TET Family Members Are Integral to Porcine Oocyte Maturation and Parthenogenetic Pre-Implantation Embryogenesis
Abstract
:1. Introduction
2. Results
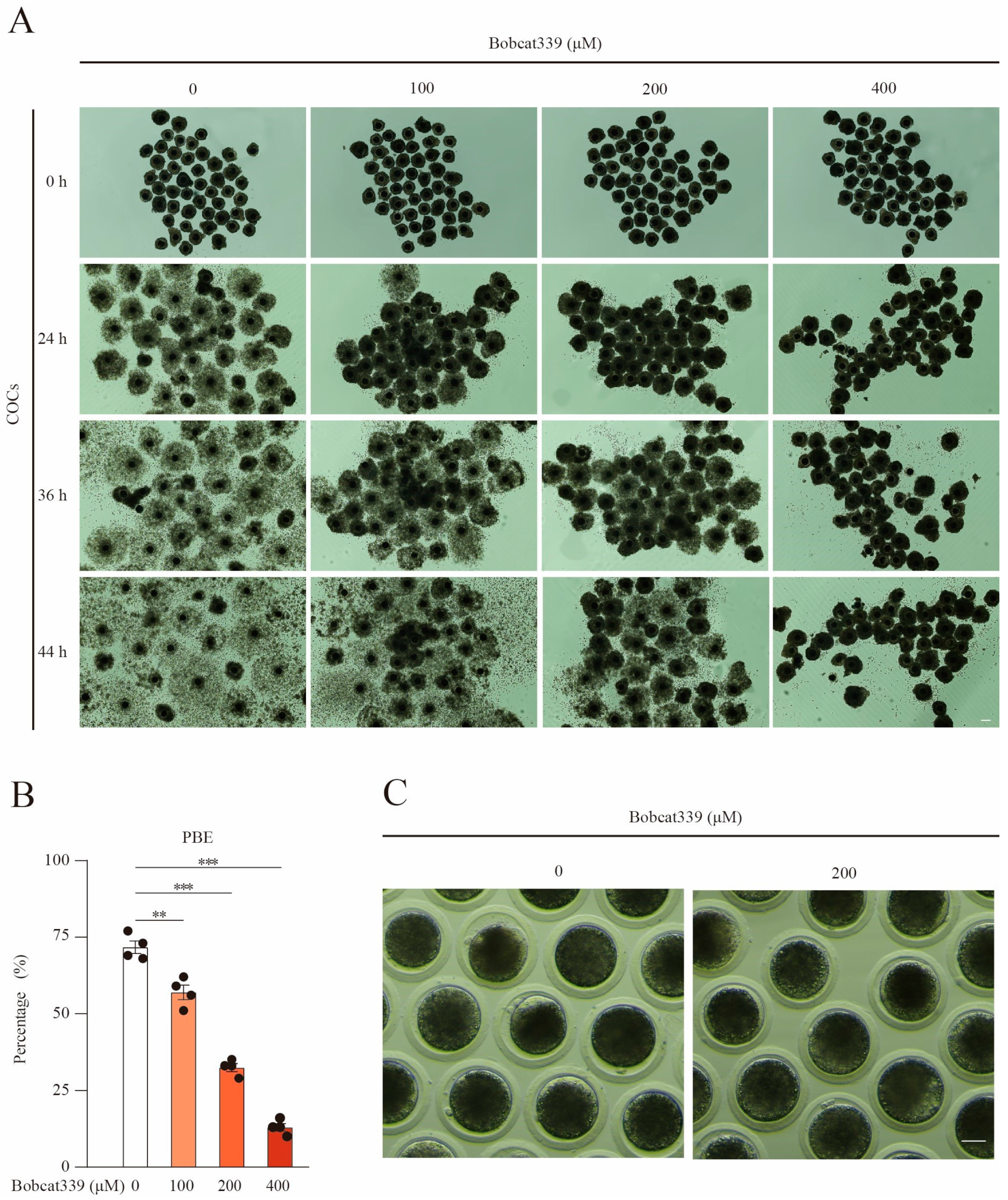
2.1. Bobcat339 Treatment Obstructed the First Polar Body Extrusion in Porcine Oocytes
2.2. Bobcat339 Treatment Triggered Apoptosis of Porcine Oocytes
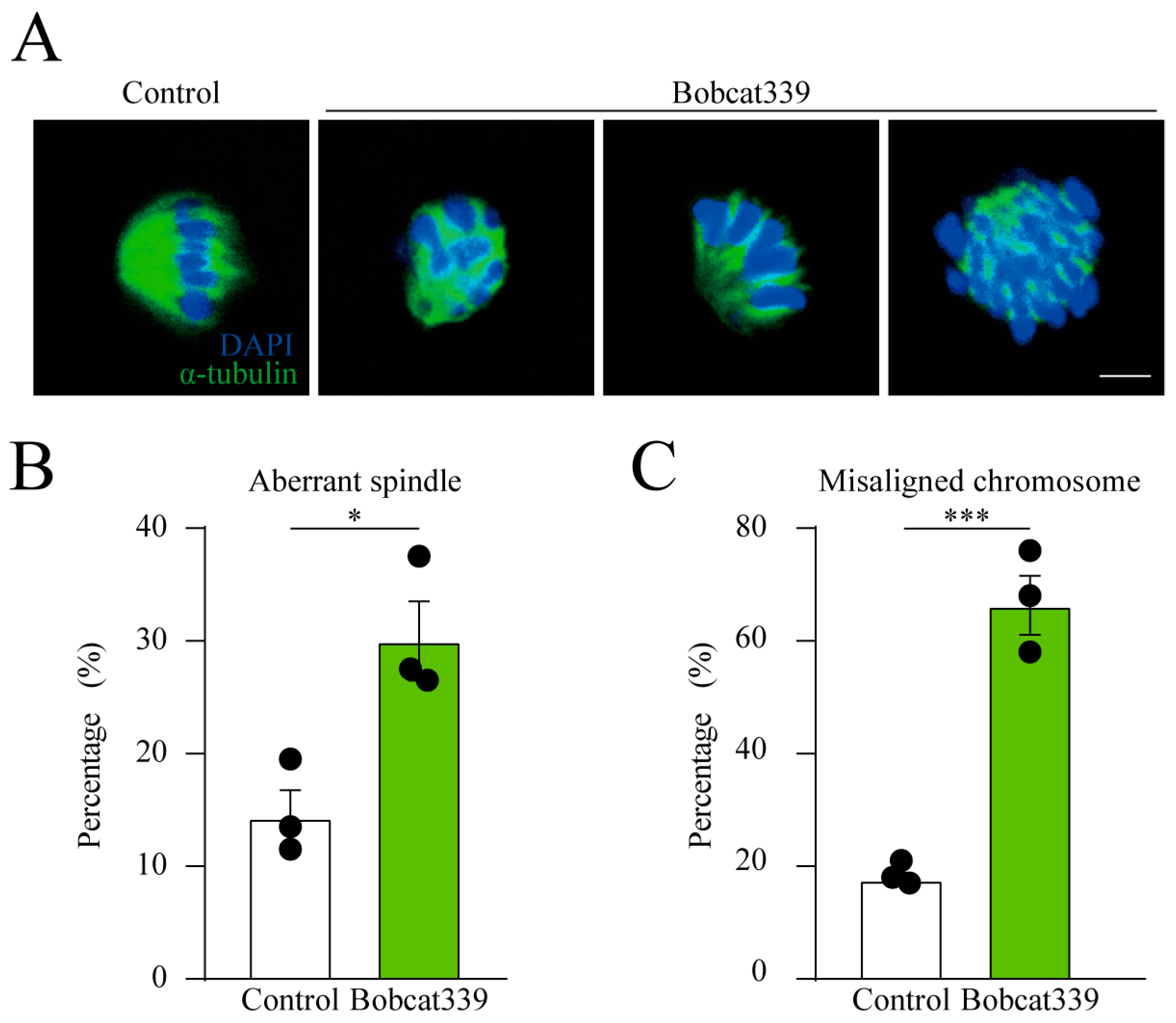
2.3. Effect of Bobcat339 on Spindle Architecture and Chromosomes Alignment in Oocytes
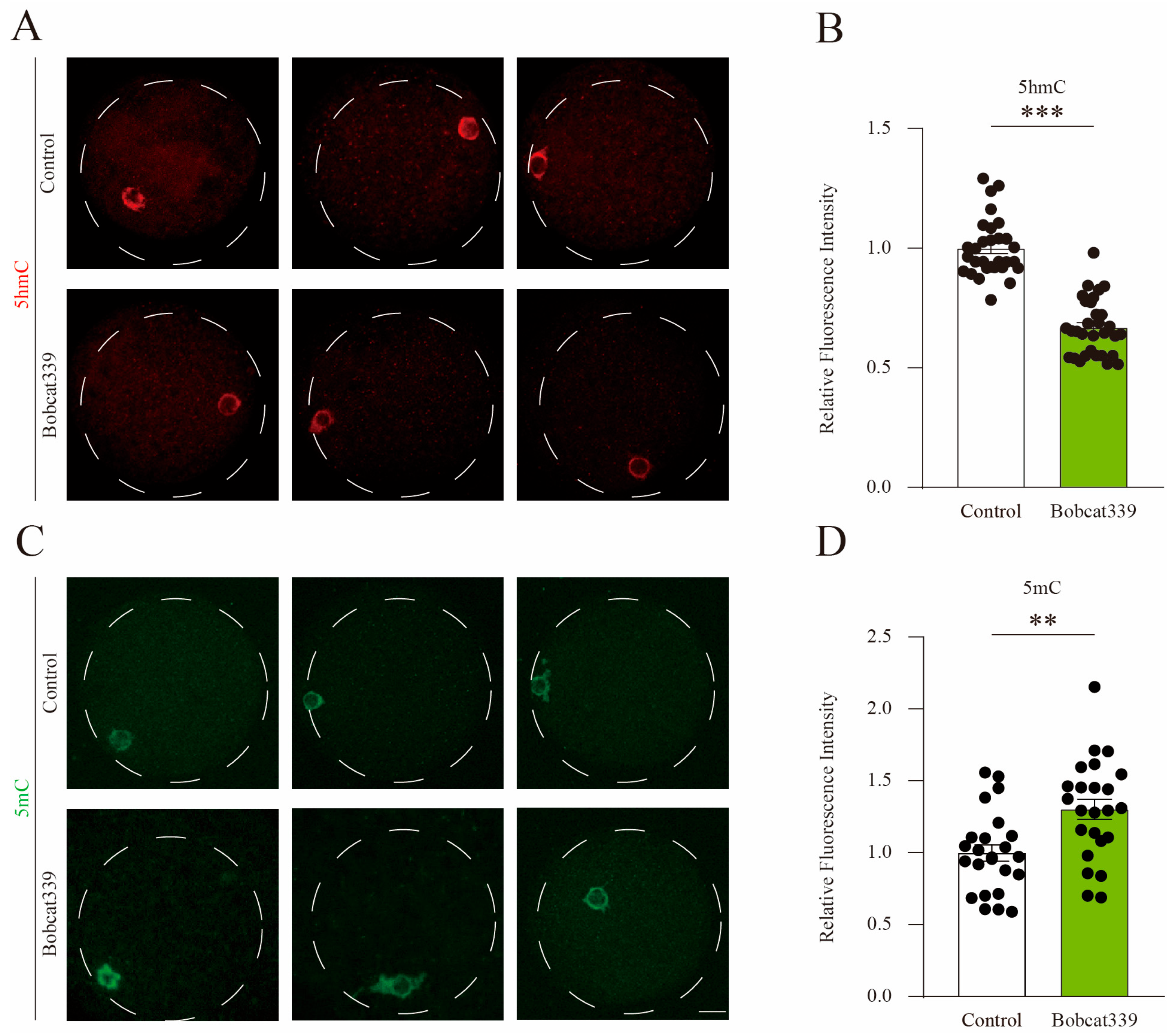
2.4. Bobcat339 Treatment Altered 5mC/5hmC Levels in Porcine Oocytes
2.5. Effect of Bobcat339 on Preimplantation Embryo Development
2.6. Bobcat339 Treatment Decreased the Expression of ZGA and Pluripotency-Related Genes
2.7. Bobcat339 Treatment Disrupted 5mC/5hmC Levels in Porcine Embryos
2.8. Comparative Analyses of Transcriptomic Data
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Porcine Oocyte Collection and In Vitro Maturation (IVM)
4.3. Parthenogenetic Activation and In Vitro Culture (IVC)
4.4. Chemical Treatment
4.5. AnnexinV-FITC Staining
4.6. RNA Isolation and RT-PCR
4.7. Immunofluorescent Staining
4.8. RNA Sequencing and Data Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, A.J.; Sweatt, J.D. Drugging the methylome: DNA methylation and memory. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.F.; Li, B.Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.Q.; Ali, I.; Tang, J.; Yang, W.C. New Insights into 5hmC DNA Modification: Generation, Distribution and Function. Front. Genet. 2017, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Yan, L.; Guo, H.; Li, L.; Hu, B.; Zhao, Y.; Yong, J.; Hu, Y.; Wang, X.; Wei, Y.; et al. The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells. Cell 2015, 161, 1437–1452. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Li, C.; Liu, X.; Gao, S. Insights into epigenetic patterns in mammalian early embryos. Protein. Cell 2021, 12, 7–28. [Google Scholar] [CrossRef]
- Vincent, J.J.; Huang, Y.; Chen, P.Y.; Feng, S.; Calvopiña, J.H.; Nee, K.; Lee, S.A.; Le, T.; Yoon, A.J.; Faull, K.; et al. Stage-specific roles for tet1 and tet2 in DNA demethylation in primordial germ cells. Cell Stem. Cell 2013, 12, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Khoueiry, R.; Sohni, A.; Thienpont, B.; Luo, X.; Velde, J.V.; Bartoccetti, M.; Boeckx, B.; Zwijsen, A.; Rao, A.; Lambrechts, D.; et al. Lineage-specific functions of TET1 in the postimplantation mouse embryo. Nat. Genet. 2017, 49, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Gu, T.P.; Guo, F.; Yang, H.; Wu, H.P.; Xu, G.F.; Liu, W.; Xie, Z.G.; Shi, L.; He, X.; Jin, S.G.; et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 2011, 477, 606–610. [Google Scholar] [CrossRef]
- Iqbal, K.; Jin, S.G.; Pfeifer, G.P.; Szabó, P.E. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl. Acad. Sci. USA 2011, 108, 3642–3647. [Google Scholar] [CrossRef]
- Shen, L.; Inoue, A.; He, J.; Liu, Y.; Lu, F.; Zhang, Y. Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell Stem. Cell 2014, 15, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Sakashita, A.; Kobayashi, H.; Wakai, T.; Sotomaru, Y.; Hata, K.; Kono, T. Dynamics of genomic 5-hydroxymethylcytosine during mouse oocyte growth. Genes Cells 2014, 19, 629–636. [Google Scholar] [CrossRef]
- Tsukada, Y.; Akiyama, T.; Nakayama, K.I. Maternal TET3 is dispensable for embryonic development but is required for neonatal growth. Sci. Rep. 2015, 5, 15876. [Google Scholar] [CrossRef] [Green Version]
- Dawlaty, M.M.; Breiling, A.; Le, T.; Raddatz, G.; Barrasa, M.I.; Cheng, A.W.; Gao, Q.; Powell, B.E.; Li, Z.; Xu, M.; et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell 2013, 24, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Dawlaty, M.M.; Breiling, A.; Le, T.; Barrasa, M.I.; Raddatz, G.; Gao, Q.; Powell, B.E.; Cheng, A.W.; Faull, K.F.; Lyko, F.; et al. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev. Cell. 2014, 29, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lan, Y.; Schwartz-Orbach, L.; Korol, E.; Tahiliani, M.; Evans, T.; Goll, M.G. Overlapping Requirements for Tet2 and Tet3 in Normal Development and Hematopoietic Stem Cell Emergence. Cell Rep. 2015, 12, 1133–1143. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Huang, K.; Cai, C.; Cai, L.; Jiang, C.Y.; Feng, Y.; Liu, Z.; Zeng, Q.; Cheng, L.; Sun, Y.E.; et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013, 500, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Yu, H.; An, X.; Zhang, Z.; Zhang, M.; Zhang, S.; Li, Q.; Li, Z. Profiling the transcriptomic signatures and identifying the patterns of zygotic genome activation-a comparative analysis between early porcine embryos and their counterparts in other three mammalian species. BMC Genomics. 2022, 23, 772. [Google Scholar] [CrossRef]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef]
- Lee, K.; Hamm, J.; Whitworth, K.; Park, K.W.; Murphy, C.N.; Prather, R.S. Dynamics of TET family expression in porcine preimplantation embryos is related to zygotic genome activation and required for the maintenance of NANOG. Dev. Biol. 2014, 386, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wossidlo, M.; Nakamura, T.; Lepikhov, K.; Marques, C.J.; Zakhartchenko, V.; Boiani, M.; Arand, J.; Nakano, T.; Reik, W.; Walter, J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun. 2011, 2, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Zhou, N.; Zhang, Y.; Zhang, Y.; Wu, R.; Li, Y.; Zhang, Y.; Li, N. Dynamic reprogramming of 5-hydroxymethylcytosine during early porcine embryogenesis. Theriogenology 2014, 81, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, L.; Wei, Q.; Zhang, S.; Cheng, H.; Zhai, Y.; Jiang, Y.; An, X.; Li, Z.; Zhang, X.; et al. TET3 overexpression facilitates DNA reprogramming and early development of bovine SCNT embryos. Reproduction 2020, 160, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Uh, K.; Ryu, J.; Farrell, K.; Wax, N.; Lee, K. TET family regulates the embryonic pluripotency of porcine preimplantation embryos by maintaining the DNA methylation level of NANOG. Epigenetics 2020, 15, 1228–1242. [Google Scholar] [CrossRef]
- Uh, K.; Lee, K. Ten-Eleven Translocation-3 CXXC domain is critical for postfertilization demethylation and expression of pluripotency genes in pig embryos. Biol. Reprod. 2022, 107, 1205–1216. [Google Scholar] [CrossRef]
- Chua, G.N.L.; Wassarman, K.L.; Sun, H.; Alp, J.A.; Jarczyk, E.I.; Kuzio, N.J.; Bennett, M.J.; Malachowsky, B.G.; Kruse, M.; Kennedy, A.J. Cytosine-Based TET Enzyme Inhibitors. ACS Med. Chem. Lett. 2019, 10, 180–185. [Google Scholar] [CrossRef]
- Kang, J.; Lienhard, M.; Pastor, W.A.; Chawla, A.; Novotny, M.; Tsagaratou, A.; Lasken, R.S.; Thompson, E.C.; Surani, M.A.; Koralov, S.B.; et al. Simultaneous deletion of the methylcytosine oxidases Tet1 and Tet3 increases transcriptome variability in early embryogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E4236–E4245. [Google Scholar] [CrossRef]
- Arand, J.; Chiang, H.R.; Martin, D.; Snyder, M.P.; Sage, J.; Pera, R.A.R.; Wossidlo, M. Tet enzymes are essential for early embryogenesis and completion of embryonic genome activation. EMBO Rep. 2022, 23, e53968. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Arnold, A.; Eberhart, C.; Raabe, E. Exth-15 multi-faceted inhibiton of TET pathway with cell-permeable 2HG and Bobcat339 reduces proliferation and induces apoptosis in DIPGS. Neuro.-Oncol. 2021, 23 (Suppl. 6), vi166. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Gou, M.; Huang, G.; Tian, C.; Yang, J.; Wang, H.; Xu, Q.; Xu, G.L.; Liu, L. Roles of Tet2 in meiosis, fertility and reproductive aging. Protein. Cell 2021, 12, 578–585. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, Y.; Cheng, H.; Hao, L.; Zhai, Y.; Zhang, Z.; An, X.; Ma, X.; Zhang, X.; et al. Effect of TET inhibitor on bovine parthenogenetic embryo development. PLoS ONE 2017, 12, e0189542. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Pan, H.; Doré, L.C.; Shukla, A.; Li, Q.V.; Pelham-Webb, B.; Teijeiro, V.; González, F.; Krivtsov, A.; Chang, C.J.; et al. TET proteins safeguard bivalent promoters from de novo methylation in human embryonic stem cells. Nat. Genet. 2018, 50, 83–95. [Google Scholar] [CrossRef]
- Farthing, C.R.; Ficz, G.; Ng, R.K.; Chan, C.F.; Andrews, S.; Dean, W.; Hemberger, M.; Reik, W. Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet. 2008, 4, e1000116. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Fan, A.; Ma, K.; An, X.; Ding, Y.; An, P.; Song, G.; Tang, L.; Zhang, S.; Zhang, P.; Tan, W.; et al. Effects of TET1 knockdown on gene expression and DNA methylation in porcine induced pluripotent stem cells. Reproduction 2013, 146, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Costa, Y.; Ding, J.; Theunissen, T.W.; Faiola, F.; Hore, T.A.; Shliaha, P.V.; Fidalgo, M.; Saunders, A.; Lawrence, M.; Dietmann, S.; et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature 2013, 495, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, G.; Feil, R. New insights into establishment and maintenance of DNA methylation imprints in mammals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 368, 20110336. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Kumagai, T.; Kawahara, M.; Ogawa, H.; Hiura, H.; Obata, Y.; Takano, R.; Kono, T. Regulated expression of two sets of paternally imprinted genes is necessary for mouse parthenogenetic development to term. Reproduction 2006, 131, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Kim, H.S.; Lee, S.G.; Lee, C.K. Methylation status of differentially methylated regions at Igf2/H19 locus in porcine gametes and preimplantation embryos. Genomics 2009, 93, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Zheng, Y.; Han, R.; Kuang, T.; Min, C.; Wang, H.; Zhao, Y.; Wang, J.; Yang, L.; Che, D. Effects of pyruvate on early embryonic development and zygotic genome activation in pigs. Theriogenology 2022, 189, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Picelli, S.; Faridani, O.R.; Björklund, A.K.; Winberg, G.; Sagasser, S.; Sandberg, R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014, 9, 171–181. [Google Scholar] [CrossRef] [PubMed]





| Gene | Accession | Primer Sequences (5′ to 3′) | Product Size (bp) |
|---|---|---|---|
| BCL-2 | XM_021077298.1 | F: CAGGGACAGCGTATCAGAGC R: TTGCGATCCGACTCACCAAT | 156 |
| BAX | XM_013998624.2 | F: CCAGGATCGAGCAGGGCGAAT R: CACAGGGCCTTGAGCACCAGTTT | 285 |
| DPPA2 | XM_003358822.4 | F: CCGTTCCTGCTTCTGTTGAGACC R: GGCGAACCCAACCTTCTGTATCTG | 105 |
| EIF1A | NM_001243218.1 | F: GGTGTTCAAAGAAGATGGGCAAGAG R: TTTCCCTCTGATGTGACATAACCTC | 115 |
| H19 | AY044827.1 | F: TCAAACGACAAGAGATGGTGCTA R: GACGTCTGTTCCTTTGGCTC | 118 |
| NANOG | XM_021092390.1 | F: AGGACAGCCCTGATTCTTCCACAA R: AAAGTTCTTGCATCTGCTGGAGGC | 198 |
| OCT4 | XM_021097869.1 | F: AAGCAGTGACTATTCGCAAC R: CAGGGTGGTGAAGTGAGG | 136 |
| RPLP0 | NM_001129964.2 | F: GCTAAGGTGCTCGGTTCTTC R: GTGCGGACCAATGCTAGG | 112 |
| SOX2 | NM_001123197.1 | F: CGCAGACCTACATGAACG R: TCGGACTTGACCACTGAG | 103 |
| TET1 | NM_001315772.1 | F: AGCACAGGACAAAATGAAGG R: TGGTTAGTTGGAGAGGAGG | 171 |
| TET2 | XM_013978993.2 | F: GCCAACCCTGTGAACCTCT R: GGGCTGGTAAAGTGTATGG | 270 |
| TET3 | XM_021087365.1 | F: TCAAGGCAAAGACCCGAAC R: AGACGGCAGTCAATCGCTATT | 261 |
| ZSCAN4 | XM_021097584.1 | F: GCCCAGAAAGTCTTCCCATGTGAG R: GCCTCTCATCATTGTGTCTCCTCTG | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Li, M.-G.; Hua, Z.-D.; Ren, H.-Y.; Gu, H.; Luo, A.-F.; Zhou, C.-F.; Zhu, Z.; Huang, T.; Bi, Y.-Z. TET Family Members Are Integral to Porcine Oocyte Maturation and Parthenogenetic Pre-Implantation Embryogenesis. Int. J. Mol. Sci. 2023, 24, 12455. https://doi.org/10.3390/ijms241512455
Chen F, Li M-G, Hua Z-D, Ren H-Y, Gu H, Luo A-F, Zhou C-F, Zhu Z, Huang T, Bi Y-Z. TET Family Members Are Integral to Porcine Oocyte Maturation and Parthenogenetic Pre-Implantation Embryogenesis. International Journal of Molecular Sciences. 2023; 24(15):12455. https://doi.org/10.3390/ijms241512455
Chicago/Turabian StyleChen, Fan, Ming-Guo Li, Zai-Dong Hua, Hong-Yan Ren, Hao Gu, An-Feng Luo, Chang-Fan Zhou, Zhe Zhu, Tao Huang, and Yan-Zhen Bi. 2023. "TET Family Members Are Integral to Porcine Oocyte Maturation and Parthenogenetic Pre-Implantation Embryogenesis" International Journal of Molecular Sciences 24, no. 15: 12455. https://doi.org/10.3390/ijms241512455





