Prospects of Using Chitosan-Based Biopolymers in the Treatment of Peripheral Nerve Injuries
Abstract
:1. Introduction
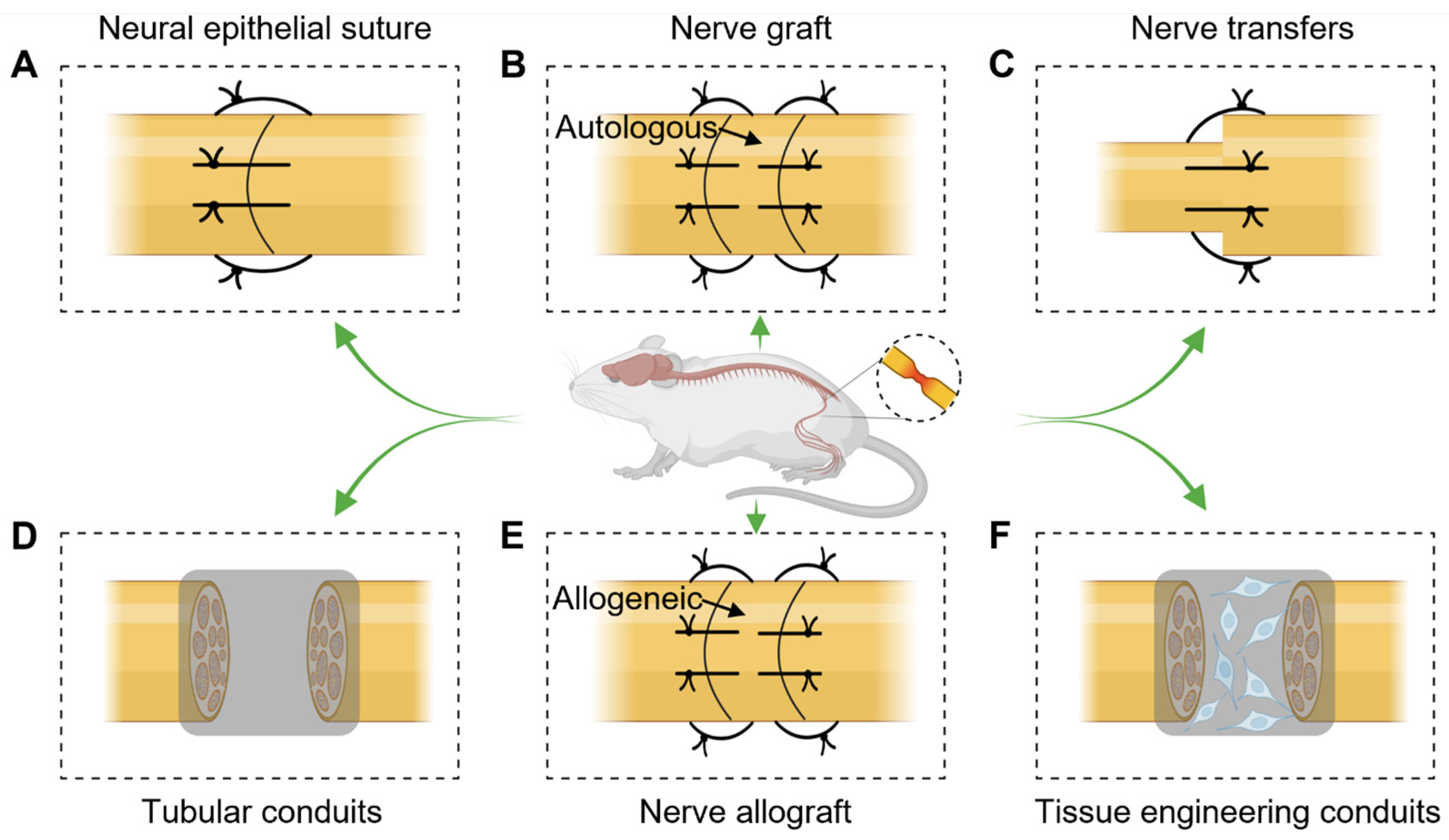
2. Treatment of Peripheral Nerve Injuries
2.1. Neural Epithelial Suture
2.2. Nerve Grafting
2.3. Nerve Transfers
2.4. Tubular Conduits
2.5. Nerve Allograft
2.6. Tissue Engineering Conduits
3. Overview of Chitosan-Based Polymers and Their Properties
3.1. Biocompatibility
3.2. Chitosan-Based Polymer Loading Factors/Cell Repair in PNI
3.3. Antimicrobial Properties
3.4. Porous Structures for Nutrient and Oxygen Transfer
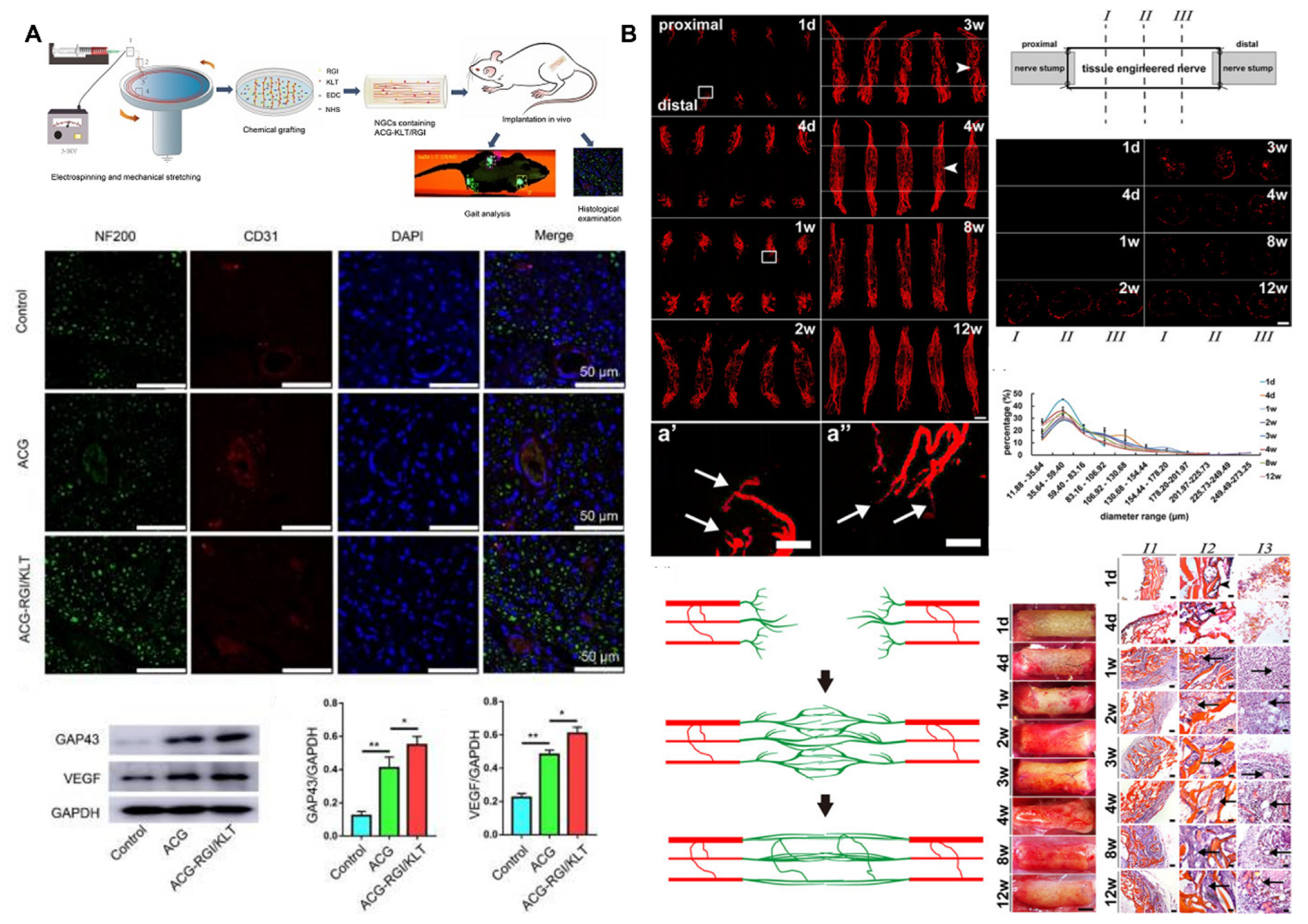
4. Chitosan-Based Polymers in Peripheral Nerve Regeneration
4.1. Chitosan-Based Polymer Nerve-Repair Conduits
4.2. Chitosan-Loaded Cells
4.3. Chitosan Slow-Release Bioactive Molecules
5. Techniques of Tissue Engineering for PNI Repair
5.1. Hydrogel for PNI Repair
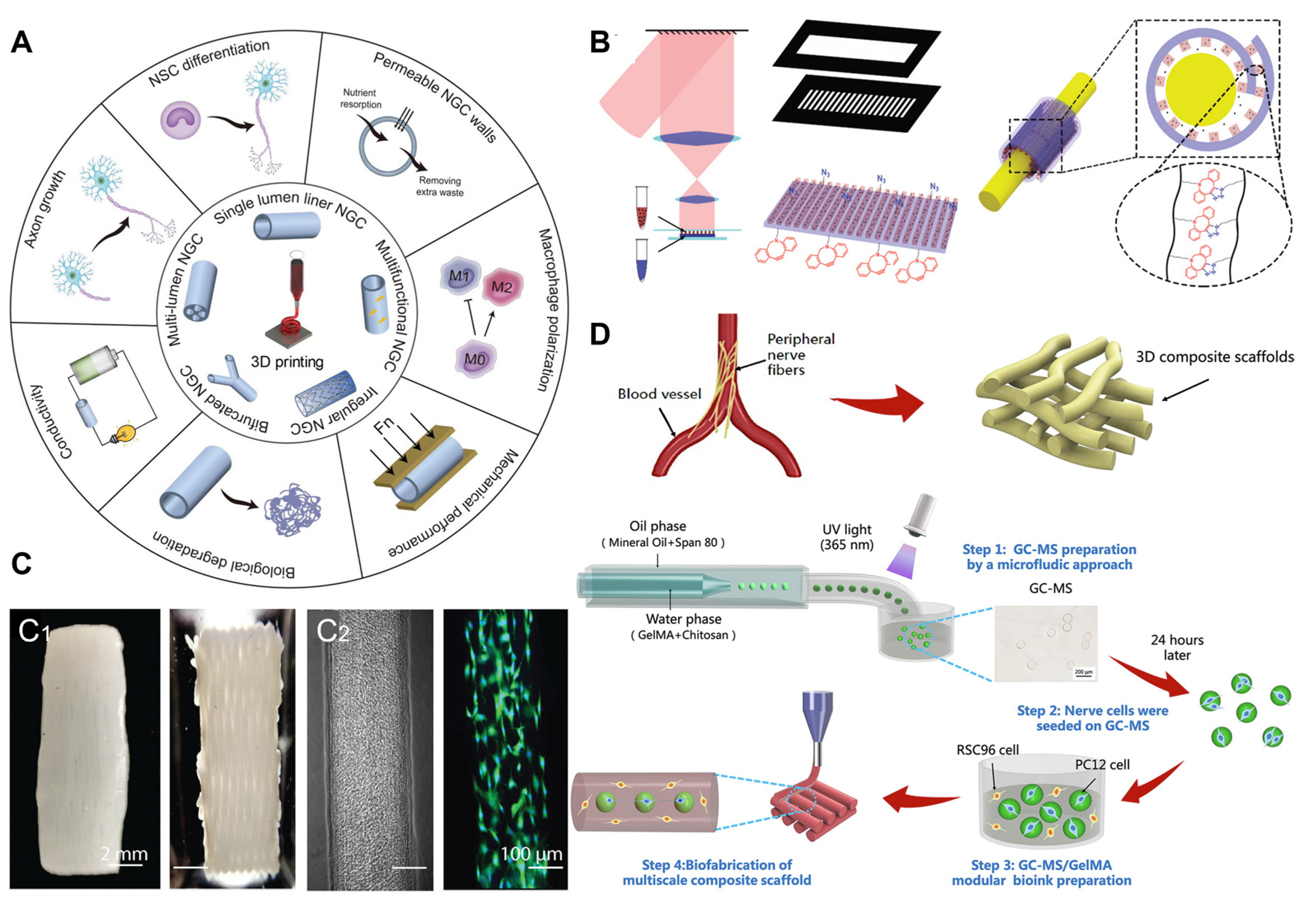
5.2. Three-Dimensional Printing Technology Applied to PNI Repair
5.3. Electrostatic Spinning for PNI Repair
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Yang, Y.; Quan, X.; Lu, L.; Xia, B.; Gao, J.; Qi, F.; Li, S.; Zhao, L.; Mei, L.; et al. Oxygen carrier in core-shell fibers synthesized by coaxial electrospinning enhances Schwann cell survival and nerve regeneration. Theranostics 2020, 10, 8957–8973. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Ge, X.; Chen, X.; Xu, Y.; Yuan, W.E.; Ouyang, Y. Enhancement of sciatic nerve regeneration with dual delivery of vascular endothelial growth factor and nerve growth factor genes. J. Nanobiotechnology 2020, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- El Hajj Abdallah, Y.; Beveridge, J.; Chan, M.; Deeb, T.; Mowafi, H.; Al-Nuaimi, S.; Easa, A.S.; Saqqur, M. Union of Medical Care and Relief Organizations. Devastating neurologic injuries in the Syrian war. Neurol. Clin. Pract. 2019, 9, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Papaloïzos, M.; Gander, B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials 2010, 31, 2323–2334. [Google Scholar] [CrossRef]
- Yucel, D.; Kose, G.T.; Hasirci, V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31, 1596–1603. [Google Scholar] [CrossRef]
- Wang, W.; Itoh, S.; Yamamoto, N.; Okawa, A.; Nagai, A.; Yamashita, K. Enhancement of nerve regeneration along a chitosan nanofiber mesh tube on which electrically polarized beta-tricalcium phosphate particles are immobilized. Acta Biomater. 2010, 6, 4027–4033. [Google Scholar] [CrossRef]
- Canales, A.; Park, S.; Kilias, A.; Anikeeva, P. Multifunctional Fibers as Tools for Neuroscience and Neuroengineering. Acc. Chem. Res. 2018, 51, 829–838. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, N.; Wang, J.; Jin, Z.; Zhu, L.; Wang, Y.; Chen, S.; Hu, Y.; Zhang, T.; Song, Q.; et al. Peripheral nerve defects repaired with autogenous vein grafts filled with platelet-rich plasma and active nerve microtissues and evaluated by novel multimodal ultrasound techniques. Biomater. Res. 2022, 26, 24. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, K.H.; Fan, C.Y.; Mo, X.M.; Ruan, H.J.; Li, F.F. Aligned natural-synthetic polyblend nanofibers for peripheral nerve regeneration. Acta Biomater. 2011, 7, 634–643. [Google Scholar] [CrossRef]
- Hosoyama, K.; Ahumada, M.; Goel, K.; Ruel, M.; Suuronen, E.J.; Alarcon, E.I. Electroconductive materials as biomimetic platforms for tissue regeneration. Biotechnol. Adv. 2019, 37, 444–458. [Google Scholar] [PubMed]
- Gu, Y.; Zhu, J.; Xue, C.; Li, Z.; Ding, F.; Yang, Y.; Gu, X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 2014, 35, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar]
- Manzari-Tavakoli, A.; Tarasi, R.; Sedghi, R.; Moghimi, A.; Niknejad, H. Fabrication of nanochitosan incorporated polypyrrole/alginate conducting scaffold for neural tissue engineering. Sci. Rep. 2020, 10, 22012. [Google Scholar] [CrossRef]
- Huang, Y.; Seitz, D.; König, F.; Müller, P.E.; Jansson, V.; Klar, R.M. Induction of Articular Chondrogenesis by Chitosan/Hyaluronic-Acid-Based Biomimetic Matrices Using Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2019, 20, 4487. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.E.; Kan, C.F.K.; Stewart, B.R.; Sanicola, H.W., 3rd; Jung, J.P.; Sulaiman, O.A.R.; Wang, D. Machine intelligence for nerve conduit design and production. J. Biol. Eng. 2020, 14, 25. [Google Scholar]
- Ghasemi Hamidabadi, H.; Rezvani, Z.; Nazm Bojnordi, M.; Shirinzadeh, H.; Seifalian, A.M.; Joghataei, M.T.; Razaghpour, M.; Alibakhshi, A.; Yazdanpanah, A.; Salimi, M. Chitosan-Intercalated Montmorillonite/Poly(vinyl alcohol) Nanofibers as a Platform to Guide Neuronlike Differentiation of Human Dental Pulp Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 11392–11404. [Google Scholar] [CrossRef]
- Liu, H.; Wen, W.; Hu, M.; Bi, W.; Chen, L.; Liu, S.; Chen, P.; Tan, X. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen. Res. 2013, 8, 3139–3147. [Google Scholar]
- Zeng, W.; Rong, M.; Hu, X.; Xiao, W.; Qi, F.; Huang, J.; Luo, Z. Incorporation of chitosan microspheres into collagen-chitosan scaffolds for the controlled release of nerve growth factor. PLoS ONE 2014, 9, e101300. [Google Scholar] [CrossRef]
- Paul, G.; Anisimov, S.V. The secretome of mesenchymal stem cells: Potential implications for neuroregeneration. Biochimie 2013, 95, 2246–2256. [Google Scholar] [CrossRef]
- Ni, H.C.; Lin, Z.Y.; Hsu, S.H.; Chiu, I.M. The use of air plasma in surface modification of peripheral nerve conduits. Acta Biomater. 2010, 6, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Yu, Y.; Chen, X.; Yang, Y.; Xiong, Y.; Im, Y.J.; Zhao, Y.; Xiao, J. Microtubes with gradient decellularized porcine sciatic nerve matrix from microfluidics for sciatic nerve regeneration. Bioact. Mater. 2023, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Chen, F.; Zhang, H.; Chen, Y.; Zhou, J. Multifunctional Double-Layer Composite Hydrogel Conduit Based on Chitosan for Peripheral Nerve Repairing. Adv. Healthc. Mater. 2022, 11, 2200115. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, H.; Huang, H.; Bi, W.; Yan, R.; Tan, X.; Wen, W.; Wang, C.; Song, W.; Zhang, Y.; et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol. Med. Rep. 2018, 17, 4360–4368. [Google Scholar] [CrossRef]
- Huang, T.W.; Li, S.T.; Wang, Y.H.; Young, T.H. Regulation of chitosan-mediated differentiation of human olfactory receptor neurons by insulin-like growth factor binding protein-2. Acta Biomater. 2019, 97, 399–408. [Google Scholar] [CrossRef]
- Xu, J.; Chen, T.Y.; Tai, C.H.; Hsu, S.H. Bioactive self-healing hydrogel based on tannic acid modified gold nano-crosslinker as an injectable brain implant for treating Parkinson’s disease. Biomater. Res. 2023, 27, 8. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chen, C.C.; Lin, Y.Y.; Chen, Y.J.; Liu, B.H.; Wong, S.C.; Wu, C.Y.; Chang, Y.T.; Chou, H.E.; Ding, S.T. Chitosan-assisted differentiation of porcine adipose tissue-derived stem cells into glucose-responsive insulin-secreting clusters. PLoS ONE 2017, 12, e0172922. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.Y.; Zhou, L.P.; Min, T.T.; Zhang, M.; Pi, W.; Wen, Y.Q.; Zhang, P.X. Polydopamine-modified chitin conduits with sustained release of bioactive peptides enhance peripheral nerve regeneration in rats. Neural Regen. Res. 2022, 17, 2544–2550. [Google Scholar]
- Hsueh, Y.Y.; Chang, Y.J.; Huang, T.C.; Fan, S.C.; Wang, D.H.; Chen, J.J.; Wu, C.C.; Lin, S.C. Functional recoveries of sciatic nerve regeneration by combining chitosan-coated conduit and neurosphere cells induced from adipose-derived stem cells. Biomaterials 2014, 35, 2234–2244. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Wieczorek, M. Epineurium-mimicking chitosan conduits for peripheral nervous tissue engineering. Carbohydr. Polym. 2016, 152, 119–128. [Google Scholar] [CrossRef]
- Ao, Q.; Fung, C.K.; Tsui, A.Y.; Cai, S.; Zuo, H.C.; Chan, Y.S.; Shum, D.K. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials 2011, 32, 787–796. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Lu, S.; Zhang, T.; Zhou, N.; Ren, P.; Wang, F.; Yang, Y.; Ji, Z. Assembled anti-adhesion polypropylene mesh with self-fixable and degradable in situ mussel-inspired hydrogel coating for abdominal wall defect repair. Biomater. Sci. 2018, 6, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Marchand, C.; Rivard, G.E.; El-Gabalawy, H.; Poubelle, P.E. Effect of chitosan and coagulation factors on the wound repair phenotype of bioengineered blood clots. Int. J. Biol. Macromol. 2017, 104 Pt B, 1916–1924. [Google Scholar] [CrossRef]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira Junior, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hou, X.; Ma, B.; Xu, H.; Yang, Y. Chitosan/gallnut tannins composite fiber with improved tensile, antibacterial and fluorescence properties. Carbohydr. Polym. 2019, 226, 115311. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved hydrophobicity, antibacterial and mechanical properties of polyvinyl alcohol/quaternary chitosan composite films for antibacterial packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef]
- Zou, Z.; Ismail, B.; Zhang, X.; Yang, Z.; Liu, D.; Guo, M. Improving barrier and antibacterial properties of chitosan composite films by incorporating lignin nanoparticles and acylated soy protein isolate nanogel. Food Hydrocoll. 2023, 134, 108091. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, N.; Wang, Q.; Wang, P.; Yuan, J.; Shen, J.; Fan, X. Disulfide bond reconstruction: A novel approach for grafting of thiolated chitosan onto wool. Carbohydr. Polym. 2019, 203, 369–377. [Google Scholar] [CrossRef]
- Hassan, M.M. Binding of a quaternary ammonium polymer-grafted-chitosan onto a chemically modified wool fabric surface: Assessment of mechanical, antibacterial and antifungal properties. RSC Adv. 2015, 5, 35497–35505. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W.; Tao, K.; Song, Y.; Xie, H.; Wang, J.; Li, X.; Shuai, X.; Gao, J.; Chang, P.; et al. Sustained Local Release of NGF from a Chitosan-Sericin Composite Scaffold for Treating Chronic Nerve Compression. ACS Appl. Mater. Interfaces 2017, 9, 3432–3444. [Google Scholar] [CrossRef]
- Irshad, A.; Sarwar, N.; Sadia, H.; Malik, K.; Javed, I.; Irshad, A.; Afzal, M.; Abbas, M.; Rizvi, H. Comprehensive facts on dynamic antimicrobial properties of polysaccharides and biomolecules-silver nanoparticle conjugate. Int. J. Biol. Macromol. 2020, 145, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Gylienė, O.; Servienė, E.; Vepštaitė, I.; Binkienė, R.; Baranauskas, M.; Lukša, J. Correlation between the sorption of dissolved oxygen onto chitosan and its antimicrobial activity against Esherichia coli. Carbohydr. Polym. 2015, 131, 218–223. [Google Scholar] [CrossRef]
- Tang, Y.; Li, N.; Duan, J.A.; Tao, W. Structure, bioactivity, and chemical synthesis of OSW-1 and other steroidal glycosides in the genus Ornithogalum. Chem. Rev. 2013, 113, 5480–5514. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Prakash, S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 2018, 186, 54–63. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Bible, E.; Qutachi, O.; Chau, D.Y.; Alexander, M.R.; Shakesheff, K.M.; Modo, M. Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials 2012, 33, 7435–7446. [Google Scholar] [CrossRef]
- Muley, A.B.; Shingote, P.R.; Patil, A.P.; Dalvi, S.G.; Suprasanna, P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.). Carbohydr. Polym. 2019, 210, 289–301. [Google Scholar] [CrossRef]
- Chen, X.M.; Chen, Y.; Hou, X.F.; Wu, X.; Gu, B.H.; Liu, Y. Sulfonato-β-Cyclodextrin Mediated Supramolecular Nanoparticle for Controlled Release of Berberine. ACS Appl. Mater. Interfaces 2018, 10, 24987–24992. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Wang, G.; Lu, P.; Qiao, P.; Zhang, P.; Cai, X.; Tang, L.; Qian, T.; Wang, H. Blood vessel remodeling in late stage of vascular network reconstruction is essential for peripheral nerve regeneration. Bioeng. Transl. Med. 2022, 7, e10361. [Google Scholar] [CrossRef]
- Ahadi, S.; Zhou, W.; Schüssler-Fiorenza Rose, S.M.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef]
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnology 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Smelcerovic, A.; Knezevic-Jugovic, Z.; Petronijevic, Z. Microbial polysaccharides and their derivatives as current and prospective pharmaceuticals. Curr. Pharm. Des. 2008, 14, 3168–3195. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, M.; Liu, S.Y.; Zhang, F.S.; Wan, T.; Ding, Z.T.; Zhang, P.X. Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration. Polymers 2021, 13, 3957. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Zen, F.; Nato, G.; Fogli, M.; Luzzati, F.; Ronchi, G.; Raimondo, S.; Gambarotta, G. Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits. Int. J. Mol. Sci. 2022, 23, 2254. [Google Scholar] [CrossRef]
- Muheremu, A.; Chen, L.; Wang, X.; Wei, Y.; Gong, K.; Ao, Q. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci. Rep. 2017, 7, 44002. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Li, L.; Yin, J.; Fu, J. Additive-lathe 3D bioprinting of bilayered nerve conduits incorporated with supportive cells. Bioact. Mater. 2021, 6, 219–229. [Google Scholar] [CrossRef]
- Millesi, F.; Weiss, T.; Mann, A.; Haertinger, M.; Semmler, L.; Supper, P.; Pils, D.; Naghilou, A.; Radtke, C. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021, 35, e21196. [Google Scholar] [CrossRef]
- Chen, Z.X.; Lu, H.B.; Jin, X.L.; Feng, W.F.; Yang, X.N.; Qi, Z.L. Skeletal muscle-derived cells repair peripheral nerve defects in mice. Neural Regen. Res. 2020, 15, 152–161. [Google Scholar] [PubMed]
- Hussin, H.M.; Lawi, M.M.; Haflah, N.H.M.; Kassim, A.Y.M.; Idrus, R.B.H.; Lokanathan, Y. Centella asiatica (L.)-Neurodifferentiated Mesenchymal Stem Cells Promote the Regeneration of Peripheral Nerve. Tissue Eng. Regen. Med. 2020, 17, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-Y.; Miao, Y.; Wang, X.H.; Wang, P.; Cheng, Z.C.; Qian, T.M. Increased levels of miR-3099 induced by peripheral nerve injury promote Schwann cell proliferation and migration. Neural Regen. Res. 2019, 14, 525–531. [Google Scholar] [PubMed]
- Jones, I.; Novikova, L.N.; Novikov, L.N.; Renardy, M.; Ullrich, A.; Wiberg, M.; Carlsson, L.; Kingham, P.J. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J. Tissue Eng. Regen. Med. 2018, 12, e2099–e2109. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhao, M.; Yi, X.; Tao, J.; Li, S.; Jiang, Z.; Cheng, B.; Yuan, H.; Zhang, F. Acellular nerve grafts supplemented with induced pluripotent stem cell-derived exosomes promote peripheral nerve reconstruction and motor function recovery. Bioact. Mater. 2022, 15, 272–287. [Google Scholar] [CrossRef]
- Huang, Z.; Powell, R.; Phillips, J.B.; Haastert-Talini, K. Perspective on Schwann Cells Derived from Induced Pluripotent Stem Cells in Peripheral Nerve Tissue Engineering. Cells 2020, 9, 2497. [Google Scholar] [CrossRef]
- Malheiro, A.; Harichandan, A.; Bernardi, J.; Seijas-Gamardo, A.; Konings, G.F.; Volders, P.G.A.; Romano, A.; Mota, C.; Wieringa, P.; Moroni, L. 3D culture platform of human iPSCs-derived nociceptors for peripheral nerve modeling and tissue innervation. Biofabrication 2022, 14, 014105. [Google Scholar] [CrossRef]
- Soman, S.S.; Vijayavenkataraman, S. Perspectives on 3D Bioprinting of Peripheral Nerve Conduits. Int. J. Mol. Sci. 2020, 21, 5792. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef]
- Shen, C.-C.; Yang, Y.-C.; Liu, B.-S. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J. Biomed. Mater. Res. Part A 2012, 100A, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, C.F.; Peng, J.; Hu, C.D.; Wang, Y. Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen. Res. 2017, 12, 2106–2112. [Google Scholar] [PubMed]
- Tokutake, K.; Takeuchi, M.; Kurimoto, S.; Saeki, S.; Asami, Y.; Onaka, K.; Saeki, M.; Aoyama, T.; Hasegawa, Y.; Hirata, H. A Therapeutic Strategy for Lower Motor Neuron Disease and Injury Integrating Neural Stem Cell Transplantation and Functional Electrical Stimulation in a Rat Model. Int. J. Mol. Sci. 2022, 23, 8760. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Y.T.; Tsang, K.S.; Sun, C.R.; Li, J.; Huang, H.; Cui, F.Z.; An, Y.H. Implantation of neural stem cells embedded in hyaluronic acid and collagen composite conduit promotes regeneration in a rabbit facial nerve injury model. J. Transl. Med. 2008, 6, 67. [Google Scholar] [CrossRef]
- Takeda, I.; Yoshihara, K.; Cheung, D.L.; Kobayashi, T.; Agetsuma, M.; Tsuda, M.; Eto, K.; Koizumi, S.; Wake, H.; Moorhouse, A.J.; et al. Controlled activation of cortical astrocytes modulates neuropathic pain-like behaviour. Nat. Commun. 2022, 13, 4100. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Cui, S.S.; Wang, X.X.; Chen, L.; Liu, F.; Gao, J.; Wang, W. Astrocytic c-Jun N-terminal kinase-histone deacetylase-2 cascade contributes to glutamate transporter-1 decrease and mechanical allodynia following peripheral nerve injury in rats. Brain Res. Bull. 2021, 175, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tong, Z.; Luo, L.; Zhao, Y.; Chen, F.; Li, Y.; Huselstein, C.; Ye, Q.; Ye, Q.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef]
- Mandemakers, W.; Zwart, R.; Jaegle, M.; Walbeehm, E.; Visser, P.; Grosveld, F.; Meijer, D. A distal Schwann cell-specific enhancer mediates axonal regulation of the Oct-6 transcription factor during peripheral nerve development and regeneration. EMBO J. 2000, 19, 2992–3003. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Wu, L.; Wu, L.; Dhanjai; Fu, L.; Gao, Y.; Chen, J. Response Characteristics of Bisphenols on a Metal-Organic Framework-Based Tyrosinase Nanosensor. ACS Appl. Mater. Interfaces 2016, 8, 16533–16539. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Rudraiah, S.; Arul, M.R.; Bartley, J.M.; Baker, J.T.; Yu, X.; Kumbar, S.G. Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: In vivo structural and functional assessment. Bioact. Mater. 2021, 6, 2881–2893. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.Y.; Zhang, M.; Pi, W.; Wang, B.; Li, Q.C.; Lu, C.F.; Zhang, P.X. Sustained release of exosomes loaded into polydopamine-modified chitin conduits promotes peripheral nerve regeneration in rats. Neural Regen. Res. 2022, 17, 2050–2057. [Google Scholar] [PubMed]
- Nawrotek, K.; Tylman, M.; Adamus-Włodarczyk, A.; Rudnicka, K.; Gatkowska, J.; Wieczorek, M.; Wach, R. Influence of chitosan average molecular weight on degradation and stability of electrodeposited conduits. Carbohydr. Polym. 2020, 244, 116484. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef]
- Lackington, W.A.; Kočí, Z.; Alekseeva, T.; Hibbitts, A.J.; Kneafsey, S.L.; Chen, G.; O’Brien, F.J. Controlling the dose-dependent, synergistic and temporal effects of NGF and GDNF by encapsulation in PLGA microparticles for use in nerve guidance conduits for the repair of large peripheral nerve defects. J. Control. Release 2019, 304, 51–64. [Google Scholar] [CrossRef]
- Qian, C.; Tan, D.; Wang, X.; Li, L.; Wen, J.; Pan, M.; Li, Y.; Wu, W.; Guo, J. Peripheral Nerve Injury-Induced Astrocyte Activation in Spinal Ventral Horn Contributes to Nerve Regeneration. Neural Plast. 2018, 2018, 8561704. [Google Scholar] [CrossRef]
- Richner, M.; Pallesen, L.T.; Ulrichsen, M.; Poulsen, E.T.; Holm, T.H.; Login, H.; Castonguay, A.; Lorenzo, L.E.; Gonçalves, N.P.; Andersen, O.M.; et al. Sortilin gates neurotensin and BDNF signaling to control peripheral neuropathic pain. Sci. Adv. 2019, 5, eaav9946. [Google Scholar] [CrossRef]
- Guénard, V.; Dinarello, C.A.; Weston, P.J.; Aebischer, P. Peripheral nerve regeneration is impeded by interleukin-1 receptor antagonist released from a polymeric guidance channel. J. Neurosci. Res. 1991, 29, 396–400. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.J.; Wang, J.; Li, D.; Ren, W.J.; Peng, J.; Wei, X.; Xu, T.; Xin, W.J.; Pang, R.P.; et al. TNF-α Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury. J. Neurosci. 2017, 37, 871–881. [Google Scholar] [CrossRef]
- Rotshenker, S.; Aamar, S.; Barak, V. Interleukin-1 activity in lesioned peripheral nerve. J. Neuroimmunol. 1992, 39, 75–80. [Google Scholar] [CrossRef]
- Feltri, M.L.; Graus Porta, D.; Previtali, S.C.; Nodari, A.; Migliavacca, B.; Cassetti, A.; Littlewood-Evans, A.; Reichardt, L.F.; Messing, A.; Quattrini, A.; et al. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 2002, 156, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Frieboes, L.S.; Forootan, M.; Palispis, W.A.; Mozaffar, T.; Jafari, M.; Steward, O.; Gall, C.M.; Gupta, R. Biophysical stimulation induces demyelination via an integrin-dependent mechanism. Ann. Neurol. 2012, 72, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-T.; Lee, C.M.; Lin, Y.C.; Chen, C.Y.; Liao, C.C.; Lee, H.C.; Day, Y.J. P-selectin is required for neutrophils and macrophage infiltration into injured site and contributes to generation of behavioral hypersensitivity following peripheral nerve injury in mice. Pain 2013, 154, 2150–2159. [Google Scholar] [CrossRef]
- Remacle, A.G.; Hullugundi, S.K.; Dolkas, J.; Angert, M.; Chernov, A.V.; Strongin, A.Y.; Shubayev, V.I. Acute- and late-phase matrix metalloproteinase (MMP)-9 activity is comparable in female and male rats after peripheral nerve injury. J. Neuroinflammation 2018, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zha, G.B.; Yu, J.; Zhang, H.H.; Yi, S. Differential temporal expression of matrix metalloproteinases following sciatic nerve crush. Neural Regen. Res. 2016, 11, 1165–1171. [Google Scholar] [PubMed]
- Scarlato, M.; Previtali, S.C.; Carpo, M.; Pareyson, D.; Briani, C.; Del Bo, R.; Nobile-Orazio, E.; Quattrini, A.; Comi, G.P. Polyneuropathy in POEMS syndrome: Role of angiogenic factors in the pathogenesis. Brain 2005, 128, 1911–1920. [Google Scholar] [CrossRef]
- Saffari, T.M.; Bedar, M.; Hundepool, C.A.; Bishop, A.T.; Shin, A.Y. The role of vascularization in nerve regeneration of nerve graft. Neural Regen. Res. 2020, 15, 1573–1579. [Google Scholar]
- Liu, F.; Hao, F.; Hao, P.; Zhao, W.; Gao, Y.; Duan, H.; Yang, Z.; Li, X. bFGF-chitosan scaffolds effectively repair 20 mm sciatic nerve defects in adult rats. Biomed. Mater. 2021, 16, 025011. [Google Scholar] [CrossRef]
- Fujimaki, H.; Uchida, K.; Inoue, G.; Miyagi, M.; Nemoto, N.; Saku, T.; Isobe, Y.; Inage, K.; Matsushita, O.; Yagishita, S.; et al. Oriented collagen tubes combined with basic fibroblast growth factor promote peripheral nerve regeneration in a 15 mm sciatic nerve defect rat model. J. Biomed. Mater. Res. Part A 2017, 105, 8–14. [Google Scholar] [CrossRef]
- Runge, E.M.; Iyer, A.K.; Setter, D.O.; Kennedy, F.M.; Sanders, V.M.; Jones, K.J. CD4+ T cell expression of the IL-10 receptor is necessary for facial motoneuron survival after axotomy. J. Neuroinflammation 2020, 17, 121. [Google Scholar] [CrossRef]
- Fonseca, M.M.; Davoli-Ferreira, M.; Santa-Cecília, F.; Guimarães, R.M.; Oliveira, F.F.B.; Kusuda, R.; Ferreira, D.W.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M. IL-27 Counteracts Neuropathic Pain Development Through Induction of IL-10. Front. Immunol. 2020, 10, 3059. [Google Scholar] [CrossRef] [PubMed]
- Mietto, B.S.; Kroner, A.; Girolami, E.I.; Santos-Nogueira, E.; Zhang, J.; David, S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J. Neurosci. 2015, 35, 16431–16442. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, C.; Wu, J.; Chen, X.; He, H. Effect of TGF-β1-Mediated Exercise Analgesia in Spared Nerve Injury Mice. Neural Plast. 2022, 2022, 7382327. [Google Scholar] [CrossRef]
- Morris, A.D.; Lewis, G.M.; Kucenas, S. Perineurial Glial Plasticity and the Role of TGF-β in the Development of the Blood–Nerve Barrier. J. Neurosci. 2017, 37, 4790–4807. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Hao, M.; Wang, D.; Jiang, Z.; Sun, L.; Gao, Y.; Jin, Y.; Lei, P.; Zhuo, Y. The application of collagen in the repair of peripheral nerve defect. Front. Bioeng. Biotechnol. 2022, 10, 973301. [Google Scholar] [CrossRef] [PubMed]
- Zainul, Z.; Heikkinen, A.; Koivisto, H.; Rautalahti, I.; Kallio, M.; Lin, S.; Härönen, H.; Norman, O.; Rüegg, M.A.; Tanila, H.; et al. Collagen XIII Is Required for Neuromuscular Synapse Regeneration and Functional Recovery after Peripheral Nerve Injury. J. Neurosci. 2018, 38, 4243–4258. [Google Scholar] [CrossRef]
- Mao, W.; Lee, E.; Cho, W.; Kang, B.J.; Yoo, H.S. Cell-directed assembly of luminal nanofibril fillers in nerve conduits for peripheral nerve repair. Biomaterials 2023, 301, 122209. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Liu, Z.; Liu, Z.H.; Chen, S.P.; Li, M.; Shahveranov, A.; Ye, D.W.; Tian, Y.K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflammation 2016, 13, 141. [Google Scholar] [CrossRef]
- Qin, H.-J.; Li, H.; Chen, J.Z.; Zhang, K.R.; Zhao, X.Q.; Qin, J.Q.; Yu, B.; Yang, J. Artificial nerve graft constructed by coculture of activated Schwann cells and human hair keratin for repair of peripheral nerve defects. Neural Regen. Res. 2023, 18, 1118–1123. [Google Scholar]
- Eskilsson, A.; Shionoya, K.; Engblom, D.; Blomqvist, A. Fever During Localized Inflammation in Mice Is Elicited by a Humoral Pathway and Depends on Brain Endothelial Interleukin-1 and Interleukin-6 Signaling and Central EP3 Receptors. J. Neurosci. 2021, 41, 5206–5218. [Google Scholar] [CrossRef]
- Eccleston, P.A.; Jessen, K.R.; Mirsky, R. Transforming growth factor-β and γ-interferon have dual effects on growth of peripheral glia. J. Neurosci. Res. 1989, 24, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kong, Y.; Shi, W.; Kuss, M.; Liao, K.; Hu, G.; Xiao, P.; Sankarasubramanian, J.; Guda, C.; Wang, X.; et al. Exosomes derived from differentiated human ADMSC with the Schwann cell phenotype modulate peripheral nerve-related cellular functions. Bioact. Mater. 2022, 14, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Hussain, A.; Behfar, A.; Moran, S.L.; Zhao, C. The Therapeutic Potential of Exosomes in Soft Tissue Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 3869. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Hao, D.; Zhang, L.; Qin, J.; Tian, G.; Ma, B.; Zhou, X. Endocytosis-associated patterns in nerve regeneration after peripheral nerve injury. J. Orthop. Transl. 2021, 31, 10–19. [Google Scholar] [CrossRef]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; Strauß, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Lin, H. Fabrication and characterization of a self-crosslinking chitosan hydrogel under mild conditions without the use of strong bases. Carbohydr. Polym. 2017, 156, 372–379. [Google Scholar] [CrossRef]
- Rickett, T.A.; Amoozgar, Z.; Tuchek, C.A.; Park, J.; Yeo, Y.; Shi, R. Rapidly photo-cross-linkable chitosan hydrogel for peripheral neurosurgeries. Biomacromolecules 2011, 12, 57–65. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Luo, Y.; Mills, D.K. The Effect of Halloysite Addition on the Material Properties of Chitosan-Halloysite Hydrogel Composites. Gels 2019, 5, 40. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, N.; Xiong, L.; Sun, Q. Rapid gelling, self-healing, and fluorescence-responsive chitosan hydrogels formed by dynamic covalent crosslinking. Carbohydr. Polym. 2020, 246, 116586. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Liu, A.; Wu, L.; Wang, D.; Han, Q.; Zheng, T.; Wang, F.; Kong, Y.; Li, G.; et al. Development of a polyacrylamide/chitosan composite hydrogel conduit containing synergistic cues of elasticity and topographies for promoting peripheral nerve regeneration. Biomater. Sci. 2022, 10, 4915–4932. [Google Scholar] [CrossRef] [PubMed]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan-Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, H.; Yang, Y.Y.; Huang, J.X.; Chen, Y.J.; Yang, F.; Yan, J.Z. A double-network hydrogel for the dynamic compression of the lumbar nerve root. Neural Regen. Res. 2020, 15, 1724–1731. [Google Scholar]
- Bakhshandeh, S.; Gorgin Karaji, Z.; Lietaert, K.; Fluit, A.C.; Boel, C.H.E.; Vogely, H.C.; Vermonden, T.; Hennink, W.E.; Weinans, H.; Zadpoor, A.A.; et al. Simultaneous Delivery of Multiple Antibacterial Agents from Additively Manufactured Porous Biomaterials to Fully Eradicate Planktonic and Adherent Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 25691–25699. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Bettini, R.; Elviri, L. 3D Printed Chitosan/Alginate Hydrogels for the Controlled Release of Silver Sulfadiazine in Wound Healing Applications: Design, Characterization and Antimicrobial Activity. Micromachines 2023, 14, 137. [Google Scholar] [CrossRef]
- Elango, J.; Saravanakumar, K.; Rahman, S.U.; Henrotin, Y.; Regenstein, J.M.; Wu, W.; Bao, B. Chitosan-Collagen 3D Matrix Mimics Trabecular Bone and Regulates RANKL-Mediated Paracrine Cues of Differentiated Osteoblast and Mesenchymal Stem Cells for Bone Marrow Macrophage-Derived Osteoclastogenesis. Biomolecules 2019, 9, 173. [Google Scholar] [CrossRef]
- Liu, K.; Yan, L.; Li, R.; Song, Z.; Ding, J.; Liu, B.; Chen, X. 3D Printed Personalized Nerve Guide Conduits for Precision Repair of Peripheral Nerve Defects. Adv. Sci. 2022, 9, e2103875. [Google Scholar] [CrossRef]
- Yang, J.; Yang, K.; Man, W.; Zheng, J.; Cao, Z.; Yang, C.Y.; Kim, K.; Yang, S.; Hou, Z.; Wang, G.; et al. 3D bio-printed living nerve-like fibers refine the ecological niche for long-distance spinal cord injury regeneration. Bioact. Mater. 2023, 25, 160–175. [Google Scholar] [CrossRef]
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv. Funct. Mater. 2020, 30, 1906237. [Google Scholar] [CrossRef]
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid. Interface Sci. 2020, 574, 162–173. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Huang, Y.; Wu, W.; Deng, X.; Liu, H.; Li, R.; Tao, J.; Li, X.; Liu, X.; et al. A 3D-Printed Self-Adhesive Bandage with Drug Release for Peripheral Nerve Repair. Adv. Sci. 2020, 7, 2002601. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.Y.; Kuo, T.Y.; Hung, S.C.; Lin, C.M.; Hsien, T.Y.; Wang, D.M.; Hsieh, H.J. Use of gum arabic to improve the fabrication of chitosan-gelatin-based nanofibers for tissue engineering. Carbohydr. Polym. 2015, 115, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Hsueh, Y.Y.; Koo, J.; Yang, Q.; Avila, R.; Hu, B.; Xie, Z.; Lee, G.; Ning, Z.; Liu, C.; et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 2020, 11, 5990. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Potential of electrospun core–shell structured gelatin–chitosan nanofibers for biomedical applications. Carbohydr. Polym. 2016, 136, 1098–1107. [Google Scholar] [CrossRef]
- Qi, T.; Zhang, X.; Gu, X.; Cui, S. Experimental Study on Repairing Peripheral Nerve Defects with Novel Bionic Tissue Engineering. Adv. Healthc. Mater. 2023, 12, e2203199. [Google Scholar] [CrossRef]





| Cell Types | Origins | Characteristics | Applications | Reference |
|---|---|---|---|---|
| Adult Neural Stem Cells | Brain and bone marrow | Self-renew and differentiate into various types of neural cells | Promote neural regeneration and repair | [63] |
| Human Embryonic Stem Cells | Early-stage human embryos | Broad differentiation potential, capable of generating various cell types | Repair peripheral nerves | [64,65] |
| Induced Pluripotent Stem Cells | Reprogrammed from adult body cells | Induced to differentiate into neural cells | Treat neural injuries and diseases | [66,67,68,69] |
| Adipose-Derived Stem Cells | Human adipose tissue | Relatively easy to obtain and expand | Promote neural regeneration | [70,71] |
| Peripheral Neural Stem Cells | Peripheral nervous system | Differentiate into various types of neural cells | Transplantation and facilitating neural regeneration and repair | [72,73,74] |
| Astrocytes | Neural progenitor cells | Provide support and nourishment for neuronal survival | Provide structural support, regulate the chemical environment | [75,76] |
| Schwann Cells | Neural crest | Generate myelin sheaths and promote neuronal regeneration and repair | Wrap around peripheral nerve fibers and form myelin sheaths | [77,78] |
| Factor Types | Including | Origins | Characteristics | Reference |
|---|---|---|---|---|
| Neurotrophic Factors | Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), etc. | Neurons, astrocytes, and immune cells. | Facilitate neurite outgrowth, enhance neuronal cell survival and function | [85,86,87] |
| Inflammatory Factors | Tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β) | Macrophages and lymphocytes. | Promote the occurrence and regulation of inflammatory responses, influencing neural repair | [88,89,90] |
| Cell Adhesion Molecules | Neural cell adhesion molecule (NCAM), integrins, and selectins | Nerve cells | Regulating neuronal migration, positioning, and connectivity | [91,92,93] |
| Matrix Metalloproteinases | Enzymes | Macrophages, astrocytes, and endothelial cells | Cell migration and neurite formation | [94,95] |
| Angiogenic Factors | Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) | Endothelial cells, astrocytes, and inflammatory cells | Promote the growth of blood vessels, increasing the supply of oxygen and nutrients | [96,97,98,99] |
| Immunomodulatory Factors | Interleukin-10 (IL-10), transforming growth factor-beta (TGF-β) | Immune cells, astrocytes | Regulate immune responses, inhibit excessive immune reactions and inflammation | [100,101,102,103,104] |
| Fibrotic Factors | Collagen proteins, fibronectin, | Fibroblasts and inflammatory cells | Hinder scar formation and impede neural regeneration | [105,106,107] |
| Cytokines | Immune cells, astrocytes, and neurons | Interleukins, interferons, | Regulation of immune and inflammatory responses | [108,109,110,111] |
| Exosomes | Proteins, nucleic acids, and lipids | Neural cells, astrocytes, and immune cells | Intercellular communication, transferring functional molecules and signals between cells | [66,112,113,114,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; An, H.; Zhang, F.; Jiang, H.; Wan, T.; Wen, Y.; Han, N.; Zhang, P. Prospects of Using Chitosan-Based Biopolymers in the Treatment of Peripheral Nerve Injuries. Int. J. Mol. Sci. 2023, 24, 12956. https://doi.org/10.3390/ijms241612956
Zhang M, An H, Zhang F, Jiang H, Wan T, Wen Y, Han N, Zhang P. Prospects of Using Chitosan-Based Biopolymers in the Treatment of Peripheral Nerve Injuries. International Journal of Molecular Sciences. 2023; 24(16):12956. https://doi.org/10.3390/ijms241612956
Chicago/Turabian StyleZhang, Meng, Heng An, Fengshi Zhang, Haoran Jiang, Teng Wan, Yongqiang Wen, Na Han, and Peixun Zhang. 2023. "Prospects of Using Chitosan-Based Biopolymers in the Treatment of Peripheral Nerve Injuries" International Journal of Molecular Sciences 24, no. 16: 12956. https://doi.org/10.3390/ijms241612956





