miR-143-3p Promotes Ovarian Granulosa Cell Senescence and Inhibits Estradiol Synthesis by Targeting UBE2E3 and LHCGR
Abstract
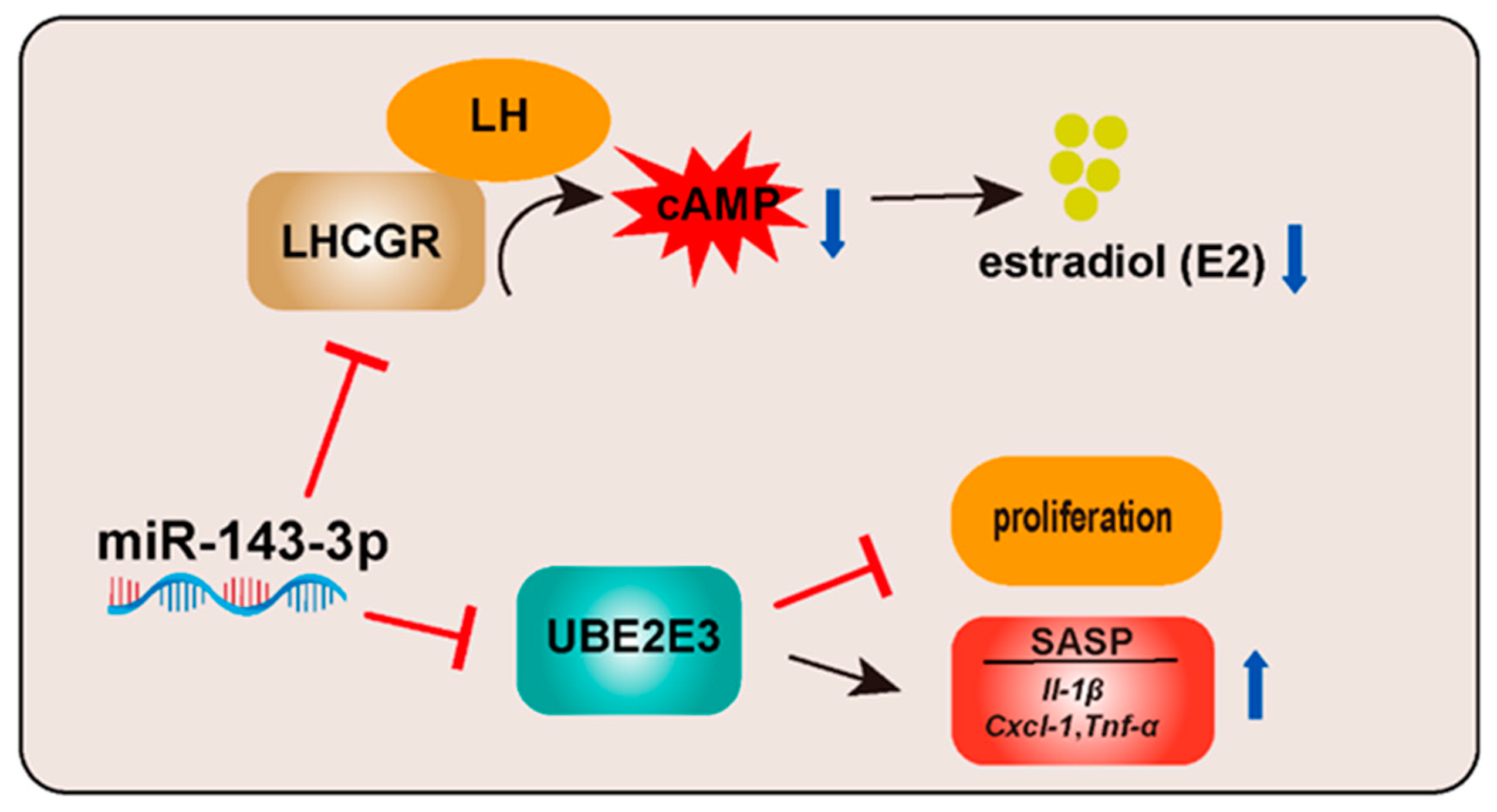
:1. Introduction
2. Results
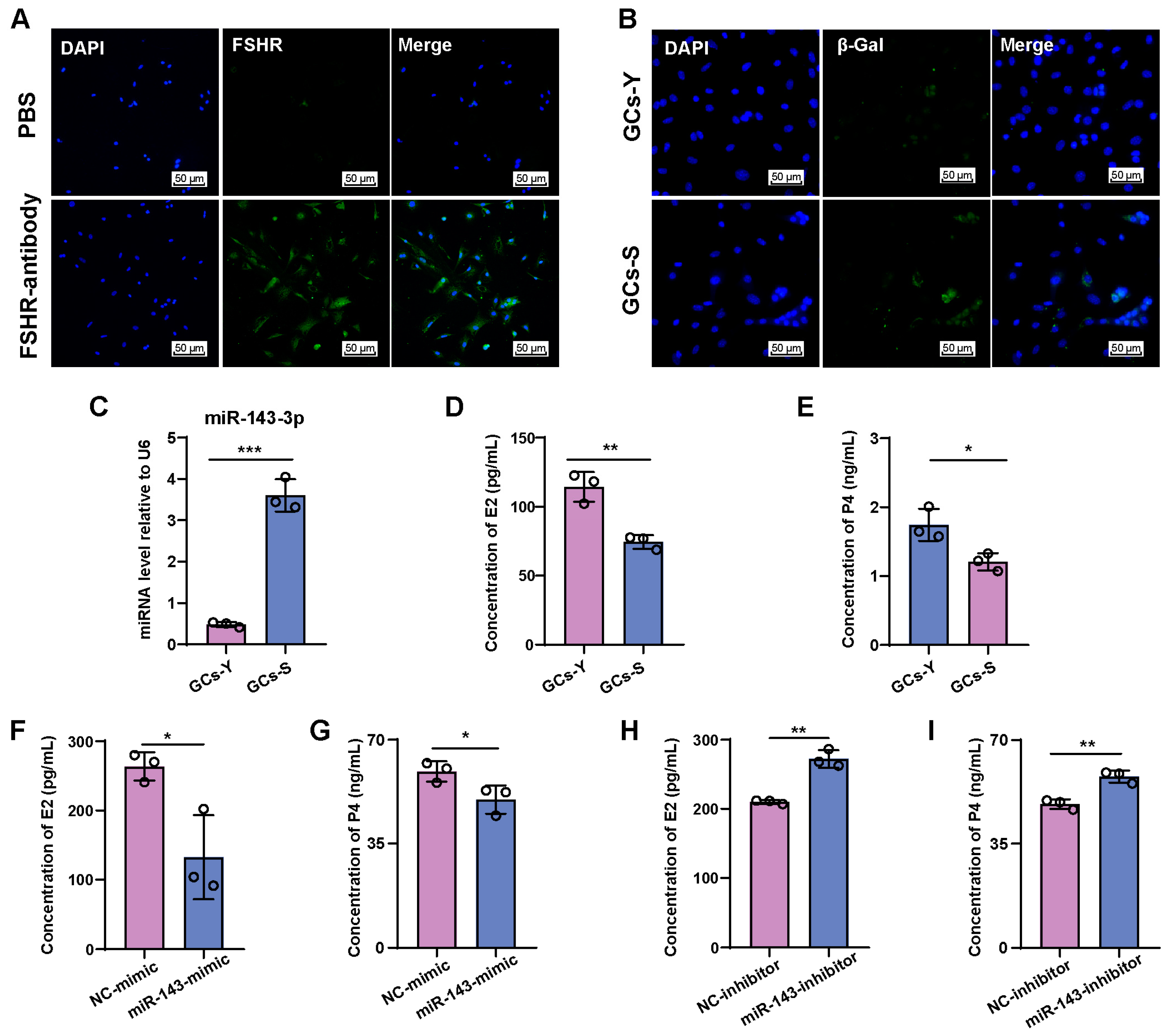
2.1. miR-143-3p Is Highly Expressed in the Ovaries of Aged Mice
2.2. miR-143-3p Inhibits E2 and P4 Synthesis in GCs
2.3. Increased miR-143-3p Expression Inhibits GCs’ Proliferation and Triggers Senescence Characterized by a Distinct SASP
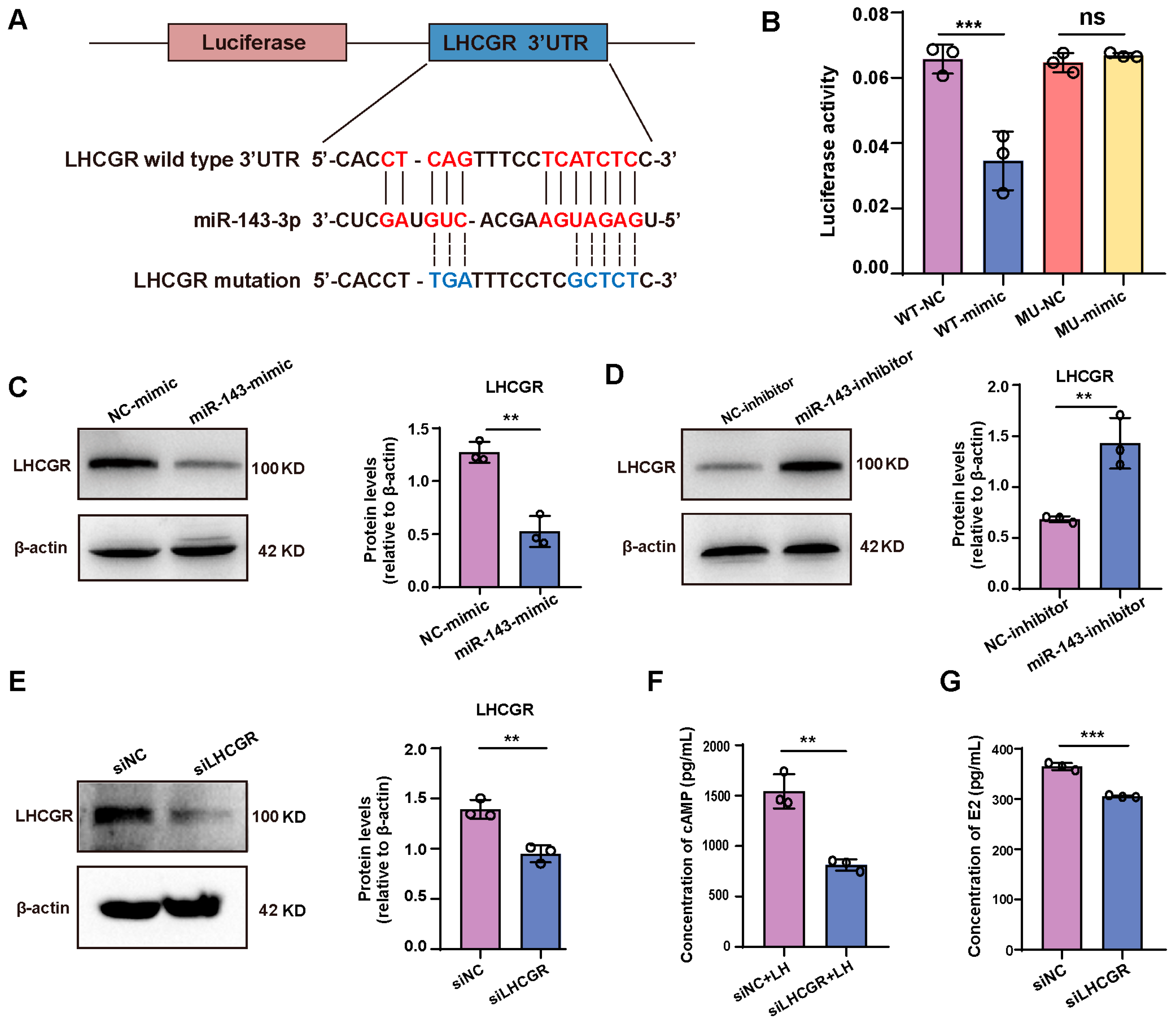
2.4. miR-143-3p Inhibits E2 Synthesis in GCs by Targeting Lhcgr
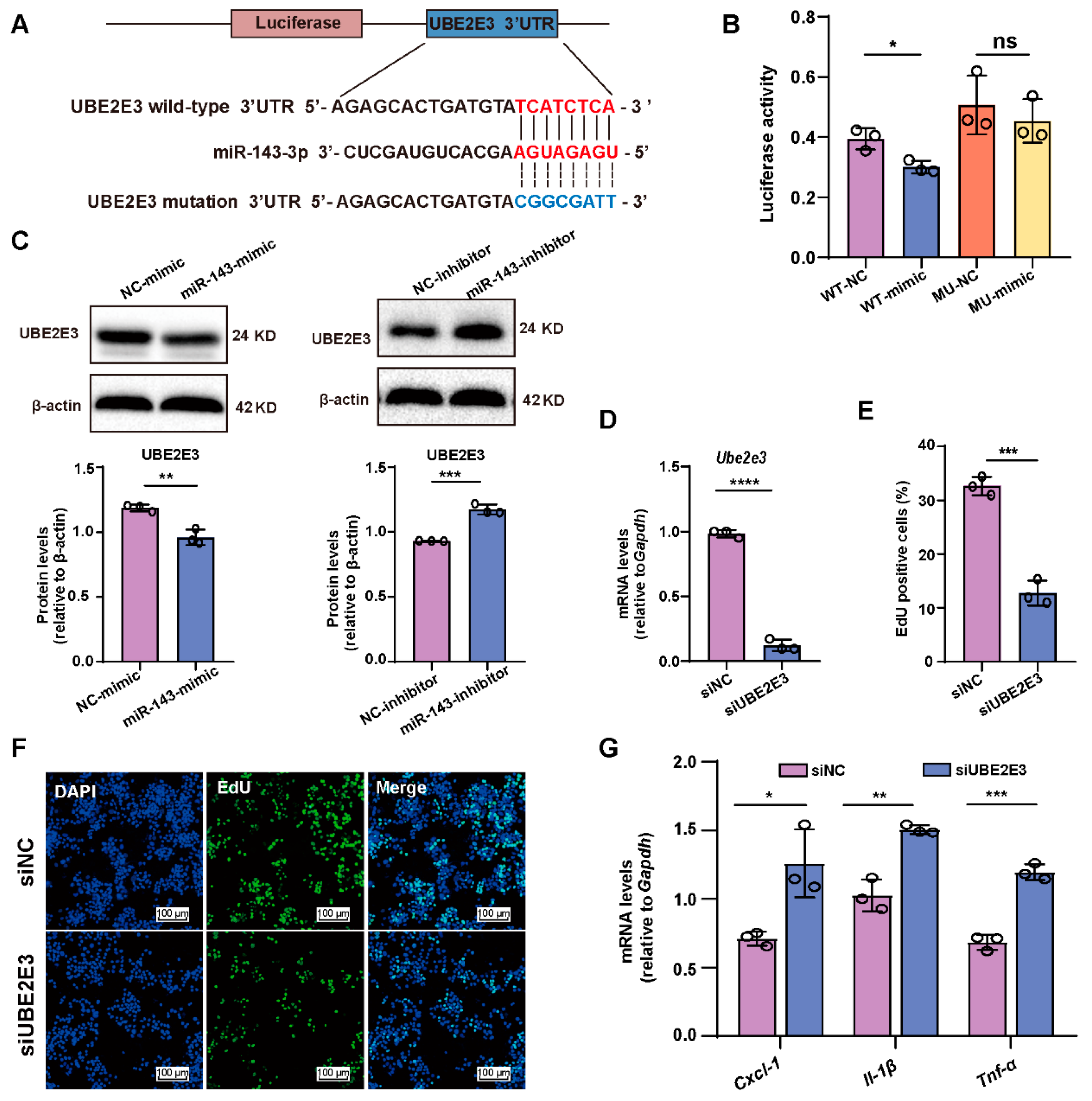
2.5. miR-143-3p Inhibits GCs’ proliferation and Induces SASP Factor Upregulation by Targeting Ube2e3
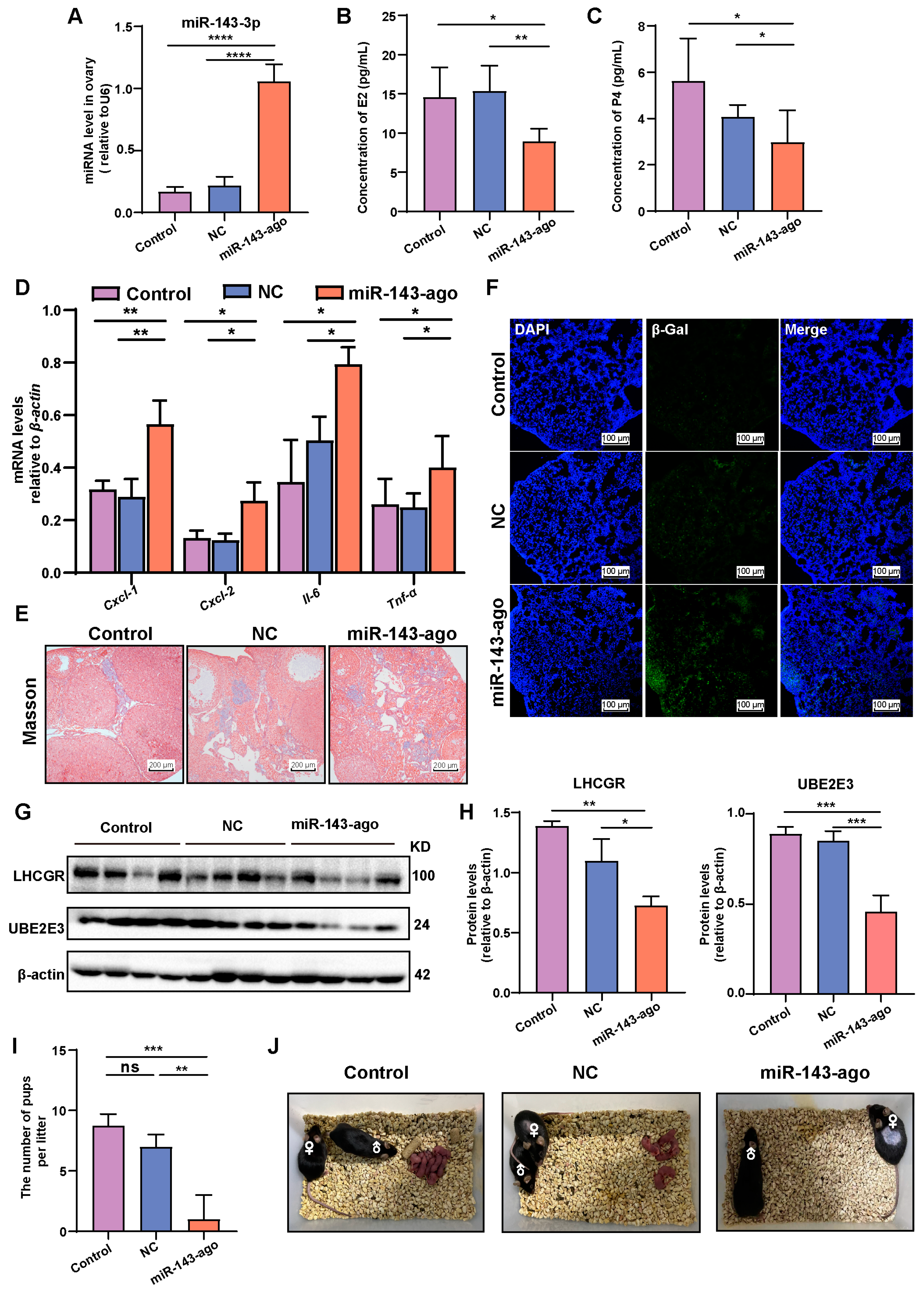
2.6. Overexpression of miR-143-3p Accelerated Ovarian Aging In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. GCs Isolation and Culture
4.3. Protein Extraction and Western Blot
4.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Oligonucleotide Transfection
4.6. Hormone Assay
4.7. Intraperitoneal Injection of miR-143-3p Agomir
4.8. Luciferase Reporter Assay
4.9. Fluorescence In Situ Hybridization (FISH)
4.10. EdU Assay
4.11. SPIDER-β-Gal Staining
4.12. Mating Experiment
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Secomandi, L.; Borghesan, M.; Velarde, M.; Demaria, M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum. Reprod. Update 2022, 28, 172–189. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice; The Practice Committee of the American Society for Reproductive Medicine. Female age-related fertility decline: Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Vollenhoven, B.; Hunt, S. Ovarian ageing and the impact on female fertility. F1000Res 2018, 7, 1835. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.X.; Wang, Y.N.; Li, Z.Y.; Dai, Z.H.; He, Y.; Chu, K.; Gu, J.Y.; Ji, Y.X.; Sun, N.X.; Yang, F.; et al. The m6A mRNA demethylase FTO in granulosa cells retards FOS-dependent ovarian aging. Cell Death Dis. 2021, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhou, Y.; Tang, W.; Ren, C.; Jiang, A.; Wang, X.; Qian, X.; Zhou, Z.; Gong, A. Long-term treatment of Nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J. Nutr. Biochem. 2022, 101, 108911. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Wang, G.; Yao, Y.; Liang, J.; Tenny Chung, C.Y.; Chuai, M.; Lee, K.K.; Yang, X. BRE modulates granulosa cell death to affect ovarian follicle development and atresia in the mouse. Cell Death Dis. 2017, 8, e2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Mol. Cell 2018, 72, 1021–1034.e1024. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, M.; Liu, C.; Wang, L.; Wei, H.; Zhang, R.; Ren, Z.; Chen, Y.; Luo, M.; Zhao, J.; et al. Epg5 deficiency leads to primary ovarian insufficiency due to WT1 accumulation in mouse granulosa cells. Autophagy 2023, 19, 644–659. [Google Scholar] [CrossRef]
- Fan, X.; Moustakas, I.; Bialecka, M.; Del Valle, J.S.; Overeem, A.W.; Louwe, L.A.; Pilgram, G.S.K.; van der Westerlaken, L.A.J.; Mei, H.; Chuva de Sousa Lopes, S.M. Single-Cell Transcriptomics Analysis of Human Small Antral Follicles. Int. J. Mol. Sci. 2021, 22, 11955. [Google Scholar] [CrossRef]
- Shao, T.; Ke, H.; Liu, R.; Xu, L.; Han, S.; Zhang, X.; Dang, Y.; Jiao, X.; Li, W.; Chen, Z.J.; et al. Autophagy regulates differentiation of ovarian granulosa cells through degradation of WT1. Autophagy 2022, 18, 1864–1878. [Google Scholar] [CrossRef]
- Farquhar, C.M.; Bhattacharya, S.; Repping, S.; Mastenbroek, S.; Kamath, M.S.; Marjoribanks, J.; Boivin, J. Female subfertility. Nat. Rev. Dis. Prim. 2019, 5, 7. [Google Scholar] [CrossRef]
- Suzuki, E.; Miyado, M.; Kuroki, Y.; Fukami, M. Genetic variants of G-protein coupled receptors associated with pubertal disorders. Reprod. Med. Biol. 2023, 22, e12515. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, L.; Li, X.; Pan, Z.; Liu, H.; Li, Q. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis. 2016, 7, e2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Convissar, S.; Winston, N.J.; Fierro, M.A.; Scoccia, H.; Zamah, A.M.; Stocco, C. Sp1 regulates steroidogenic genes and LHCGR expression in primary human luteinized granulosa cells. J. Steroid. Biochem. Mol. Biol. 2019, 190, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Y.; Li, X.; Li, C.; Zhang, L.; Liu, Z.; Cao, Y.; Li, W.; Zhang, X.; Zhang, J.; et al. The Corticosterone-Glucocorticoid Receptor-AP1/CREB Axis Inhibits the Luteinizing Hormone Receptor Expression in Mouse Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 12454. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Yang, Z.X.; Wang, Y.; Du, L.; Li, Y.R.; Zhang, N.N.; Gao, W.Y.; Peng, R.R.; Zhu, F.Y.; Wang, L.L.; et al. Single xenotransplant of rat brown adipose tissue prolonged the ovarian lifespan of aging mice by improving follicle survival. Aging Cell 2019, 18, e13024. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Li, Y.; Song, D.; Ding, J.; Mei, S.; Sun, S.; Cheng, W.; Yu, J.; Zhou, L.; Kuang, Y.; et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. 2022, 13, 579. [Google Scholar] [CrossRef]
- Hoque, S.A.M.; Umehara, T.; Kawai, T.; Shimada, M. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radic. Biol. Med. 2021, 163, 344–355. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gu, L.; Lin, Q.; Ma, Y.; Liu, C.; Pei, X.; Li, P.A.; Yang, Y. The Immp2l Mutation Causes Ovarian Aging Through ROS-Wnt/beta-Catenin-Estrogen Pathway: Preventive Effect of Melatonin. Endocrinology 2020, 161, bqaa119. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, Y.; Xue, Z.; Wu, R.; Li, J.; Niu, X.; Yuan, J.; Wang, Y.; Wu, Q. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Y.; Xu, J.; Cao, X.; Zhang, D.; Liu, M.; Liu, S.; Dong, X.; Shi, H. Mitochondrial dysfunction in cumulus cells is related to decreased reproductive capacity in advanced-age women. Fertil. Steril. 2022, 118, 393–404. [Google Scholar] [CrossRef]
- Wang, C.; Fei, X.; Zhang, H.; Zhou, W.; Cheng, Z.; Feng, Y. Proteomic Analysis of the Alterations in Follicular Fluid Proteins During Oocyte Maturation in Humans. Front. Endocrinol. 2021, 12, 830691. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Accelerated Ovarian Aging Among Type 2 Diabetes Patients and Its Association with Adverse Lipid Profile. Front. Endocrinol. 2022, 13, 780979. [Google Scholar] [CrossRef]
- Rowley, J.E.; Amargant, F.; Zhou, L.T.; Galligos, A.; Simon, L.E.; Pritchard, M.T.; Duncan, F.E. Low Molecular Weight Hyaluronan Induces an Inflammatory Response in Ovarian Stromal Cells and Impairs Gamete Development In Vitro. Int. J. Mol. Sci. 2020, 21, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uri-Belapolsky, S.; Shaish, A.; Eliyahu, E.; Grossman, H.; Levi, M.; Chuderland, D.; Ninio-Many, L.; Hasky, N.; Shashar, D.; Almog, T.; et al. Interleukin-1 deficiency prolongs ovarian lifespan in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 12492–12497. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, J.; Lang, H.; Wang, W.; Liu, X.; Liu, H.; Tan, C.; Li, X.; Zhao, Y.; Wu, X. MicroRNA-205 affects mouse granulosa cell apoptosis and estradiol synthesis by targeting CREB1. J. Cell Biochem. 2019, 120, 8466–8474. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, X.; Mu, H.; Mei, Q.; Liu, Y.; Min, Z.; Zhang, L.; Su, P.; Xiang, W. Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via YAP1-mediated mitochondrial function and apoptosis. Int. J. Biol. Sci. 2022, 18, 1008–1021. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Pan, Z.; Du, X.; Li, X.; Ma, B.; Yao, W.; Li, Q.; Liu, H. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-beta type 1 receptor. Mol. Cell Endocrinol. 2015, 409, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-P.; Hu, Y.-P.; Wu, X.-S.; Wu, Y.-S.; Ye, Y.-Y.; Li, H.-F.; Liu, Y.-C.; Jiang, L.; Liu, F.-T.; Zhang, Y.-J.; et al. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018, 9, 182. [Google Scholar] [CrossRef]
- Troidl, K.; Hammerschick, T.; Albarran-Juarez, J.; Jung, G.; Schierling, W.; Tonack, S.; Kruger, M.; Matuschke, B.; Troidl, C.; Schaper, W.; et al. Shear Stress-Induced miR-143-3p in Collateral Arteries Contributes to Outward Vessel Growth by Targeting Collagen V-alpha2. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e126–e137. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Q.; Lv, Z.; Ling, Y.; Hou, X.; Chen, Z.; Dinglin, X.; Ma, S.; Li, D.; Wu, Y.; et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol. Cancer 2019, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Bock, J.; Northoff, B.H.; Klein, M.; et al. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef]
- Cao, J.; Huo, P.; Cui, K.; Wei, H.; Cao, J.; Wang, J.; Liu, Q.; Lei, X.; Zhang, S. Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome. Cell. Commun. Signal. 2022, 20, 61. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, S.; Li, Y.; Wu, X. Exosomal miR-143-3p derived from follicular fluid promotes granulosa cell apoptosis by targeting BMPR1A in polycystic ovary syndrome. Sci. Rep. 2022, 12, 4359. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Kim, S.R.; Kim, S.K.; Park, J.R.; Hong, I.S. A novel role of follicle-stimulating hormone (FSH) in various regeneration-related functions of endometrial stem cells. Exp. Mol. Med. 2022, 54, 1524–1535. [Google Scholar] [CrossRef]
- Sreerangaraja Urs, D.B.; Wu, W.H.; Komrskova, K.; Postlerova, P.; Lin, Y.F.; Tzeng, C.R.; Kao, S.H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, H.; Zhu, Y.; Sun, Q.; Ji, Y.; Xue, A.; Wang, Y.; Chen, W.; Yu, X.; Wang, L.; et al. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020, 30, 574–589. [Google Scholar] [CrossRef]
- Guerrero, F.; Carmona, A.; Jimenez, M.J.; Obrero, T.; Pulido, V.; Moreno, J.A.; Soriano, S.; Martin-Malo, A.; Aljama, P. Passage Number-Induced Replicative Senescence Modulates the Endothelial Cell Response to Protein-Bound Uremic Toxins. Toxins 2021, 13, 738. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Shi, C.; Lin, J.; Chen, G.; Wu, B.; Wu, L.; Shi, H.; Yuan, Y.; Zhou, W.; et al. Altered microRNAs expression profiling in cumulus cells from patients with polycystic ovary syndrome. J. Transl. Med. 2015, 13, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, D.M.; Hathaway, T.J.; Ramsey, P.S. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst. Rev. 2019, 11, CD003511. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Lokman, N.A.; Abraham, R.D.; Macpherson, A.M.; Lee, E.; Grutzner, F.; Ghinea, N.; Oehler, M.K.; Ricciardelli, C. Reduced Gonadotrophin Receptor Expression Is Associated with a More Aggressive Ovarian Cancer Phenotype. Int. J. Mol. Sci. 2020, 22, 10071. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Deng, L. Chrysin reduces inflammation and oxidative stress and improves ovarian function in D-gal-induced premature ovarian failure. Bioengineered 2022, 13, 8291–8301. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Z.; Weng, H.; Chen, Y.; Zhang, J.; Mei, S.; Wei, J.; Zhu, X.; Nong, Y.; Ruan, J.; et al. Folic Acid Preconditioning Alleviated Radiation-Induced Ovarian Dysfunction in Female Mice. Front. Nutr. 2022, 9, 854655. [Google Scholar] [CrossRef] [PubMed]
- Plafker, K.S.; Zyla, K.; Berry, W.; Plafker, S.M. Loss of the ubiquitin conjugating enzyme UBE2E3 induces cellular senescence. Redox. Biol. 2018, 17, 411–422. [Google Scholar] [CrossRef]
- Plafker, K.S.; Farjo, K.M.; Wiechmann, A.F.; Plafker, S.M. The human ubiquitin conjugating enzyme, UBE2E3, is required for proliferation of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5611–5618. [Google Scholar] [CrossRef] [Green Version]
- Ambivero, C.T.; Cilenti, L.; Main, S.; Zervos, A.S. Mulan E3 ubiquitin ligase interacts with multiple E2 conjugating enzymes and participates in mitophagy by recruiting GABARAP. Cell Signal 2014, 26, 2921–2929. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, G.; Chen, P.; Jiang, T.; Xia, Z. UBE2E3 regulates cellular senescence and osteogenic differentiation of BMSCs during aging. PeerJ 2021, 9, e12253. [Google Scholar] [CrossRef]
- Plafker, K.S.; Plafker, S.M. The ubiquitin-conjugating enzyme UBE2E3 and its import receptor importin-11 regulate the localization and activity of the antioxidant transcription factor NRF2. Mol. Biol. Cell 2015, 26, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Q.; Xu, C.; Gogol, M.; Tang, J.; Chen, W.; Yu, X.; Workman, J.L.; Li, S. Set1-catalyzed H3K4 trimethylation antagonizes the HIR/Asf1/Rtt106 repressor complex to promote histone gene expression and chronological life span. Nucleic Acids Res. 2019, 47, 3434–3449. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Blake, J. Menopause: Evidence-based practice. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 799–839. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol. Ther. 2020, 28, 1200–1213. [Google Scholar] [CrossRef]


| Name | Manufacturers | Usage and Dosage |
|---|---|---|
| UBE2E3 | Proteintech | WB (1:1000) |
| LHCGR | Proteintech | WB (1:1000) |
| CCND2 | CST | WB (1:1000) |
| β-actin | Fude bio | WB (1:5000) |
| Primers | Sence Primer (5’-3’) | Anti-Sence Primer |
|---|---|---|
| Cxcl-1 | TGCACCCAAACCGAAGTCAT | CTCCGTTACTTGGGGACACC |
| Cxcl-2 | TCATAGCCACTCTCAAGGGC | TCAGGTACGATCCAGGCTTC |
| Il-1β | GCCACCTTTTGACAGTGATGAG | GACAGCCCAGGTCAAAGGTT |
| Il-6 | CACTTCACAAGTCGGAGGCT | CTGCAAGTGCATCATCGTTGT |
| Il-8 | CTTTGTCCATTCCCACTTCTGA | TCCCTAACGGTTGCCTTTGTAT |
| Tnf-α | ATGTCTCAGCCTCTTCTCATTC | GCTTGTCACTCGAATTTTGAGA |
| Fluorescence In Situ Hybridization | Sence primer (5’-3’) | |
| miR-143-3p | GAGCTACAGTGCTTCATCTCA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Tang, Y.; Li, L.; Huang, R.; Wang, Z.; Ye, T.; Xiao, Z.; Hu, M.; Wei, S.; Wang, Y.; et al. miR-143-3p Promotes Ovarian Granulosa Cell Senescence and Inhibits Estradiol Synthesis by Targeting UBE2E3 and LHCGR. Int. J. Mol. Sci. 2023, 24, 12560. https://doi.org/10.3390/ijms241612560
Deng J, Tang Y, Li L, Huang R, Wang Z, Ye T, Xiao Z, Hu M, Wei S, Wang Y, et al. miR-143-3p Promotes Ovarian Granulosa Cell Senescence and Inhibits Estradiol Synthesis by Targeting UBE2E3 and LHCGR. International Journal of Molecular Sciences. 2023; 24(16):12560. https://doi.org/10.3390/ijms241612560
Chicago/Turabian StyleDeng, Jingxian, Yan Tang, Lu Li, Rufei Huang, Zhaoyang Wang, Tao Ye, Ziyan Xiao, Meirong Hu, Siying Wei, Yuxin Wang, and et al. 2023. "miR-143-3p Promotes Ovarian Granulosa Cell Senescence and Inhibits Estradiol Synthesis by Targeting UBE2E3 and LHCGR" International Journal of Molecular Sciences 24, no. 16: 12560. https://doi.org/10.3390/ijms241612560





