4.4. Microscopy and Quantification of Fluorescence Signals
4.5. Statistical Analyses and Figure Preparation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Yilmaz, S.E. Neuroprotective approach in acute ischemic stroke: A systematic review of clinical and experimental studies. Brain Circ. 2022, 8, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H.; Rosenberg, G.A. The neurovascular unit in health and disease: Introduction. Stroke 2009, 40 (Suppl. 3), 2–3. [Google Scholar] [CrossRef] [Green Version]
- del Zoppo, G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010, 267, 156–171. [Google Scholar] [CrossRef] [Green Version]
- del Zoppo, G.J. Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture. Stroke 2013, 44, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Michalski, D.; Grosche, J.; Pelz, J.; Schneider, D.; Weise, C.; Bauer, U.; Kacza, J.; Gärtner, U.; Hobohm, C.; Härtig, W. A novel quantification of blood-brain barrier damage and histochemical typing after embolic stroke in rats. Brain Res. 2010, 1359, 186–200. [Google Scholar] [CrossRef]
- del Zoppo, G.J. Relationship of neurovascular elements to neuron injury during ischemia. Cerebrovasc. Dis. 2009, 27 (Suppl. 1), 65–76. [Google Scholar] [CrossRef] [Green Version]
- del Zoppo, G.J. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 2009, 158, 972–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Härtig, W.; Mages, B.; Aleithe, S.; Nitzsche, B.; Altmann, S.; Barthel, H.; Krueger, M.; Michalski, D. Damaged Neocortical Perineuronal Nets Due to Experimental Focal Cerebral Ischemia in Mice, Rats and Sheep. Front. Integr. Neurosci. 2017, 11, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobohm, C.; Günther, A.; Grosche, J.; Rossner, S.; Schneider, D.; Brückner, G. Decomposition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J. Neurosci. Res. 2005, 80, 539–548. [Google Scholar] [CrossRef]
- Michalski, D.; Spielvogel, E.; Puchta, J.; Reimann, W.; Barthel, H.; Nitzsche, B.; Mages, B.; Jäger, C.; Martens, H.; Horn, A.K.E.; et al. Increased Immunosignals of Collagen IV and Fibronectin Indicate Ischemic Consequences for the Neurovascular Matrix Adhesion Zone in Various Animal Models and Human Stroke Tissue. Front. Physiol. 2020, 11, 575598. [Google Scholar] [CrossRef]
- Dewar, D.; Dawson, D.A. Changes of cytoskeletal protein immunostaining in myelinated fibre tracts after focal cerebral ischaemia in the rat. Acta Neuropathol. 1997, 93, 71–77. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments at a glance. J. Cell Sci. 2012, 125, 3257–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Härtig, W.; Krueger, M.; Hofmann, S.; Preißler, H.; Märkel, M.; Frydrychowicz, C.; Mueller, W.C.; Bechmann, I.; Michalski, D. Up-regulation of neurofilament light chains is associated with diminished immunoreactivities for MAP2 and tau after ischemic stroke in rodents and in a human case. J. Chem. Neuroanat. 2016, 78, 140–148. [Google Scholar] [CrossRef]
- Mages, B.; Aleithe, S.; Altmann, S.; Blietz, A.; Nitzsche, B.; Barthel, H.; Horn, A.K.E.; Hobusch, C.; Härtig, W.; Krueger, M.; et al. Impaired Neurofilament Integrity and Neuronal Morphology in Different Models of Focal Cerebral Ischemia and Human Stroke Tissue. Front. Cell. Neurosci. 2018, 12, 161. [Google Scholar] [CrossRef] [Green Version]
- Mages, B.; Fuhs, T.; Aleithe, S.; Blietz, A.; Hobusch, C.; Härtig, W.; Schob, S.; Krueger, M.; Michalski, D. The Cytoskeletal Elements MAP2 and NF-L Show Substantial Alterations in Different Stroke Models While Elevated Serum Levels Highlight Especially MAP2 as a Sensitive Biomarker in Stroke Patients. Mol. Neurobiol. 2021, 58, 4051–4069. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef]
- Kikuchi, S.; Ninomiya, T.; Tatsumi, H.; Sawada, N.; Kojima, T. Tricellulin is expressed in autotypic tight junctions of peripheral myelinating Schwann cells. J. Histochem. Cytochem. 2010, 58, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, A.K.; Rittner, H.L. Barrier function in the peripheral and central nervous system—A review. Pflüg. Arch. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- van den Goor, L.; Miller, A.L. Closing the gap: Tricellulin/α-catenin interaction maintains epithelial integrity at vertices. J. Cell Biol. 2022, 221, e202202009. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Haraguchi, D.; Shigetomi, K.; Matsuzawa, K.; Uchida, S.; Ikenouchi, J. Tricellulin secures the epithelial barrier at tricellular junctions by interacting with actomyosin. J. Cell Biol. 2022, 221, e202009037. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Miller, A.L. Tricellular junctions: How to build junctions at the TRICkiest points of epithelial cells. Mol. Biol. Cell 2017, 28, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Wada, Y.; Watanabe, T.; Nagafuchi, A.; Shibata, M. α-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010, 12, 533–542. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, L.; Bennett, S.; Xu, H.; He, D.; Xu, J. Molecular structure and function of microfibrillar-associated proteins in skeletal and metabolic disorders and cancers. J. Cell. Physiol. 2021, 236, 41–48. [Google Scholar] [CrossRef]
- Vaittinen, M.; Kolehmainen, M.; Rydén, M.; Eskelinen, M.; Wabitsch, M.; Pihlajamäki, J.; Uusitupa, M.; Pulkkinen, L. MFAP5 is related to obesity-associated adipose tissue and extracellular matrix remodeling and inflammation. Obesity 2015, 23, 1371–1378. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yin, J.X.; Zhang, H.; Liao, Y. High glucose stimulating ECM remodeling and an inflammatory phenotype in the IPFP via upregulation of MFAP5 expression. Biochem. Biophys. Res. Commun. 2022, 601, 93–100. [Google Scholar] [CrossRef]
- Kujawa, K.A.; Zembala-Nożynska, E.; Syrkis, J.P.; Cortez, A.J.; Kupryjańczyk, J.; Lisowska, K.M. Microfibril Associated Protein 5 (MFAP5) Is Related to Survival of Ovarian Cancer Patients but Not Useful as a Prognostic Biomarker. Int. J. Mol. Sci. 2022, 23, 15994. [Google Scholar] [CrossRef]
- Smetanina, M.A.; Kel, A.E.; Sevost’ianova, K.S.; Maiborodin, I.V.; Shevela, A.I.; Zolotukhin, I.A.; Stegmaier, P.; Filipenko, M.L. DNA methylation and gene expression profiling reveal MFAP5 as a regulatory driver of extracellular matrix remodeling in varicose vein disease. Epigenomics 2018, 10, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhong, J.P.; Fu, W.J.; Chen, H.J.; Fang, L.; Li, G.L.; Li, J.W.; Wen, M.H.; Lv, Y.B.; Wang, H.B. Microfiber-associated protein 5 (MFAP5): A promising approach to discover new biomarkers for heart failure and cardiac remodeling. Int. J. Cardiol. 2022, 366, 68–69. [Google Scholar] [CrossRef]
- Endres, M.; Engelhardt, B.; Koistinaho, J.; Lindvall, O.; Meairs, S.; Mohr, J.P.; Planas, A.; Rothwell, N.; Schwaninger, M.; Schwab, M.E.; et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovasc. Dis. 2008, 25, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Young, A.R.; Ali, C.; Duretête, A.; Vivien, D. Neuroprotection and stroke: Time for a compromise. J. Neurochem. 2007, 103, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Chiba, H. Molecular organization, regulation and function of tricellular junctions. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183143. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Higashi, T.; Furuse, M. Localization of angulin-1/LSR and tricellulin at tricellular contacts of brain and retinal endothelial cells in vivo. Cell Struct. Funct. 2014, 39, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornabene, E.; Helms, H.C.C.; Pedersen, S.F.; Brodin, B. Effects of oxygen-glucose deprivation (OGD) on barrier properties and mRNA transcript levels of selected marker proteins in brain endothelial cells/astrocyte co-cultures. PLoS ONE 2019, 14, e0221103. [Google Scholar] [CrossRef]
- Mariano, C.; Palmela, I.; Pereira, P.; Fernandes, A.; Falcão, A.S.; Cardoso, F.L.; Vaz, A.R.; Campos, A.R.; Gonçalves-Ferreira, A.; Kim, K.S.; et al. Tricellulin expression in brain endothelial and neural cells. Cell Tissue Res. 2013, 351, 397–407. [Google Scholar] [CrossRef]
- Aleithe, S.; Blietz, A.; Mages, B.; Hobusch, C.; Härtig, W.; Michalski, D. Transcriptional Response and Morphological Features of the Neurovascular Unit and Associated Extracellular Matrix After Experimental Stroke in Mice. Mol. Neurobiol. 2019, 56, 7631–7650. [Google Scholar] [CrossRef] [Green Version]
- Mecham, R.P.; Gibson, M.A. The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche. Matrix Biol. 2015, 47, 13–33. [Google Scholar] [CrossRef]
- Gibson, M.A.; Leavesley, D.I.; Ashman, L.K. Microfibril-associated glycoprotein-2 specifically interacts with a range of bovine and human cell types via αVβ3 integrin. J. Biol. Chem. 1999, 274, 13060–13065. [Google Scholar] [CrossRef] [Green Version]
- Guell, K.; Bix, G.J. Brain endothelial cell specific integrins and ischemic stroke. Expert Rev. Neurother. 2014, 14, 1287–1292. [Google Scholar] [CrossRef]
- Prehn, A.; Hobusch, C.; Härtig, W.; Michalski, D.; Krueger, M.; Flachmeyer, B. Increasing reproducibility in preclinical stroke research: The correlation of immunofluorescence intensity measurements and Western blot analyses strongly depends on antibody clonality and tissue pre-treatment in a mouse model of focal cerebral ischemia. Front. Cell. Neurosci. 2023, 17, 1183232. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, 1000412. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.L.; Chopp, M.; Zhang, Z.G.; Jiang, Q.; Ewing, J.R. A rat model of focal embolic cerebral ischemia. Brain Res. 1997, 766, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Menzies, S.A.; Hoff, J.T.; Betz, A.L. Middle cerebral artery occlusion in rats: A neurological and pathological evaluation of a reproducible model. Neurosurgery 1992, 31, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, W. StatistikGuru: Rechner zur Adjustierung des α-Niveaus. 2016. Available online: https://statistikguru.de/rechner/adjustierung-des-alphaniveaus.html (accessed on 17 April 2023).


| Antigen | Host | Dilution | Supplier | Product Number | Stock Concen-tration | Clone | Secondary Antibodies * |
|---|---|---|---|---|---|---|---|
| Fibronectin | chicken | 1:100 | Agrisera | IMS02-060-314 | 1 mg/mL | polyclonal | donkey anti-chicken Cy2 Dianova 703-225-155 |
| Collagen IV | goat | 1:40,000 | Merck | AB769 | 0.4 mg/mL | polyclonal | donkey anti-goat Cy5 Dianova 705-175-147 |
| NF-L | mouse | 1:100 | Life Technologies | 130400 | 0.5 mg/mL | DA2 (IgG1k) | donkey anti-mouse Cy3 Dianova 715-165-151 donkey anti-mouse-Cy2 Dianova 715-225-150 |
| MAP2 | guinea pig | 1:500 | Synaptic Systems | 188004 | not available | polyclonal | donkey anti-guinea pig Cy3 Dianova 706-165-148 donkey anti-guinea pig Cy5 Dianova 706-175-148 |
| MFAP5 | rabbit | 1:250 | Sigma | HPA010553 | 1 mg/mL | polyclonal | donkey anti-rabbit Cy3 Dianova 711-165-152 |
| Tricellulin | rabbit | 1:100 | Invitrogen | 700191 | 0.5 mg/mL | 54H19L38 (IgG) | donkey anti-rabbit Cy3 Dianova 711-165-152 |
| α-catenin (biotin conjugated) | mouse | 1:100 | Antibodies -online | ABIN6178165 | 0.1 mg/mL | 1G5 (IgG1) | Streptavidin Cy3 Dianova 016-160-084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höfling, C.; Roßner, S.; Flachmeyer, B.; Krueger, M.; Härtig, W.; Michalski, D. Tricellulin, α-Catenin and Microfibrillar-Associated Protein 5 Exhibit Concomitantly Altered Immunosignals along with Vascular, Extracellular and Cytoskeletal Elements after Experimental Focal Cerebral Ischemia. Int. J. Mol. Sci. 2023, 24, 11893. https://doi.org/10.3390/ijms241511893
Höfling C, Roßner S, Flachmeyer B, Krueger M, Härtig W, Michalski D. Tricellulin, α-Catenin and Microfibrillar-Associated Protein 5 Exhibit Concomitantly Altered Immunosignals along with Vascular, Extracellular and Cytoskeletal Elements after Experimental Focal Cerebral Ischemia. International Journal of Molecular Sciences. 2023; 24(15):11893. https://doi.org/10.3390/ijms241511893
Chicago/Turabian StyleHöfling, Corinna, Steffen Roßner, Bianca Flachmeyer, Martin Krueger, Wolfgang Härtig, and Dominik Michalski. 2023. "Tricellulin, α-Catenin and Microfibrillar-Associated Protein 5 Exhibit Concomitantly Altered Immunosignals along with Vascular, Extracellular and Cytoskeletal Elements after Experimental Focal Cerebral Ischemia" International Journal of Molecular Sciences 24, no. 15: 11893. https://doi.org/10.3390/ijms241511893




