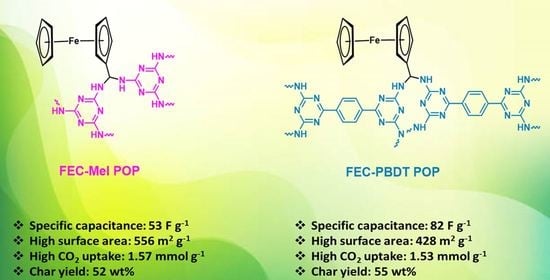
Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage
Abstract
:1. Introduction
2. Results and Discussion
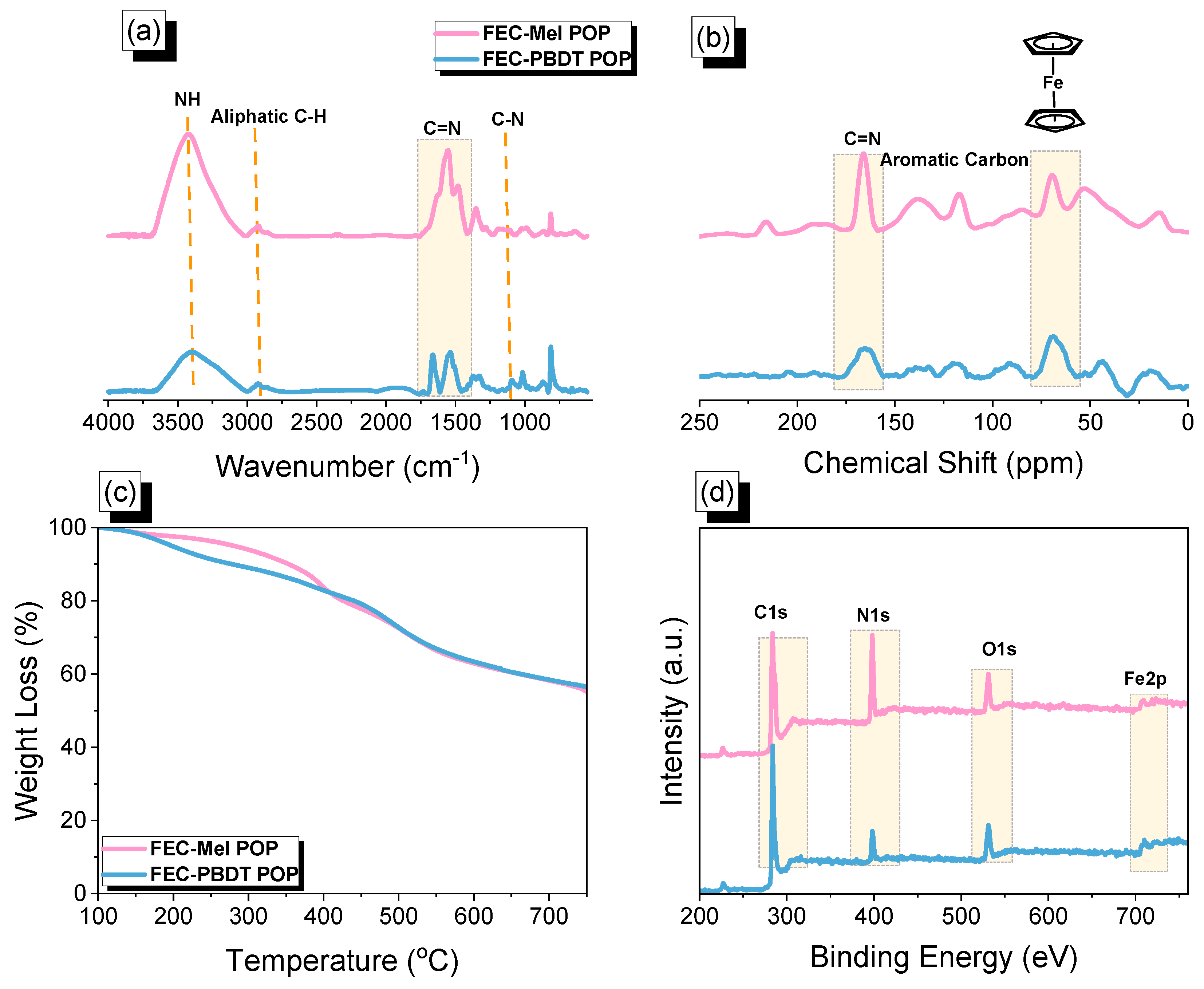
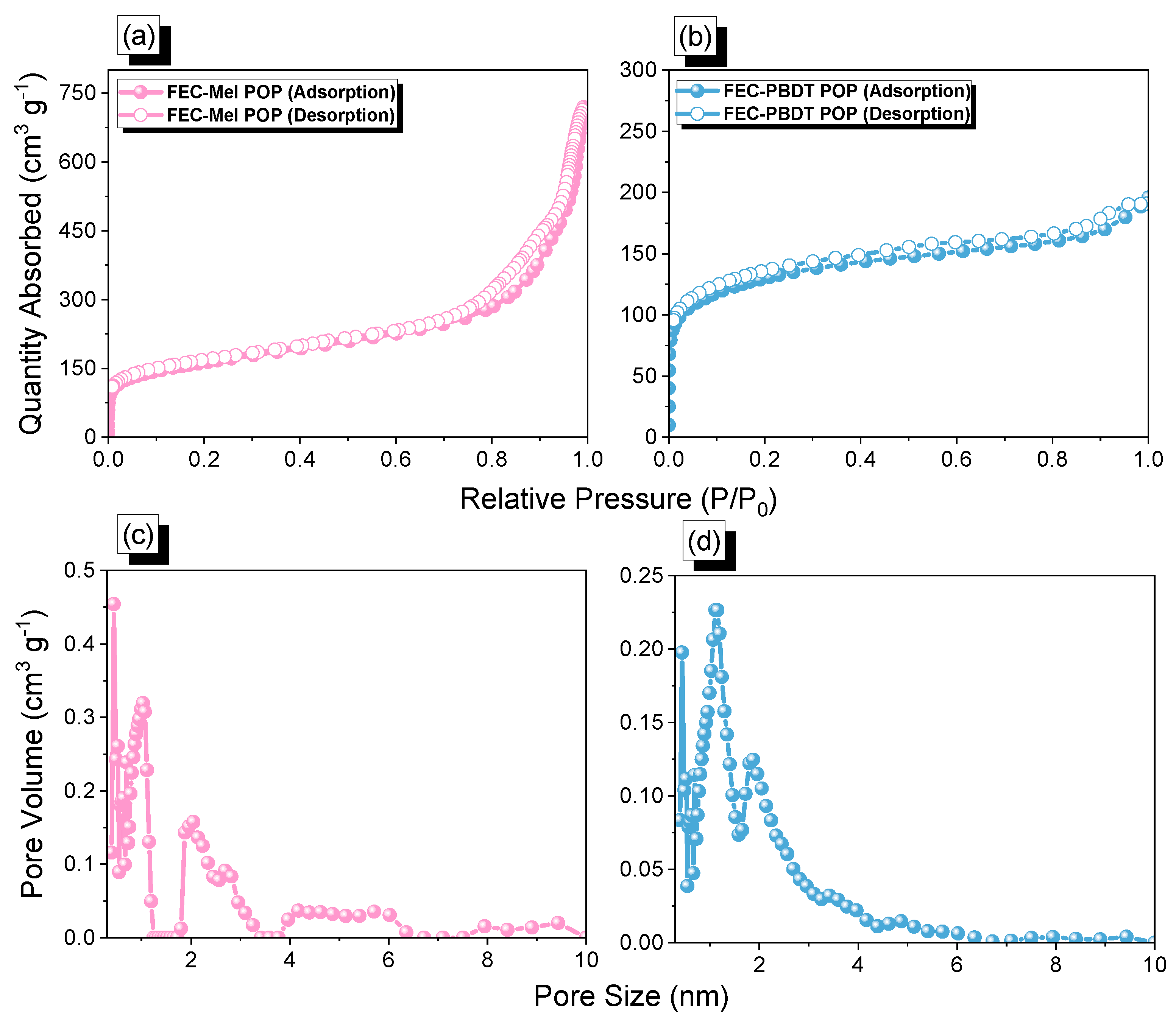
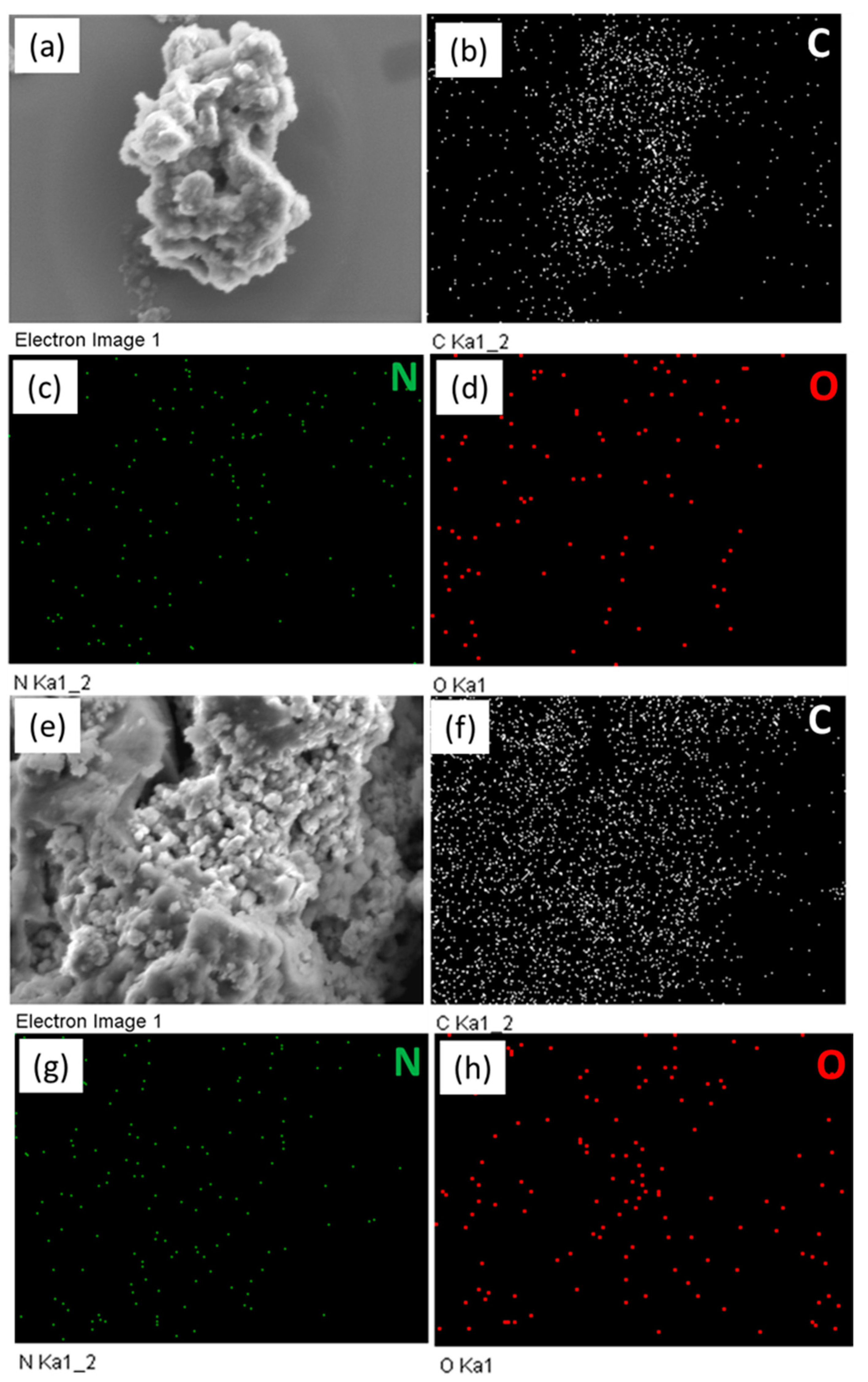
2.1. Synthesis and Molecular Characterization of FEC-CHO, PBDT, and FEC-POPs
2.2. CO2 Uptake of FEC-Mel and FEC-PBDT POPs
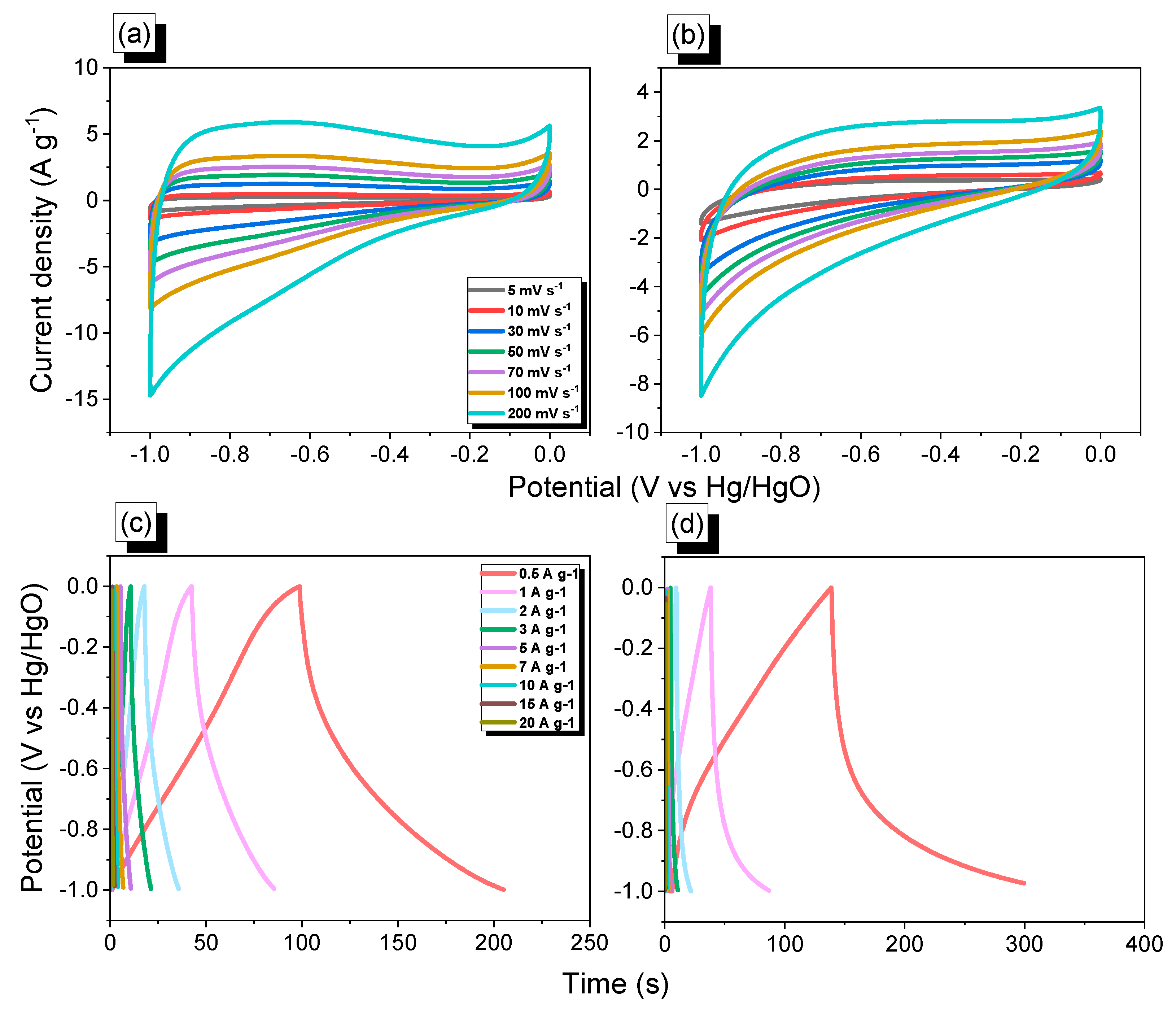
2.3. Electrochemical Analysis of FEC-POPs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ferrocenecarboxaldehyde (FEC-CHO)
3.3. Synthesis of 6,6′-(1,4-Phenylene)bis(1,3,5-Triazine-2,4-Diamine) (PBDT)
3.4. Synthesis of FEC-Mel POP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhanja, P.; Das, S.K.; Bhunia, K.; Pradhan, D.; Hayashi, T.; Hijikata, Y.; Irle, S.; Bhaumik, A. A new porous polymer for highly efficient capacitive energy storage. ACS Sustain. Chem. Eng. 2018, 6, 202–209. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Elewa, A.M.; Li, M.S.; Kuo, S.W. Construction and multifunctional of hypercrosslinked porous organic polymers containing ferrocene unit for high-performance iodine adsorption and supercapacitor. J. Taiwan Inst. Chem. Eng. 2023, 150, 10545. [Google Scholar] [CrossRef]
- Ejaz, M.; Mohamed, M.G.; Chang, W.C.; Kuo, S.W. Synthesis and design of hypercrosslinked porous organic frameworks containing tetraphenylpyrazine unit for high-performance supercapacitor. J. Polym. Sci. 2023. [Google Scholar] [CrossRef]
- Yu, K.; Pan, X.; Zhang, G.; Liao, X.; Zhou, X.; Yan, M.; Xu, L.; Mai, L. Nanowires in energy storage devices: Structures, synthesis, and applications. Adv. Energy Mater. 2018, 8, 1802369. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.-L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Jiang, K.; Huang, T.; Shen, G. Recent advances in fiber supercapacitors: Materials, device configurations, and applications. Adv. Mater. 2020, 32, 1901806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, J.; Zheng, Y.; Li, J.; Han, X.; He, G.; Du, Y. Organic thiocarboxylate electrodes for a room-temperature sodium-ion battery delivering an ultrahigh capacity. Angew. Chem. 2017, 129, 15536–15540. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fang, B. Electrochemical hydrogen storage: Opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrogen Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Sarangapani, S.; Tilak, B.; Chen, C.P. Materials for electrochemical capacitors: Theoretical and experimental constraints. J. Electrochem. Soc. 1996, 143, 3791. [Google Scholar] [CrossRef]
- Kim, D.; Kang, J.; Yan, B.; Seong, K.-d.; Piao, Y. Ambient temperature synthesis of iron-doped porous nickel pyrophosphate nanoparticles with long-term chemical stability for high-performance oxygen evolution reaction catalysis and supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 2843–2853. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Fathoni, K.B.; Wang, J.; Ide, Y.; Yuliarto, B.; Dipojono, H.K.; Nanjundan, A.K.; Golberg, D.; Bando, Y. Self-assembly of nickel phosphate-based nanotubes into two-dimensional crumpled sheet-like architectures for high-performance asymmetric supercapacitors. Nano Energy 2020, 67, 104270. [Google Scholar] [CrossRef]
- Shi, R.; Han, C.; Duan, H.; Xu, L.; Zhou, D.; Li, H.; Li, J.; Kang, F.; Li, B.; Wang, G. Redox-active organic sodium anthraquinone-2-sulfonate (AQS) anchored on reduced graphene oxide for high-performance supercapacitors. Adv. Energy Mater. 2018, 8, 1802088. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.-W. Directly synthesized nitrogen-and-oxygen–doped microporous carbons derived from a bio-derived polybenzoxazine exhibiting high-performance supercapacitance and CO2 uptake. Eur. Polym. J. 2020, 138, 109954. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Das, M.; Manna, A.; Krishna, R.; Das, S. Dual strategic approach to prepare defluorinated triazole-embedded covalent triazine frameworks with high gas uptake performance. Chem. Mater. 2019, 31, 3929–3940. [Google Scholar] [CrossRef]
- Lv, H.; Wang, W.; Li, F. Porous organic polymers with built-in N-heterocyclic carbenes: Selective and efficient heterogeneous catalyst for the reductive N-formylation of amines with CO2. Chem. Eur. J. 2018, 24, 16588–16594. [Google Scholar] [CrossRef]
- Mousa, A.O.; Mohamed, M.G.; Chuang, C.-H.; Kuo, S.-W. Carbonized Aminal-Linked Porous Organic Polymers Containing Pyrene and Triazine Units for Gas Uptake and Energy Storage. Polymers 2023, 15, 1891. [Google Scholar] [CrossRef]
- Cui, Y.; Du, J.; Liu, Y.; Yu, Y.; Wang, S.; Pang, H.; Liang, Z.; Yu, J. Design and synthesis of a multifunctional porous N-rich polymer containing s-triazine and Tröger’s base for CO2 adsorption, catalysis and sensing. Polym. Chem. 2018, 9, 2643–2649. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M.; Book, D. Microporous polymers as potential hydrogen storage materials. Macromol. Rapid Commun. 2007, 28, 995–1002. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, P.; Zhang, X.; Wu, D. Emerging porous organic polymers for biomedical applications. Chem. Soc. Rev. 2022, 51, 1377–1414. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure–function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Yuan, D.; Lu, W.; Zhao, D.; Zhou, H.C. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar] [CrossRef]
- Wang, T.-X.; Liang, H.-P.; Anito, D.A.; Ding, X.; Han, B.-H. Emerging applications of porous organic polymers in visible-light photocatalysis. J. Mater. Chem. A 2020, 8, 7003–7034. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, N.; Feng, S.; Zhang, C.; Wang, F.; Chen, Y.; Zeng, J.; Jiang, J.X. Perylene-Containing Conjugated Microporous Polymers for Photocatalytic Hydrogen Evolution. Macromol. Chem. Phys. 2017, 218, 1700049. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Tsai, M.-Y.; Wang, C.-F.; Huang, C.-F.; Danko, M.; Dai, L.; Chen, T.; Kuo, S.-W. Multifunctional polyhedral oligomeric silsesquioxane (POSS) based hybrid porous materials for CO2 uptake and iodine adsorption. Polymers 2021, 13, 221. [Google Scholar] [CrossRef]
- Mousa, A.O.; Lin, Z.-I.; Chuang, C.-H.; Chen, C.-K.; Kuo, S.-W.; Mohamed, M.G. Rational Design of Bifunctional Microporous Organic Polymers Containing Anthracene and Triphenylamine Units for Energy Storage and Biological Applications. Int. J. Mol. Sci. 2023, 24, 8966. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Elsayed, M.H.; Ye, Y.; Samy, M.M.; Hassan, A.E.; Mansoure, T.H.; Wen, Z.; Chou, H.-H.; Chen, K.-H.; Kuo, S.-W. Construction of Porous Organic/Inorganic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxane for Energy Storage and Hydrogen Production from Water. Polymers 2023, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013, 62, 345–352. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Ren, S. Progress on CO2 capture by porous organic polymers. Curr. Opin. Green Sustain. Chem. 2019, 16, 33–38. [Google Scholar] [CrossRef]
- Chen, D.; Fu, Y.; Yu, W.; Yu, G.; Pan, C. Versatile Adamantane-based porous polymers with enhanced microporosity for efficient CO2 capture and iodine removal. Chem. Eng. J. 2018, 334, 900–906. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Yu, Z.; Gong, L.; Wang, H.; Yu, B.; Zhang, W.; Jiang, J. Transformation of porous organic cages and covalent organic frameworks with efficient iodine vapor capture performance. J. Am. Chem. Soc. 2022, 144, 12390–12399. [Google Scholar] [CrossRef]
- Abid, A.; Razzaque, S.; Hussain, I.; Tan, B. Eco-Friendly Phosphorus and Nitrogen-Rich Inorganic-Organic Hybrid Hypercross-linked Porous Polymers via a Low-Cost Strategy. Macromolecules 2021, 54, 5848–5855. [Google Scholar] [CrossRef]
- Mohamed, M.G.; EL-Mahdy, A.F.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, Y.; He, X.; Chen, P.; Zhao, S.; Huang, C.; Wang, Y.; Chen, J. Robust porous polymers bearing phosphine oxide/chalcogenide ligands for volatile iodine capture. Chem. Eng. J. 2020, 379, 122365. [Google Scholar] [CrossRef]
- Zou, X.; Ren, H.; Zhu, G. Topology-directed design of porous organic frameworks and their advanced applications. Chem. Commun. 2013, 49, 3925–3936. [Google Scholar] [CrossRef] [Green Version]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Ding, L.; Gao, H.; Xie, F.; Li, W.; Bai, H.; Li, L. Porosity-enhanced polymers from hyper-cross-linked polymer precursors. Macromolecules 2017, 50, 956–962. [Google Scholar] [CrossRef]
- Sun, L.; Zou, Y.; Liang, Z.; Yu, J.; Xu, R. A one-pot synthetic strategy via tandem Suzuki–Heck reactions for the construction of luminescent microporous organic polymers. Polym. Chem. 2014, 5, 471–478. [Google Scholar] [CrossRef]
- Pan, L.; Chen, Q.; Zhu, J.-H.; Yu, J.-G.; He, Y.-J.; Han, B.-H. Hypercrosslinked porous polycarbazoles via one-step oxidative coupling reaction and Friedel-Crafts alkylation. Polym. Chem. 2015, 6, 2478–2487. [Google Scholar] [CrossRef]
- Fang, D.; Li, X.; Zou, M.; Guo, X.; Zhang, A. Carbazole-functionalized hyper-cross-linked polymers for CO2 uptake based on Friedel-Crafts polymerization on 9-phenylcarbazole. Beilstein J. Org. Chem. 2019, 15, 2856–2863. [Google Scholar] [CrossRef] [Green Version]
- Kuo, S.W. Construction Archimedean tiling patterns based on soft materials from block copolymers and covalent organic frameworks. Giant 2023, 15, 100170. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Chung, W.T.; Mekhemer, I.M.A.; Mohamed, M.G.; Elewa, A.M.; EL-Mahdy, A.F.M.; Chou, H.H.; Kuo, S.W.; Wu, K.C.W. Recent advances in metal/covalent organic frameworks based materials: Their synthesis, structure design and potential applications for hydrogen production. Coord. Chem. Rev. 2023, 483, 215066. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.-W. Conjugated Microporous Polymers Based on Ferrocene Units as Highly Efficient Electrodes for Energy Storage. Polymers 2023, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Sato, K.; Sukegawa, T.; Oyaizu, K.; Ho, C.-L.; Xiang, J.; Feng, Y.-H.; Lo, Y.H.; Nishide, H.; Wong, W.-Y. Metallopolyyne polymers with ferrocenyl pendant ligands as cathode-active materials for organic battery application. J. Organomet. Chem. 2016, 812, 51–55. [Google Scholar] [CrossRef]
- Cong, G.; Zhou, Y.; Li, Z.; Lu, Y.-C. A highly concentrated catholyte enabled by a low-melting-point ferrocene derivative. ACS Energy Lett. 2017, 2, 869–875. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, Y.; Ding, Y.; Zhang, L.; Guo, X.; Zhao, Y.; Yu, G. Biredox eutectic electrolytes derived from organic redox-active molecules: High-energy storage systems. Angew. Chem. Int. Ed. 2019, 58, 7045–7050. [Google Scholar] [CrossRef] [PubMed]
- Elsler, B.; Schollmeyer, D.; Dyballa, K.M.; Franke, R.; Waldvogel, S.R. Metal-and Reagent-Free Highly Selective Anodic Cross-Coupling Reaction of Phenols. Angew. Chem. Int. Ed. 2014, 53, 5210–5213. [Google Scholar] [CrossRef] [PubMed]
- Mandai, H.; Fujii, K.; Yasuhara, H.; Abe, K.; Mitsudo, K.; Korenaga, T.; Suga, S. Enantioselective acyl transfer catalysis by a combination of common catalytic motifs and electrostatic interactions. Nat. Commun. 2016, 7, 11297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, T.-L.; Lee, K.-H.; Joo, W.-J.; Lee, S.; Lee, T.-W.; Chae, M.Y. Synthesis and nonvolatile memory behavior of redox-active conjugated polymer-containing ferrocene. J. Am. Chem. Soc. 2007, 129, 9842–9843. [Google Scholar] [CrossRef]
- Dong, Q.; Meng, Z.; Ho, C.-L.; Guo, H.; Yang, W.; Manners, I.; Xu, L.; Wong, W.-Y. A molecular approach to magnetic metallic nanostructures from metallopolymer precursors. Chem. Soc. Rev. 2018, 47, 4934–4953. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Li, H.; Hu, X.; Abdullah, R.; Xie, S.; Zhang, L.; Zhao, M.; Luo, Q.; Li, Y.; Sun, Z. Size-tunable assemblies based on ferrocene-containing DNA polymers for spatially uniform penetration. Chem 2019, 5, 1775–1792. [Google Scholar] [CrossRef]
- Xiang, J.; Li, X.; Ma, Y.; Zhao, Q.; Ho, C.-L.; Wong, W.-Y. Efficient flash memory devices based on non-conjugated ferrocene-containing copolymers. J. Mater. Chem. C 2018, 6, 11348–11355. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Xiong, S.; Lu, P.; Tang, J.; He, J.; Javaid, M.U.; Pan, C.; Yu, G. Ferrocene-based porous organic polymers for high-affinity iodine capture. Chem. Eng. J. 2020, 380, 122420. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Liu, Y.; Jiang, S.; Li, P.; Hao, Y.; Shao, P.; Yin, A.; Feng, X.; Wang, B. Ferrocene-linkage-facilitated charge separation in conjugated microporous polymers. Angew. Chem. Int. Ed. 2019, 58, 4221–4226. [Google Scholar] [CrossRef]
- Tan, Z.; Su, H.; Guo, Y.; Liu, H.; Liao, B.; Amin, A.M.; Liu, Q. Ferrocene-based conjugated microporous polymers derived from yamamoto coupling for gas storage and dye removal. Polymers 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samy, M.M.; Sharma, S.U.; Mohamed, M.G.; Mohammed, A.A.; Chaganti, S.V.; Lee, J.-T.; Kuo, S.-W. Conjugated microporous polymers containing ferrocene units for high carbon dioxide uptake and energy storage. Mater. Chem. Phys. 2022, 287, 126177. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Mansoure, T.H.; Meng, T.S.; Khan, M.A.R.; Liaw, C.-C.; Kuo, S.-W. Solid state chemical transformations through ring-opening polymerization of ferrocene-based conjugated microporous polymers in host–guest complexes with benzoxazine-linked cyclodextrin. J. Taiwan Inst. Chem. Eng. 2022, 132, 104110. [Google Scholar] [CrossRef]
- Mei, L.; Cui, X.; Duan, Q.; Li, Y.; Lv, X.; Wang, H.-g. Metal phthalocyanine-linked conjugated microporous polymer hybridized with carbon nanotubes as a high-performance flexible electrode for supercapacitors. Int. J. Hydrogen Energy 2020, 45, 22950–22958. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Wu, T.-H.; Alston, B.M.; Briggs, M.E.; Hasell, T.; Hu, C.-C.; Cooper, A.I. Porosity-engineered carbons for supercapacitive energy storage using conjugated microporous polymer precursors. J. Mater. Chem. A 2016, 4, 7665–7673. [Google Scholar] [CrossRef] [Green Version]
- Samy, M.M.; Mohamed, M.G.; El-Mahdy, A.F.M.; Mansoure, T.H.; Wu, K.C.-W.; Kuo, S.-W. High-performance supercapacitor electrodes prepared from dispersions of tetrabenzonaphthalene-based conjugated microporous polymers and carbon nanotubes. ACS Appl. Mater. Interfaces 2021, 13, 51906–51916. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Mansoure, T.H.; Takashi, Y.; Samy, M.M.; Chen, T.; Kuo, S.-W. Ultrastable porous organic/inorganic polymers based on polyhedral oligomeric silsesquioxane (POSS) hybrids exhibiting high performance for thermal property and energy storage. Microporous Mesoporous Mater. 2021, 328, 111505. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chang, S.-Y.; Ejaz, M.; Samy, M.M.; Mousa, A.O.; Kuo, S.-W. Design and Synthesis of Bisulfone-Linked Two-Dimensional Conjugated Microporous Polymers for CO2 adsorption and Energy Storage. Molecules 2023, 28, 3234. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Sharma, S.U.; Chaganti, S.V.; Lee, J.T.; Kuo, S.W. An Ultrastable Tetrabenzonaphthalene-Linked conjugated microporous polymer functioning as a high-performance electrode for supercapacitors. J. Taiwan Inst. Chem. Eng. 2023, 104750. [Google Scholar] [CrossRef]
- Ejaz, M.; Mohamed, M.G.; Kuo, S.W. Solid state chemical transformation provides a fully benzoxazine-linked porous organic polymer displaying enhanced CO2 capture and supercapacitor performance. Polym. Chem. 2023, 14, 2494–2509. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Sharma, S.U.; Chaganti, S.V.; Mansoure, T.H.; Lee, J.T.; Chen, T.; Kuo, S.W. Constructing conjugated microporous polymers containing triphenylamine moieties for high-performance capacitive energy storage. Polymer 2023, 264, 125541. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Y.; Liu, J.; Wu, D.; Bai, X.; Chen, D.; Li, H. Outstanding supercapacitor performance of nitrogen-doped activated carbon derived from shaddock peel. J. Energy Storage 2021, 39, 102640. [Google Scholar] [CrossRef]
- Liu, F.; Niu, J.; Chuan, Y.; Zhao, Y. Nitrogen and sulfur co-doping carbon in different dimensions as electrode for supercapacitor applications. J. Alloys Compd. 2023, 947, 169654. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Christov, N.; Zhao, X.S. Intercalation of mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes. Energy Environ. Sci. 2011, 4, 1866–1873. [Google Scholar] [CrossRef]




| Material | Surface Area (m2 g−1) | Capacitance | References |
|---|---|---|---|
| FEC-Mel POP | 556 | 53 F g−1/0.5 A g−1 | This work |
| FEC-PBDT POP | 428 | 82 F g−1/0.5 A g−1 | This work |
| TBN-Py-CMP | 473 | 31 F g−1/0.5 A g−1 | [67] |
| CoPc-CMP | - | 13.7 F g−1/1.0 A g–1 | [65] |
| Py-BSU CMP | 42 | 38 F g−1/0.5 A g−1 | [69] |
| POSS-F-POIP | 452 | 36.2 F g−1/0.5 A g−1 | [68] |
| TPE-FFC-CMP | 8 | 4.8 F g−1/0.5 A g−1 | [64] |
| Py-FFC-CMP | 50 | 5.07 F g−1/0.5 A g−1 | [64] |
| C1-CMP-1 | 608 | 31 F g−1 at 20 mV s𢈒1 | [66] |
| Py-PDT POP | 76 | 28 F g−1/0.5 A g−1 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, A.O.; Chuang, C.-H.; Kuo, S.-W.; Mohamed, M.G. Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage. Int. J. Mol. Sci. 2023, 24, 12371. https://doi.org/10.3390/ijms241512371
Mousa AO, Chuang C-H, Kuo S-W, Mohamed MG. Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage. International Journal of Molecular Sciences. 2023; 24(15):12371. https://doi.org/10.3390/ijms241512371
Chicago/Turabian StyleMousa, Aya Osama, Cheng-Hsin Chuang, Shiao-Wei Kuo, and Mohamed Gamal Mohamed. 2023. "Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage" International Journal of Molecular Sciences 24, no. 15: 12371. https://doi.org/10.3390/ijms241512371