Involvement of Innate Immune System in the Pathogenesis of Sepsis-Associated Acute Kidney Injury
Abstract
:1. Introduction
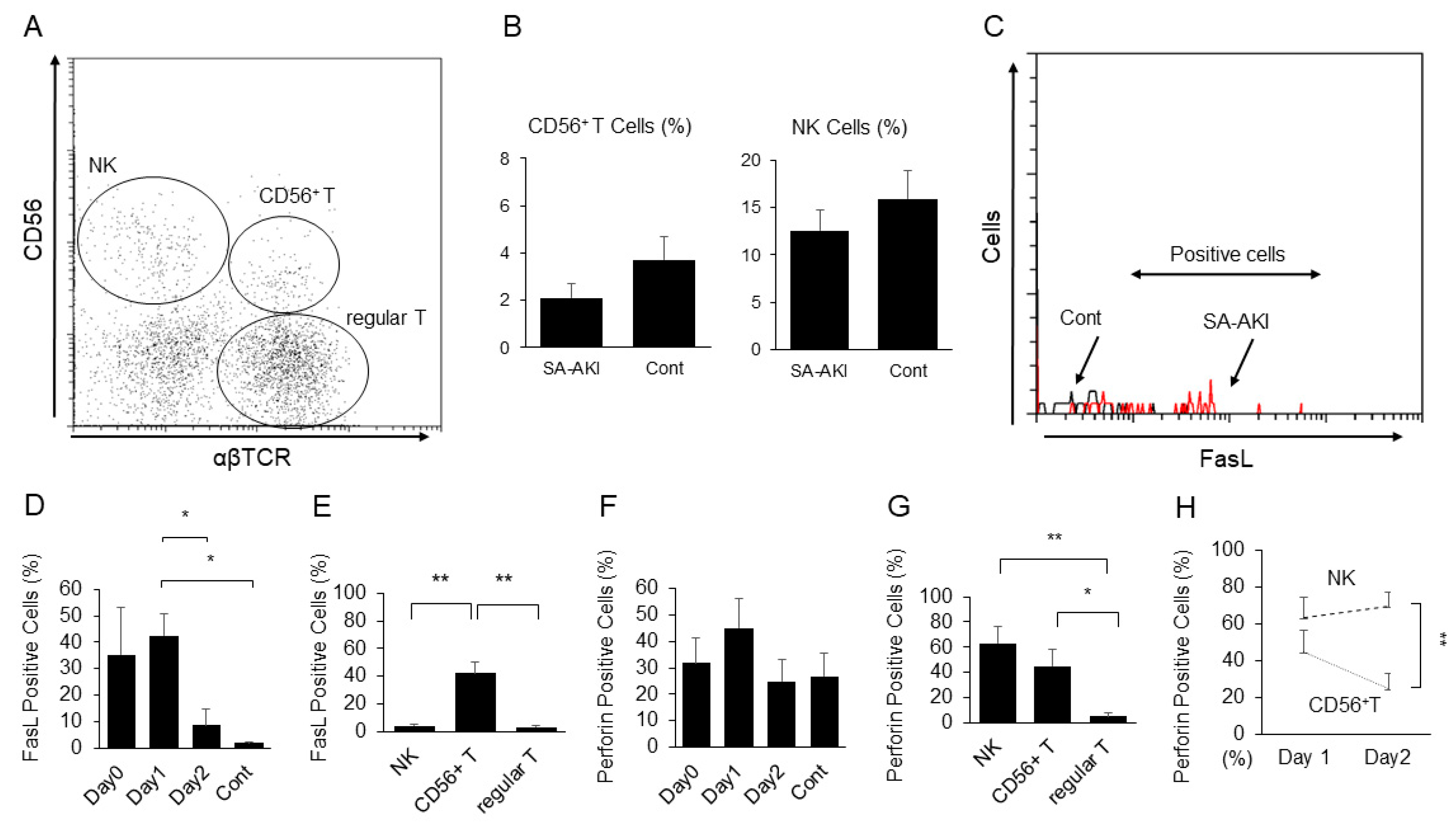
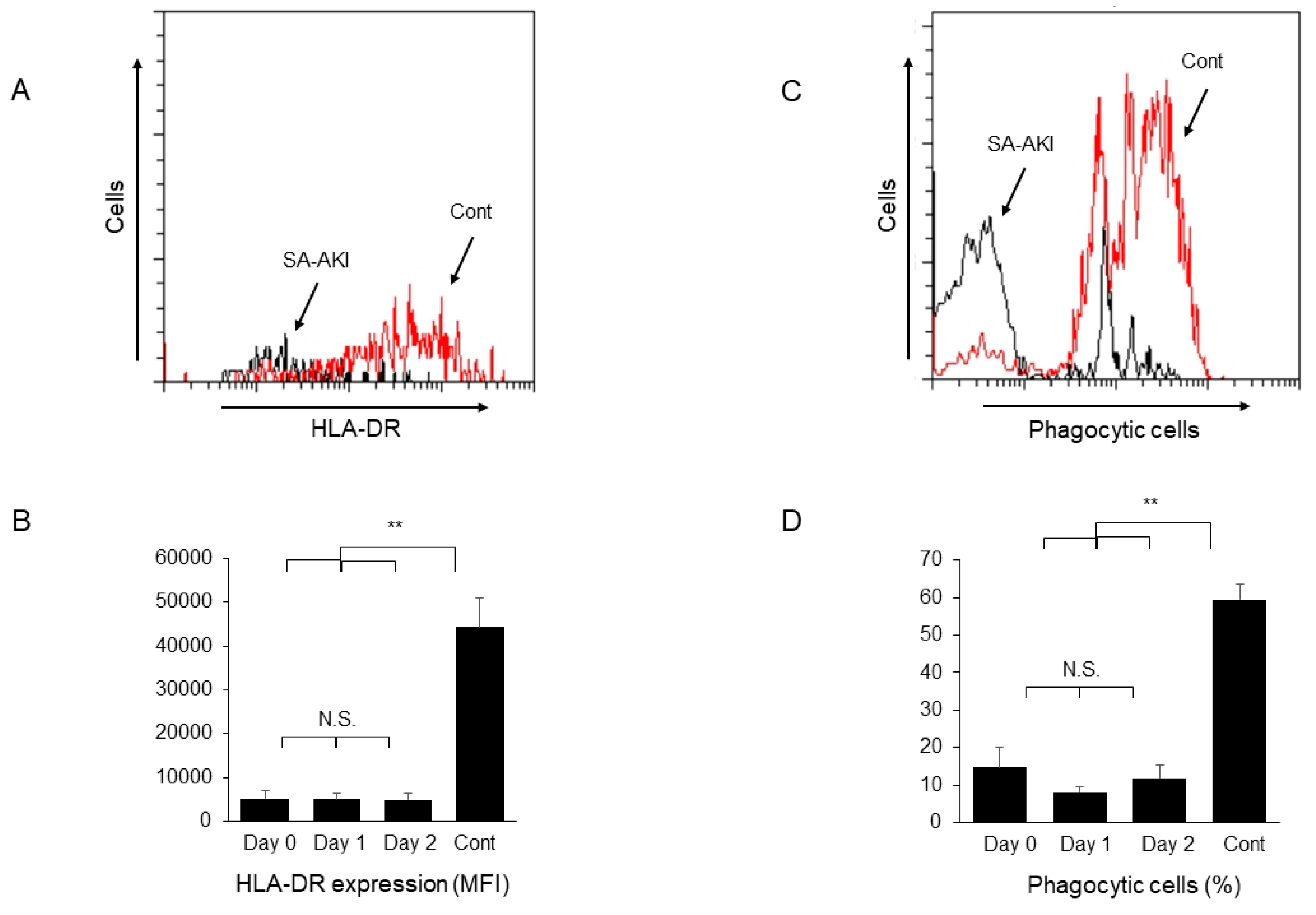
2. Results and Discussion
3. Materials and Methods
3.1. Patients and Their Clinical and Laboratory Data
3.2. Measurements of Serum TNF-α, Circulating Immune Complexes (CIC), and Serum Complement Components
3.3. Flow Cytometry
3.4. Evaluation of Phagocytic Activity
3.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, E.; Masereeuw, R.; Pickkers, P. The potential of alkaline phosphatase as a treatment for sepsis-associated acute kidney injury. Nephron Clin. Pr. 2014, 127, 144–148. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Wilkens, L.; Schraml, B.; Marschner, J. One concept does not fit all: The immune system in different forms of acute kidney injury. Nephrol. Dial. Transpl. 2021, 36, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Stenson, E.K.; Kendrick, J.; Dixon, B.; Thurman, J.M. The complement system in pediatric acute kidney injury. Pediatr. Nephrol. 2022, 38, 1411–1425. [Google Scholar] [CrossRef]
- Seki, S.; Nakashima, H.; Nakashima, M.; Kinoshita, M. Antitumor immunity produced by the liver Kupffer cells, NK cells, NKT cells, and CD8 CD122 T cells. Clin. Dev. Immunol. 2011, 2011, 868345. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Nakashima, H.; Ito, S.; Ishikiriyama, T.; Nakashima, M.; Seki, S.; Kumagai, H.; Oshima, N. Activated natural killer T cells in mice induce acute kidney injury with hematuria through possibly common mechanisms shared by human CD56(+) T cells. Am. J. Physiol. Ren. Physiol. 2018, 315, F618–F627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawarabayashi, N.; Seki, S.; Hatsuse, K.; Ohkawa, T.; Koike, Y.; Aihara, T.; Habu, Y.; Nakagawa, R.; Ami, K.; Hiraide, H.; et al. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology 2000, 32, 962–969. [Google Scholar] [CrossRef]
- Kumar, V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J. Hepatol. 2013, 59, 618–620. [Google Scholar] [CrossRef] [Green Version]
- Seki, S.; Habu, Y.; Kawamura, T.; Takeda, K.; Dobashi, H.; Ohkawa, T.; Hiraide, H. The liver as a crucial organ in the first line of host defense: The roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev. 2000, 174, 35–46. [Google Scholar] [CrossRef]
- Sato, A.; Nakashima, H.; Kinoshita, M.; Nakashima, M.; Ogawa, Y.; Shono, S.; Ikarashi, M.; Seki, S. The effect of synthetic C-reactive protein on the in vitro immune response of human PBMCs stimulated with bacterial reagents. Inflammation 2013, 36, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Ito, S.; Kumagai, H.; Oda, T.; Nakashima, H.; Seki, S. Roles of Natural Killer T Cells and Natural Killer Cells in Kidney Injury. Int. J. Mol. Sci. 2019, 20, 2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, H.; Adebiyi, A. Early septic insult in neonatal pigs increases serum and urinary soluble Fas ligand and decreases kidney function without inducing significant renal apoptosis. Ren. Fail 2017, 39, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, T.; Seki, S.; Oda, T. Infections, Reactions of Natural Killer T Cells and Natural Killer Cells, and Kidney Injury. Int. J. Mol. Sci. 2022, 23, 479. [Google Scholar] [CrossRef]
- Shono, S.; Habu, Y.; Nakashima, M.; Sato, A.; Nakashima, H.; Miyazaki, H.; Kinoshita, M.; Tsumatori, G.; Shinomiya, N.; Seki, S. The immunologic outcome of enhanced function of mouse liver lymphocytes and Kupffer cells by high-fat and high-cholesterol diet. Shock 2011, 36, 484–493. [Google Scholar] [CrossRef]
- Ohkawa, T.; Seki, S.; Dobashi, H.; Koike, Y.; Habu, Y.; Ami, K.; Hiraide, H.; Sekine, I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology 2001, 103, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Raffray, L.; Burton, R.J.; Baker, S.E.; Morgan, M.P.; Eberl, M. Zoledronate rescues immunosuppressed monocytes in sepsis patients. Immunology 2020, 159, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Srisawat, N.; Tungsanga, S.; Lumlertgul, N.; Komaenthammasophon, C.; Peerapornratana, S.; Thamrongsat, N.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit. Care 2018, 22, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honore, P.M.; Jacobs, R.; Joannes-Boyau, O.; De Regt, J.; De Waele, E.; van Gorp, V.; Boer, W.; Verfaillie, L.; Spapen, H.D. Newly designed CRRT membranes for sepsis and SIRS—A pragmatic approach for bedside intensivists summarizing the more recent advances: A systematic structured review. ASAIO J. 2013, 59, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Stasi, A.; Franzin, R.; Divella, C.; Sallustio, F.; Curci, C.; Picerno, A.; Pontrelli, P.; Staffieri, F.; Lacitignola, L.; Crovace, A.; et al. PMMA-Based Continuous Hemofiltration Modulated Complement Activation and Renal Dysfunction in LPS-Induced Acute Kidney Injury. Front. Immunol. 2021, 12, 605212. [Google Scholar] [CrossRef]
- Kojima, T.; Inoue, D.; Wajima, T.; Uchida, T.; Yamada, M.; Ohsawa, I.; Oda, T. Circulating immune-complexes and complement activation through the classical pathway in myeloperoxidase-ANCA-associated glomerulonephritis. Ren. Fail. 2022, 44, 714–723. [Google Scholar] [CrossRef]
- Ito, S.; Nakashima, M.; Ishikiriyama, T.; Nakashima, H.; Yamagata, A.; Imakiire, T.; Kinoshita, M.; Seki, S.; Kumagai, H.; Oshima, N. Effects of L-Carnitine Treatment on Kidney Mitochondria and Macrophages in Mice with Diabetic Nephropathy. Kidney Blood Press Res. 2022, 47, 277–290. [Google Scholar] [CrossRef] [PubMed]
| Data | |
|---|---|
| Number | 19 |
| Age (years) | 71.3 ± 2.2 |
| Sex (M/F) | 12/7 |
| Serum creatinine at enrollment (mg/dL) | 3.66 ± 0.40 |
| SOFA score | 12.8 ± 0.8 |
| Mean blood pressure (mmHg) | 64.0 ± 2.1 |
| Vasopressor use | 100% |
| Isolated organism (GPC/GNR/other) | 5/15/0 |
| Site of infection (GI/UTI/lung/skin) | 10/4/2/1 |
| AN69 ST membrane use | 68.4% |
| Polymyxin B hemoperfusion treatment | 15.8% |
| CRP (mg/dL) | 18.2 ± 2.1 |
| Procalcitonin (ng/mL) | 74.4 ± 21.0 |
| TNF-α (pg/mL) | 22.3 ± 5.4 |
| CIC-mRF (≧4.2 μg/mL) | 40% |
| C3 (mg/dL) | 81.6 ± 10.1 |
| C4 (mg/dL) | 19.6 ± 1.8 |
| C5a (ng/mL) | 24.1 ± 4.5 |
| C5b-9 (ng/mL) | 2201 ± 678 |
| Healthy Controls | Day 0 | Day 1 | Day 2 | |
|---|---|---|---|---|
| C5a (ng/mL) | 13.2 ± 1.4 * | 24.1 ± 4.5 | 20.6 ± 7.5 | 22.8 ± 6.1 |
| C5b-9 (ng/mL) | 1046 ± 181 * | 2201 ± 678 | 2326 ± 1077 | 2214 ± 960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, T.; Yamada, M.; Inoue, D.; Kojima, T.; Yoshikawa, N.; Suda, S.; Kamohara, H.; Oda, T. Involvement of Innate Immune System in the Pathogenesis of Sepsis-Associated Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 12465. https://doi.org/10.3390/ijms241512465
Uchida T, Yamada M, Inoue D, Kojima T, Yoshikawa N, Suda S, Kamohara H, Oda T. Involvement of Innate Immune System in the Pathogenesis of Sepsis-Associated Acute Kidney Injury. International Journal of Molecular Sciences. 2023; 24(15):12465. https://doi.org/10.3390/ijms241512465
Chicago/Turabian StyleUchida, Takahiro, Muneharu Yamada, Dan Inoue, Tadasu Kojima, Noriko Yoshikawa, Shingo Suda, Hidenobu Kamohara, and Takashi Oda. 2023. "Involvement of Innate Immune System in the Pathogenesis of Sepsis-Associated Acute Kidney Injury" International Journal of Molecular Sciences 24, no. 15: 12465. https://doi.org/10.3390/ijms241512465





